Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Copeptin as a Biomarker of Microcirculation Alterations in Systemic Sclerosis
Authors Maciejewska M , Stec A , Zaremba M, Maciejewski C, Rudnicka L, Sikora M
Received 27 February 2023
Accepted for publication 3 May 2023
Published 25 May 2023 Volume 2023:16 Pages 1351—1361
DOI https://doi.org/10.2147/CCID.S409490
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Magdalena Maciejewska,1 Albert Stec,2 Michał Zaremba,2 Cezary Maciejewski,3 Lidia Rudnicka,2 Mariusz Sikora4
1Department of Dermatology, Doctoral School of Medical University of Warsaw, Warsaw, Poland; 2Department of Dermatology, Medical University of Warsaw, Warsaw, Poland; 3 1st Department of Cardiology, Medical University of Warsaw, Warsaw, Poland; 4National Institute of Geriatrics, Rheumatology and Rehabilitation, Warsaw, Poland
Correspondence: Mariusz Sikora, National Institute of Geriatrics, Rheumatology and Rehabilitation, Spartańska 1, Warsaw, 02-637, Poland, Email [email protected]
Background: Systemic sclerosis is a connective tissue disease characterized by vasculopathy and progressive fibrosis, leading to multiorgan dysfunction. Given the complex and not fully elucidated pathogenesis, biomarkers of rapid disease progression and therapeutic response are lacking. Copeptin, which reflects vasopressin activity in serum, is used in diagnosing or prognosing different cardiometabolic conditions.
Objective: The aim of study was to investigate the concentration of copeptin in patients with systemic sclerosis and correlate it with specific clinical symptoms.
Patients and Methods: Serum copeptin was measured in patients with systemic sclerosis (34 women and 3 men; mean age 57.6 years) and in healthy individuals (n=30) using commercially available ELISA kits. According to the criteria of LeRoy our systemic sclerosis cohort consisted of 17 patients with limited cutaneous systemic sclerosis (45.9%) and 20 diffuse cutaneous systemic sclerosis patients (54.1%). According to the criteria of LeRoy our systemic sclerosis cohort consisted of 17 patients with limited cutaneous systemic sclerosis (45.9%) and 20 diffuse cutaneous systemic sclerosis patients (54.1%). The median duration of the disease was 10 [4– 14] years.
Results: We found significantly higher copeptin concentration in patients with systemic sclerosis (4.21 pmol/L [3.04– 5.42]) in comparison to control group (3.40 pmol/L [2.38– 3.76], p< 0.01). Copeptin significantly correlated with Raynaud’s condition score (r=0.801, p< 0.05). Patients with “late” capillaroscopic patterns had higher copeptin concentrations (5.37 pmol/L [4.29– 8.06]) than patients with “early” (2.43 pmol/L [2.25– 3.20], p< 0.05) and “active” patterns (3.93 pmol/L [2.92– 5.16], p< 0.05]). Copeptin was found to be significantly higher in SSc patients with DUs (5.71 pmol/L [IQR 4.85– 8.06]) when compared to SSc patients without DUs (3.31 pmol/L, [2.28– 4.30], p< 0.05). Additionally, copeptin concentration had good diagnostic accuracy in discriminating between patients with and without digital ulcers (AUC=0.863). Alprostadil decreased copeptin concentration from 4.96 [4.02– 6.01] to 3.86 pmol/L [3.17– 4.63] (p< 0.01) after 4– 6 cycles of administration.
Conclusion: Our findings suggest that copeptin may be a promising biomarker of microcirculation alterations in systemic sclerosis.
Keywords: serum biomarkers, vasculopathy, endothelial dysfunction, digital ulcers, systemic sclerosis
Introduction
Systemic sclerosis (SSc) is an autoimmune connective tissue disease associated with progressive fibrosis of the skin and internal organs, widespread peripheral vasculopathy, and immune dysfunction.1 Although tissue fibrosis is the hallmark of SSc, vascular damage plays an important role in the early stages of disease pathogenesis and may precede fibrosis by months or years.2 SSc-associated microvasculopathy clinically manifests as Raynaud’s phenomenon, refractory ischemic digital ulcers (DUs), scleroderma renal crisis (SRC), or pulmonary arterial hypertension (PAH).3–5 Digital ulcers occur in up to 50% of all SSc patients.6 These disabling and painful lesions are recurrent, refractory to treatment and may lead to auto-amputation, infection, impaired hand function, and decreased quality of life.7,8
Vascular alteration in SSc involves vasculopathy, thrombosis and deregulation of vascular tone control.9 Blood vessel resistance is regulated by a complex interplay between sympathetic modulation, local and systemic vasoactive mediators, such as angiotensin II, endothelin-1 and vasopressin.10 Angiotensin II (Ang II) is a highly active octapeptide involved in the regulation of blood pressure. Angiotensin II has two major receptor subtypes, type 1 (AT1R) and type 2 (AT2R), members of the seven transmembranes spanning the G protein-coupled receptor superfamily.11 Although classically known for its role in the maintenance of circulatory system homeostasis, Ang II is now recognized as a mediator of inflammation and fibrosis through stimulation of AT1R.12,13 Pathophysiological significance of the disturbances in the angiotensin I/angiotensin II/angiotensin-(1-7) axis has been confirmed in SSc.14 Endothelin-1 (ET-1) is another potent endogenous vasoconstrictor. Moreover, ET-1 causes fibrosis of the vascular cells, stimulates production of reactive oxygen species and proinflammatory cytokines.15,16 Agonistic autoantibodies targeted against AT1 and ET-1 type A receptors in patients with SSc predict mortality and are associated with more severe organ involvement.17
Vasopressin (AVP) is a vasoactive peptide that, like Ang II and ET-1, may be involved in vasoconstriction, inflammation, and fibrosis.18 So far, AVP has been intensively studied in various pathologies of the cardiovascular system.19 To the best of our knowledge, there were no studies of vasopressinergic system in SSc. However, the measurements of serum vasopressin concentrations are limited in clinical practice due to the short half-life time, low molecular stability and binding to platelets, which leads to a decrease in the actual peptide concentration.20 Copeptin is the C-terminal part of pro-vasopressin, which is released together with AVP in equimolar amounts, making the copeptin an increasingly studied molecule to determine the vasopressinergic system activity. The role of copeptin as a biomarker has been examined in a variety of cardiovascular, kidney and metabolic disorders.21–23
Despite recent advances in SSc treatment, it is still challenging to identify patients at risk of rapid disease progression and development of severe organ complications. There is an urgent need to identify potential biomarkers of incipient vasculopathy before tissue damage becomes clinically apparent and irreversible.24 The primary aim of this study was to assess copeptin concentration in patients with SSc in the context of its use as a biomarker reflecting the severity of SSc-related peripheral microvasculopathy.
Materials and Methods
Study Design
The study was cross-sectional and prospective in design. A cohort of consecutive outpatients and inpatients diagnosed with SSc from the tertiary referral dermatological and rheumatological centers in Warsaw were enrolled in this study from September 2019 to May 2022.
Patients
The study population consisted of adults with SSc, fulfilling the American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) 2013 classification criteria25 and age- and sex-matched healthy volunteers.
The exclusion criteria for patients and control group were as follows: heart failure (NYHA class III and IV or decompensated), unstable ischemic heart disease, history of acute cardiovascular disease event in the last 6 months, hypotension, uncontrolled arterial hypertension or diabetes mellitus, pulmonary hypertension, hepatic or renal failure, active malignancies or neoplastic disease whose treatment has been completed in the last 5 years (except for basal cell carcinoma), active or chronic infection, pregnancy and breastfeeding. Patients with SSc and overlapped autoimmune, rheumatic or connective tissue diseases were not enrolled in this study. Primary Raynaud’s phenomenon was considered as an additional exclusion criterion for the control group.
Demographic, clinical, laboratory and immunological data were assessed according to polish and international expert recommendations.26,27
Clinical Assessment
All SSc patients underwent a thorough clinical examination with assessment of the following features: gender, age, body mass index (BMI), SSc subsets according to LeRoy et al28 time of disease since the onset of Raynaud’s phenomenon, time of disease since the first non-Raynaud symptom, presence of digital ulcers, synovitis, joint contractures, muscle involvement, esophageal and gastrointestinal symptoms, dyspnea, arrhythmia, impaired exercise tolerance, comorbidities, smoking history, current and previous treatment.
The extent of skin involvement was evaluated by the modified Rodnan Skin Score (mRSS).29 The skin involvement was assessed by only one assessor (M.S.) certified with Scleroderma Clinical Trials Consortium training.
Lung involvement was assessed via high-resolution computed tomography (HRCT) scan of the chest and pulmonary function tests (PFT), including forced vital capacity (FVC) and diffusing capacity for carbon monoxide (DLCO). Interstitial lung disease (ILD) was defined as the presence of pulmonary fibrosis on an HRCT (the study did not include a quantitative assessment of the lesions). Doppler echocardiography was used to screen for PAH. Gastrointestinal tract involvement was assessed with barium contrast X-ray of the esophagus and the stomach. These specialist diagnostic procedures were carried out in all patients with SSc as a part of regular follow-ups.
Laboratory Procedures
Laboratory parameters: whole blood count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) concentration, lipid profile, liver and kidney function tests, N-terminal pro-B-type natriuretic peptide (NT-proBNP) were quantified from peripheral blood during clinical routines. Antinuclear antibodies were evaluated by indirect immunofluorescence pattern on HEp-2 cells and detected by immunoblot analysis. Data on laboratory and immunological parameters were extracted from patients’ medical records.
All blood samples were collected from the peripheral vein, between 6 a.m. and 7 a.m. (after at least 8h fasting period). Serum aliquots obtained by centrifugation (3000 rpm for 10 min at 4°C) were stored at −80°C until further analysis.
Serum copeptin concentrations were determined at baseline and after 4–6 cycles of alprostadil administration. Thirty-two patients received intravenous alprostadil (Prostavasin®, UCB Pharma GmbH, Monheim, Germany) for 3 consecutive days at a daily dose of 60 μg diluted in 50 mL of 0.9% sodium chloride administered as a 5-h infusion. Alprostadil was administered every 4–6 weeks for Raynaud phenomenon as well as for the prevention and treatment of digital ulcers. Copeptin was assessed using a commercial ELISA kit (Human Copeptin ELISA Kit, Bioassay Technology Laboratory, Shanghai, China), according to the manufacturer’s instructions. All samples were tested in duplicate and performed in the same laboratory with the same instruments. The analytical sensitivity was 0.059 pmol/L. The coefficient of intra- and inter-assay repeatability was <8% and <10%, respectively.
Microvascular Assessment
The severity of Raynaud’s phenomenon was assessed with the validated Raynaud’s condition score (RCS).30 It is completed every day by patients as an 11-point Likert scale and average scores are taken over a 1-week period. We analyzed mean nailfold video-capillaroscopy (NVC) was performed by the same investigator (MM) with USB digital microscope Dino-Lite CapillaryScope 200 MEDL4HMA (AnMo Electronics Corporation, Taiwan) in all SSc patients. Microvascular abnormalities were classified into early, active or late scleroderma pattern according to Cutolo et al.31
Ethical Approval
All patients participating in the study gave their written informed consent before entering the study. The study was conducted in accordance with the principles of the Helsinki Declaration and the protocol approved by the local ethical committee (KB/90/2018; Bioethics Committee at the Medical University of Warsaw, Warsaw, Poland).
Statistical Analysis
Statistical analysis was conducted with Statistica software v.13.3 (TIBCO, Palo Alto, CA, USA). Data were tested for normal distribution using the Shapiro–Wilk test. Descriptive statistics for continuous variables were reported as the mean and standard deviation (SD) or median and interquartile range (IQR), as appropriate. Categorical variables were expressed as a number and percentage. Due to non-normal data distribution, non-parametric tests were used as follows: Mann–Whitney U for two independent samples, Kruskal–Wallis test with post hoc Dunn analysis for multiple independent comparisons, Wilcoxon rank test for two paired samples. Correlations between continuous variables were evaluated using the Spearman’s rank-sum coefficient. Receiver operating characteristic (ROC) analysis was performed to define cut-off values. The p-values of <0.05 were statistically significant.
Results
Patients’ Characteristics
Thirty-seven SSc patients (34 women and 3 men; mean age 57.6 years) and 30 age- and sex-matched control individuals (28 women, 2 men; mean age 53.7 years) were enrolled in this study. According to the criteria of LeRoy our SSc cohort consisted of 17 patients with limited cutaneous SSc (lcSSc; 45.9%) and 20 diffuse cutaneous SSc patients (dcSSc; 54.1%). The median duration of the disease was 10 years (IQR 4–14 years). Patients were treated with following vasoactive therapy: alprostadil (86.5%), calcium channel antagonists (21.6%), phosphodiesterase 5 inhibitors (51.3%), and sulodexide (67.6%). A summary of the main demographic, clinical, laboratory and serological characteristics of patients with SSc is shown in Table 1.
 |
Table 1 The Characteristics of Patients with SSc and Control Group |
Copeptin in Systemic Sclerosis
In patients with SSc serum copeptin concentration was 4.21 pmol/L (IQR 3.04–5.42) and it was significantly higher than in control group (median 3.40 pmol/L, IQR 2.38–3.76, p<0.01, Figure 1).
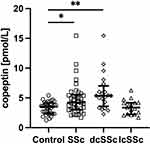 |
Figure 1 Serum concentration of copeptin in control group, patients with systemic sclerosis (SSc), diffuse cutaneous SSc (dcSSc) and limited cutaneous SSc (lcSSc), *p<0.05, **p<0.01. |
Further, we examined the possible association of copeptin with different SSc clinical phenotypes. When SSc cutaneous subsets are concerned, patients with dcSSc (median 5.06 pmol/L, IQR 3.15–5.60) had higher serum copeptin concentration compared with lcSSc (median 4.02 pmol/L, IQR 2.96–4.71, p<0.05) and control group (p<0.01, Figure 1). There was no significant difference between lcSSc and control group. No significant correlation was found between copeptin concentration and age, BMI and disease duration.
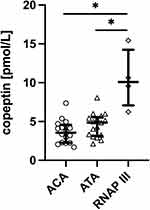
Sixteen (43.2%) SSc patients had anticentromere (ACA) antibodies, 17 (46.0%) were positive for antitopoisomerase I (ATA) antibodies and 4 (10.8%) patients had anti-RNA polymerase III antibodies (anti-RNAP III). When patients were stratified according to autoantibodies, there was significant difference between anti-RNAP-patients (median 10.09 pmol/L, IQR 7.90–13.04) and other antibody types: ACA-patients (median 3.56 pmol/L, IQR 2.27–4.46, p<0.05) or ATA-patients (median 4.85 pmol/L, IQR 3.25–5.42, p<0.05, Figure 2).
When analyzing copeptin concentration in SSc patients according to the different organ manifestations, no differences were found regarding the extent of skin fibrosis measured by mRSS, prevalence of interstitial lung disease (ILD) as well as patients with esophageal dysmotility.
Copeptin and Microvascular Impairment in SSc
All patients reported the presence of Raynaud’s phenomenon. We found a significant correlation between serum copeptin concentration and Raynaud’s condition score (rho = 0.801, p<0.05, Figure 3).
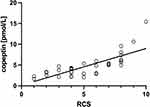 |
Figure 3 Correlation of copeptin with Raynaud’s condition score (RCS; r=0.801, p<0.05). |
The NVC pattern was found “early” in 6 (16.2%), “active” in 16 (43.2%) and “late” in 15 (40.6%) patients. When comparing copeptin concentration in SSc patients with different NVC patterns, the values of copeptin were significantly higher in the “late” pattern (median 5.37 pmol/L, IQR 4.29–8.06) in comparison to “early” (median 2.43 pmol/L, IQR 2.25–3.20, p<0.05) and “active” patterns (median 3.93 pmol/L, IQR 2.92–5.16, p<0.05, Figure 4).
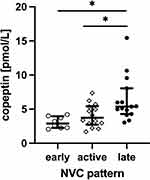 |
Figure 4 Serum concentration of copeptin in systemic sclerosis patients with “early”, “active” and “late” nailfold video-capillaroscopy (NVC) patterns, *p<0.05. |
In patients receiving alprostadil, serum concentration of copeptin significantly decreased from 4.96 pmol/L (IQR 4.02–6.01) to 3.86 pmol/L (IQR 3.17–4.63, p<0.01) after 4–6 cycles of drug administration (Figure 5).
 |
Figure 5 Serum concentration of copeptin in systemic sclerosis patients before and after 4–6 cycles of intravenous alprostadil administration. **p<0.01. |
As far as digital ulcers are concerned, copeptin was found to be significantly higher in SSc patients with DUs (median 5.71 pmol/L, IQR 4.85–8.06) when compared to SSc patients without DUs (median 3.31 pmol/L, IQR 2.28–4.30, p<0.05) and control group (p<0.05, Figure 6).
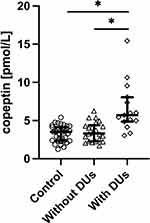 |
Figure 6 Serum concentration of copeptin in control group, patients with systemic sclerosis without and with digital ulcers (DUs). *p<0.05. |
The ROC curve analysis revealed copeptin cut-off value of 4.86 pmol/L in discriminating between SSc patients with and without DUs (sensitivity 80.0%, specificity 81.0%, area under the curve [AUC] = 0.863, Youden’s index 0.61, Figure 7).
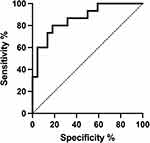 |
Figure 7 ROC curve analysis of copeptin in SSc patients without digital ulcers (DUs) vs SSc patients with DUs. |
Copeptin and Immunosuppression
Twenty patients with SSc (54.1%) received immunosuppressive therapy, while 17 (45.9%) were without immunosuppression. Among patients treated with immunosuppressive agents, 12 (60%) have dcSSc, 8 (40%) ACA, 8 (40%) ATA, 4 (20%) anti-RNAP III antibodies, 3 (15%) early, 8 (40%) active and 9 (45%) late NVC pattern. Respectively, in patients without immunosuppression, 8 (47.1%) have dcSSc, 9 (52.9%) ACA, 8 (47.1%) ATA, 0 anti-RNAP III antibodies, 3 (17.6%) early, 8 (47.1%) active and 6 (35.3%) late NVC pattern. There were no changes in copeptin concentration between patients currently treated with immunosuppressive drugs (median 4.25 pmol/L, IQR 3.56–5.40) and patients without immunosuppressive treatment (median 3.25 pmol/L, IQR 2.58–5.71, p=0.437).
Discussion
The key finding of this study is that increased copeptin concentration in patients with SSc is significantly associated with microcirculation dysfunction which presents as Raynaud’s phenomenon severity, nailfold capillary abnormalities and the presence of digital ulcers. Determination of serum copeptin concentration reflects changes in the activity of vasopressinergic system.32 Vasopressin is known to play an essential role in microcirculation regulation through activation of three receptor subtypes (V1a, V1b and V2). V1a receptors are expressed on the vascular smooth muscle cells and promote vasoconstriction. Activation of V1b receptors is part of the adaptive response to stress leading to stimulation of the hypothalamic-pituitary-adrenal axis. V2 receptors are associated with water reabsorption in the kidneys and release of coagulation factors from endothelial cells, such as von Willebrand factor and coagulation factor VIII.33,34
We found a significant correlation between serum copeptin concentration and Raynaud’s condition score, which is a validated outcome measure. Raynaud’s phenomenon (RP) is caused by an imbalance between endogenous vasoconstrictive agents (endothelin-1, angiotensin II) and endothelium-derived vasodilators (nitric oxide, prostacyclin).35 Vasopressin is another potent vasoconstrictor that is hypothesized to contribute to excessive vasoconstriction in RP.36,37
Moreover, relcovaptan (SR-49059), selective V1a receptor antagonist, has shown initial positive results in the treatment of Raynaud’s disease.37 Our study is the first evaluating the copeptin/vasopressin system in SSc-related microcirculation alterations. However, the diagnostic and prognostic value of copeptin has been reported also in other systemic diseases characterized by microangiopathy. Several studies indicated that AVP contributes to the development and progression of diabetic microangiopathy and serum copeptin may serve as an independent marker of renal, heart or retinal microcirculation involvement in type 2 diabetes patients.38
In our SSc group, higher concentrations of serum copeptin were observed in patients presenting “late” NVC pattern, which is characterized by irregular enlargement of the capillaries, few or absent giant capillaries and haemorrhages, ramified and bushy capillaries and progressive capillary loss (50–70%) with formation of extensive avascular areas (vascular desertification). These alterations, namely abnormal vessel morphology, changes in capillary number and disorganization of microcirculation architecture observed in the “late” NVC pattern stem from insufficient angio- and vasculogenesis.39,40 Inadequate processes of new blood vessels formation do not allow the regeneration of functional microcirculation and are related to imbalance between pro-angiogenic and angiostatic factors.41 Increased concentration of copeptin in SSc patients presenting “late” NVC pattern suggests that AVP might contribute to insufficient angiogenic response. This hypothesis is in accordance with the results from preclinical breast and colorectal cancer models showing that desmopressin, vasopressin peptide analog and a strong agonist of V2 receptor, reduces tumor-induced angiogenesis by production of endogenous angiostatic molecules (angiostatin) and reduction of the expression of different promoters of endothelial growth.42,43 Impaired neovascularization in SSc contributes to the development of DUs.44 In our cohort of SSc patients increased copeptin concentration was related to the presence of ischemic DUs. This finding confirms the importance of vasopressin in peripheral microcirculation disorders in the course of SSc and suggests that monitoring of copeptin concentrations may help to predict the prognosis of microangiopathy. While copeptin has not been studied in SSc so far, its properties as a biomarker of microcirculation abnormalities were studied in diabetic angiopathy.38 Baseline serum copeptin is associated with cumulative incidence of lower-extremity amputations in cohorts of patients with type 1 and type 2 diabetes and may help to identify high-risk individuals.45 Further studies are needed to confirm prospectively that copeptin may serve as a diagnostic tool for discriminating increased risk of DUs development. Noteworthy, we document that in SSc patients receiving alprostadil (prostaglandin E1 analog), serum concentration of copeptin significantly decreased after 4–6 cycles of drug administration. Intravenous prostaglandin analogs, such as alprostadil and iloprost (prostaglandin I2 analog) have been reported to be effective in RP secondary to SSc as well as improve DUs healing and prevent DUs in patients with history of DUs.46,47 The known effects of alprostadil include inhibition of vasoconstrictive effects of noradrenaline, Ang II, ET-1 and vasopressin.48 Additionally, PGE may modulate vasopressin release and antagonizes AVP at intracellular signaling pathway level.49,50
To the best of our knowledge, this is the first study evaluating the serum concentration of copeptin in patients with systemic sclerosis. We observed increased copeptin concentration in dcSSc when compared to the lcSSc and control group. Diffuse cutaneous SSc is the subtype characterized by rapid progression and a high prevalence of early internal-organ involvement with interstitial lung disease and cardiac involvement (including pulmonary arterial hypertension) being the main causes of SSc-associated mortality.1 No differences between the control and lcSSc, despite the fact that microcirculation disorders are an important component of this subtype, may result from the applied rheological treatment or the small study group. Autoantibody profiles in SSc are predictive of disease course and organ involvement.51 When patients were stratified according to autoantibodies there was a significant increase in copeptin concentration in anti-RNAP III-positive patients. This is in accordance with several observations showing that SSc patients with positive anti-RNAP III antibodies had more frequently rapidly progressive diffuse skin thickening and higher frequency of vascular involvement, such as scleroderma renal crisis, pulmonary hypertension and ischemic damage associated with RP.52 We did not find significant differences in copeptin concentration in SSc patients regarding the age, BMI, extent of skin fibrosis, prevalence of ILD and esophageal dysmotility. The possible reason for this could be that the number of participants was small. However, strict entry criteria limiting the number of study participants with severe organ damage which, independently of SSc, are associated with elevated copeptin concentration were obligatory to reduce possible factors that might influence baseline copeptin.
Based on our analysis, it may be suggested that evaluation of serum copeptin is useful for identifying SSc patients who are at risk of vascular complications. Thus, it may be hypothesized that determining the copeptin level may help individualize the management of SSc by intensification of rheological treatment in patients with high-risk of certain complications such as progression of NVC abnormalities or the DU development. There may be some possible limitations such as cross-sectional and single-center type of study as well as a relatively small sample size. However, these data may pave the way for subsequent prospective investigations which clarify copeptin significance as a biomarker in SSc.
Conclusions
Vascular involvement plays an essential role in SSc pathogenesis. The unmet need in translational investigations is to obtain biomarkers which will be useful in disease prevention, prognosis and treatment. We demonstrate that increased copeptin concentration in SSc is associated with more severe peripheral microcirculation impairment, such as Raynaud’s phenomenon, NVC changes and digital ulcers. Copeptin may represent a promising potential biomarker in SSc, which allows for personalized therapy. Further prospective studies on larger cohorts of patients with evaluation of how different vascular therapies impact copeptin concentration are needed before its measurements may be used in clinical practice.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Medical University of Warsaw (KB/90/2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Funding
This research was funded by the Medical University of Warsaw, grant number 1M4/1/MBM/N/21.
Disclosure
The authors declare no conflict of interest.
References
1. Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390(10103):1685–1699.
2. Bruni C, Frech T, Manetti M, et al. Vascular leaking, a pivotal and early pathogenetic event in systemic sclerosis: should the door be closed? Front Immunol. 2018;9:2045.
3. Maciejewska M, Sikora M, Maciejewski C, Alda-Malicka R, Czuwara J, Rudnicka L. Raynaud’s phenomenon with focus on systemic sclerosis. J Clin Med. 2022;11(9):2490.
4. Lechartier B, Humbert M. Pulmonary arterial hypertension in systemic sclerosis. Presse Med. 2021;50(1):104062.
5. Chrabaszcz M, Małyszko J, Sikora M, et al. Renal involvement in systemic sclerosis: an update. Kidney Blood Press Res. 2020;45(4):532–548.
6. Polito P, Zanatta E, Felicetti M, et al. Skin ulcers in systemic sclerosis: correlation with clinical phenotype in a monocentric cohort from the north-east of Italy. Clin Exp Rheumatol. 2020;38(3):148–153.
7. Nihtyanova SI, Brough GM, Black CM, Denton CP. Clinical burden of digital vasculopathy in limited and diffuse cutaneous systemic sclerosis. Ann Rheum Dis. 2008;67(1):120–123.
8. Shenavandeh S, Sepaskhah M, Dehghani S, Nazarinia M. A 4-week comparison of capillaroscopy changes, healing effect, and cost-effectiveness of botulinum toxin-A vs prostaglandin analog infusion in refractory digital ulcers in systemic sclerosis. Clin Rheumatol. 2022;41(1):95–104.
9. Pattanaik D, Brown M, Postlethwaite AE. Vascular involvement in systemic sclerosis (scleroderma). J Inflamm Res. 2011;4:105–125.
10. Kostov K. The causal relationship between endothelin-1 and hypertension: focusing on endothelial dysfunction, arterial stiffness, vascular remodeling, and blood pressure regulation. Life. 2021;11(9):986.
11. Szczepanska-Sadowska E, Czarzasta K, Cudnoch-Jedrzejewska A. Dysregulation of the renin-angiotensin system and the vasopressinergic system interactions in cardiovascular disorders. Curr Hypertens Rep. 2018;20(3):19.
12. Marchesi C, Paradis P, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci. 2008;29(7):367–374.
13. Stawski L, Han R, Bujor AM, Trojanowska M. Angiotensin II induces skin fibrosis: a novel mouse model of dermal fibrosis. Arthritis Res Ther. 2012;14(4):R194.
14. Miziolek B, Bergler-Czop B, Kucharz E, et al. Significance of the angiotensin I/angiotensin II/angiotensin-(1-7) axis in the pathogenesis of systemic sclerosis. J Eur Acad Dermatol Venereol. 2020;34(3):558–564.
15. Kowalczyk A, Kleniewska P, Kolodziejczyk M, Skibska B, Goraca A. The role of endothelin-1 and endothelin receptor antagonists in inflammatory response and sepsis. Arch Immunol Ther Exp. 2015;63(1):41–52.
16. Rodriguez-Pascual F, Busnadiego O, Gonzalez-Santamaria J. The profibrotic role of endothelin-1: is the door still open for the treatment of fibrotic diseases? Life Sci. 2014;118(2):156–164.
17. Riemekasten G, Philippe A, Näther M, et al. Involvement of functional autoantibodies against vascular receptors in systemic sclerosis. Ann Rheum Dis. 2011;70(3):530–536.
18. Szczepanska-Sadowska E. The heart as a target of vasopressin and other cardiovascular peptides in health and cardiovascular diseases. Int J Mol Sci. 2022;23(22):14414.
19. Szczepanska-Sadowska E, Zera T, Sosnowski P, Cudnoch-Jedrzejewska A, Puszko A, Misicka A. Vasopressin and related peptides; potential value in diagnosis, prognosis and treatment of clinical disorders. Curr Drug Metab. 2017;18(4):306–345.
20. Lukaszyk E, Malyszko J. Copeptin: pathophysiology and potential clinical impact. Adv Med Sci. 2015;60(2):335–341.
21. Mu D, Cheng J, Qiu L, Cheng X. Copeptin as a diagnostic and prognostic biomarker in cardiovascular diseases. Front Cardiovasc Med. 2022;9:901990.
22. Niemczyk S, Niemczyk L, Zmudzki W, et al. Copeptin blood content as a diagnostic marker of chronic kidney disease. Adv Exp Med Biol. 2018;1096:83–91.
23. Rojas-Humpire R, Soriano-Moreno DR, Galindo-Yllu B, Zafra-Tanaka JH. Association between copeptin and metabolic syndrome: a systematic review. J Nutr Metab. 2022;2022:5237903.
24. Pawlik KK, Bohdziewicz A, Chrabaszcz M, et al. Biomarkers of disease activity in systemic sclerosis. Wiad Lek. 2020;73(10):2300–2305.
25. van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013;65(11):2737–2747.
26. Krasowska D, Rudnicka L, Dańczak-Pazdrowska A, et al. Twardzina układowa – rekomendacje diagnostyczno-terapeutyczne Polskiego Towarzystwa Dermatologicznego. Część 1: diagnostyka i monitorowanie. Dermatol Rev. 2017;104(5):483–498.
27. Saketkoo LA, Frech T, Varjú C, et al. A comprehensive framework for navigating patient care in systemic sclerosis: a global response to the need for improving the practice of diagnostic and preventive strategies in SSc. Best Pract Res Clin Rheumatol. 2021;35(3):101707.
28. LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15(2):202–205.
29. Khanna D, Furst DE, Clements PJ, et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J Scleroderma Relat Disord. 2017;2(1):11–18.
30. Pauling JD, Reilly E, Smith T, Frech TM. Factors influencing raynaud condition score diary outcomes in systemic sclerosis. J Rheumatol. 2019;46(10):1326–1334.
31. Cutolo M, Sulli A, Smith V. Assessing microvascular changes in systemic sclerosis diagnosis and management. Nat Rev Rheumatol. 2010;6(10):578–587.
32. Dobsa L, Edozien KC. Copeptin and its potential role in diagnosis and prognosis of various diseases. Biochem Med. 2013;23(2):172–190.
33. Kaufmann JE, Oksche A, Wollheim CB, Günther G, Rosenthal W, Vischer UM. Vasopressin-induced von Willebrand factor secretion from endothelial cells involves V2 receptors and cAMP. J Clin Invest. 2000;106(1):107–116.
34. Sparapani S, Millet-Boureima C, Oliver J, et al. The biology of vasopressin. Biomedicines. 2021;9(1):89.
35. Nawaz I, Nawaz Y, Nawaz E, Manan MR, Mahmood A. Raynaud’s phenomenon: reviewing the pathophysiology and management strategies. Cureus. 2022;14(1):e21681.
36. Roca Oporto FJ, Rocha JL. Raynaud’s phenomenon triggered by the vasopressin V(2) receptor antagonist tolvaptan in a patient with autosomal dominant polycystic kidney disease and Sjögren’s syndrome. Clin Kidney J. 2022;15(4):827–828.
37. Hayoz D, Bizzini G, Noël B, et al. Effect of SR 49059, a V1a vasopressin receptor antagonist, in Raynaud’s phenomenon. Rheumatology. 2000;39(10):1132–1138.
38. Szmygin H, Szydełko J, Matyjaszek-Matuszek B. Copeptin as a novel biomarker of cardiometabolic syndrome. Endokrynol Pol. 2021;72(5):566–571.
39. Matucci-Cerinic M, Manetti M, Bruni C, et al. The “myth” of loss of angiogenesis in systemic sclerosis: a pivotal early pathogenetic process or just a late unavoidable event? Arthritis Res Ther. 2017;19(1):162.
40. Waszczykowska A, Goś R, Waszczykowska E, Dziankowska-Bartkowiak B, Podgórski M, Jurowski P. The role of angiogenesis factors in the formation of vascular changes in scleroderma by assessment of the concentrations of VEGF and sVEGFR2 in blood serum and tear fluid. Mediators Inflamm. 2020;2020:7649480.
41. Bellando Randone S, George J, Mazzotta C, et al. Angiostatic and angiogenic chemokines in systemic sclerosis: an overview. J Scleroderma Relat Disord. 2017;2(1):1–10.
42. Ripoll GV, Garona J, Pifano M, Farina HG, Gomez DE, Alonso DF. Reduction of tumor angiogenesis induced by desmopressin in a breast cancer model. Breast Cancer Res Treat. 2013;142(1):9–18.
43. Garona J, Sobol NT, Solernó LM, Alonso DF. Potential use of desmopressin during hepatic resection for colorectal liver metastases. J Surg Res. 2019;237:1–2.
44. Hughes M, Bruni C, Ruaro B, Confalonieri M, Matucci-Cerinic M, Bellando-Randone S. Digital ulcers in systemic sclerosis. Presse Med. 2021;50(1):104064.
45. Potier L, Roussel R, Marre M, et al. Plasma copeptin and risk of lower-extremity amputation in type 1 and type 2 diabetes. Diabetes Care. 2019;42(12):2290–2297.
46. Ingegnoli F, Schioppo T, Allanore Y, et al. Practical suggestions on intravenous iloprost in Raynaud’s phenomenon and digital ulcer secondary to systemic sclerosis: systematic literature review and expert consensus. Semin Arthritis Rheum. 2019;48(4):686–693.
47. Marasini B, Massarotti M, Bottasso B, et al. Comparison between iloprost and alprostadil in the treatment of Raynaud’s phenomenon. Scand J Rheumatol. 2004;33(4):253–256.
48. Holmes SW, Horton EW, Main IHM. The effect of prostaglandin E1 on responses of smooth muscle to catechol amines, angiotensin and vasopressin. Br J Pharmacol Chemother. 1963;21(3):538–543.
49. Knepel W, Nutto D, Vlaskovska M, Kittel C. Inhibition by prostaglandin E2 of the release of vasopressin and beta-endorphin from rat pituitary neurointermediate lobe or medial basal hypothalamus in vitro. J Endocrinol. 1985;106(2):189–195.
50. Machida K, Wakamatsu S, Izumi Y, et al. Downregulation of the V2 vasopressin receptor in dehydration: mechanisms and role of renal prostaglandin synthesis. Am J Physiol. 2007;292(4):F1274–F1282.
51. Graßhoff H, Fourlakis K, Comdühr S, Riemekasten G. Autoantibodies as biomarker and therapeutic target in systemic sclerosis. Biomedicines. 2022;10(9):2150.
52. Gargiulo M, Pérez N, Khoury M, et al. Anti-RNA polymerase III antibodies in systemic sclerosis: multicentric study from Argentina. Reumatol Clin. 2021;18(6):368–373.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.