Back to Journals » Therapeutics and Clinical Risk Management » Volume 11
Copeptin: a new predictor for severe obstructive sleep apnea
Authors Cinarka H, Kayhan S, Karatas M, Yavuz A, Gumus A, Özyurt S, Cumhur Cüre M, Sahin U
Received 12 January 2015
Accepted for publication 25 March 2015
Published 10 April 2015 Volume 2015:11 Pages 589—594
DOI https://doi.org/10.2147/TCRM.S80779
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Halit Çinarka,1 Servet Kayhan,1 Mevlüt Karataş,1 Asiye Yavuz,1 Aziz Gümüş,1 Songül Özyurt,1 Medine Cumhur Cüre,2 Ünal Şahin1
1Department of Chest Diseases, 2Department of Biochemistry, Recep Tayyip Erdoğan University, Rize, Turkey
Introduction: Copeptin which is the C-terminal fragment of antidiuretic hormone (ADH), is a biomarker that has been reported to be increased in various cardiovascular disorders, cerebrovascular diseases and associated with prognosis. Patients with obstructive sleep apnea syndrome (OSAS) have a tendency to develop coronary and cerebral atherosclerotic diseases.
Objectives: The aim of the present study was to study copeptin levels in patients with obstructive sleep apnea and in a control group in order to determine whether copeptin could be used as a biomarker predicting the severity of OSAS and possible complications in this group.
Methods: A total of 116 patients with OSAS, diagnosed by polysomnography, and 27 controls were included in the study. Blood samples were collected after overnight fasting, and copeptin levels were measured with enzyme-linked immunosorbent assay.
Results: Copeptin levels were significantly higher in the OSAS group compared to control group (2,156±502; 1,845±500 pg/mL, respectively, P=0.004). Mean copeptin level of the patients having apnea-hypopnea index (AHI) ≥30 was significantly higher than that of the patients having AHI <30 (2,392±415; 2,017±500 pg/mL, respectively, P<0.001). A multivariate regression analysis showed that copeptin level, (hazard ratio: 1.58; 95% confidence interval: 1.09–2.30) was a predictor of severe OSAS (P=0.016). Copeptin levels showed significant positive correlation with AHI (r=0.32; P<0.001), desaturation index (r=0.23; P=0.012), arousal index (r=0.24; P=0.010) and CRP (r=0.26; P=0.011) respectively.
Conclusion: Copeptin levels are high in OSAS patients and copeptin is a potential marker for identifying patients with a high risk of early cardiovascular complications of OSAS. Copeptin has modest sensitivity (84%) for discriminating severe OSAS patients who are candidates for severe cardiovascular complications.
Keywords: copeptin, obstructive sleep apnea, predictor, inflammation
Introduction
Obstructive sleep apnea syndrome (OSAS) is characterized by repetitive episodes of upper airway obstruction-related oxygen desaturation and sleep fragmentation.1 Oxygen desaturation-associated apneic episodes, negative intrathoracic pressure, arousals augmented by upper airway collapse, and repetitive episodes of activation of the sympathetic nervous system might lead to abnormal humoral, neural, metabolic, thrombotic, and inflammatory responses, and when activated, these pathways contribute to cardiovascular mortality.2 The well-known gold standard for diagnosis of OSAS is polysomnography, but polysomnography has several important limitations, including cost, limited availability, and need for cooperation on the part of the patient. In addition to helping confirm a diagnosis of OSAS, elevated biochemical or inflammatory biomarker levels might also define an increased risk of cardiovascular disease in OSAS.3
Studies have shown that copeptin is a good predictor of outcome in patients with heart failure or acute myocardial infarction, and its value has even been found to be superior to that of brain natriuretic peptide.4,5 The prognostic role of copeptin in lower respiratory tract infections and chronic obstructive pulmonary disease has also been studied,6,7 and a recent study reported that copeptin is a potential prognostic marker for short-term mortality independently and in addition to natriuretic peptide levels in patients with acute dyspnea.8 Copeptin levels may reflect both the inflammatory cytokine response and the presence of hemodynamic and osmoregulatory disturbances.9 Copeptin may also be associated with cardiovascular complications in OSAS patients. The aim of this study was to assess copeptin levels in patients with OSAS and compare them with levels in controls.
Materials and methods
Patients
The study population included 116 patients with OSAS recently diagnosed by polysomnography. Patients having a personal or family history of psychiatric illness, a history of alcoholism or substance abuse, or a central nervous system disorder were excluded.
Polysomnography
All patients underwent overnight polysomnography. All variables were recorded on a computer system (Alice Sleepware, Philips Respironics, Murrysville, PA, USA). Data were collected by electroencephalography (F3M2, F4M1, C3M2, C4M1, O1M2, O2M1), bilateral electro-oculography, submental electromyography, thoracic and abdominal movements measured by uncalibrated inductive plethysmography, oxyhemoglobin saturation, airflow through the nose and mouth recorded by thermistors, electrocardiography, snoring microphone, and video monitoring using an infrared video camera. The entire recording was done by an experienced sleep technician. Polysomnography records were scored in 30-second periods for sleep, breathing, and oxygenation. Hypopnea was defined as a reduction of >50% in one of three respiratory signals, ie, the airflow signal, or the respiratory or abdominal signal of respiratory inductance plethysmography, with either an associated decrease of ≥3% in oxygen saturation or arousal. Apnea was defined as complete cessation of airflow for ≥10 seconds.1 The average number of apneas and hypopneas per hour of sleep was calculated as the apnea-hypopnea index (AHI). Patients with an AHI ≥5 events/hour were diagnosed as having OSAS. Excessive daytime sleepiness was identified using the Epworth Sleepiness Scale, and values over 10 were accepted as a pathological result.10 Arousals were scored according to accepted definitions.11
Laboratory analysis
Serum copeptin levels were quantified by enzyme-linked immunosorbent assay using commercially available matched antibodies (USCN Life Science Inc., Wuhan, People’s Republic of China). The intra-assay and interassay coefficients of variation were <10.0% and <12.0%, respectively. The sensitivity was calculated to be 6.1 pg/mL.
Statistical analysis
The Kolmogorov-Smirnov test was used to test for a normal distribution of continuous variables. Data characterized by a normal distribution were expressed as the mean ± standard deviation. Parameters without such a distribution were expressed as the median with range. The Student’s t-test (normal distribution) or the Mann–Whitney U-test (non-normal distribution) was used for comparing the two groups. Analysis of variance (normal distribution) or the Kruskal-Wallis test (non-normal distribution) was used to compare more than two groups. Discrete variables were compared using Fisher’s Exact test. The Spearman test was used to assess the correlation between variables. Multivariate regression analysis was used as a stepwise descending method from prognostic factors, with a significance of <0.1 in univariate analysis. Stepwise multivariate linear regression was also performed to determine the factors independently associated with cardiovascular disease. A criterion of P<0.05 for entry and P<0.10 for removal was imposed in this procedure. P<0.05 was considered to be statistically significant.
Results
Clinical baseline characteristics
A total of 116 patients and 27 controls were included in the study population. The clinical characteristics of the OSAS and control groups are shown in Table 1. Copeptin levels were significantly higher in the OSAS group than in the control group (2,156±502 pg/mL and 1,845±500 pg/mL, respectively, P=0.004, Figure 1).
  | Table 1 Clinical characteristics of the study group |
  | Figure 1 Comparison of copeptin levels between patients with obstructive sleep apnea and healthy control group. |
Association of copeptin levels with OSAS severity
Copeptin levels in patients with AHI ≥30 were significantly higher than those in patients with AHI <30 (2,392±415 and 2,017±500 pg/mL, respectively, P<0.001, Figure 2). The optimal cut-off copeptin value for predicting severe OSAS was 2,044 pg/mL. The area under the curve of copeptin for prediction of severe OSAS was 0.736 (95% confidence interval [CI] 0.647–0.825, P<0.001, Figure 3). In cases with copeptin levels >2,044 pg/mL, the specificity and sensitivity for severe OSAS were 58% (95% CI 0.45–0.69) and 84% (95% CI 0.69–0.93), respectively. The negative predictive value of copeptin at a cut-off of 2,044 pg/mL for predicting severe OSAS was 86% (95% CI 0.72–0.94) and the positive predictive value of copeptin at a cut-off of 2,044 pg/mL for severe OSAS was 54% (95% CI 0.41–0.66).
  | Figure 2 Comparison of copeptin levels between the patients with apnea-hypopnea index (AHI) ≥30 and the patients with AHI <30. |
Association and correlation of copeptin levels with other clinical characteristics
In univariate analysis, a significant association was found between severe OSAS and age, body mass index, copeptin level, and C-reactive protein (CRP) level. CRP and copeptin levels were analyzed from the same blood samples of the study population. A multivariate regression analysis showed that the copeptin level (hazard ratio 1.58, 95% CI 1.09–2.30) was a predictor of severe OSAS (P=0.016). Other potential predictors for severe OSAS on multivariate regression analysis are summarized in Table 2. Copeptin levels showed a significant positive correlation with AHI (r=0.32; P<0.001, Figure 4), desaturation index (r=0.23; P=0.012, Figure 5), arousal index (r=0.24; P=0.010, Figure 6), and CRP level (r=0.26; P=0.011, Figure 7).
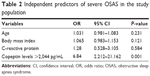  | Table 2 Independent predictors of severe OSAS in the study population |
  | Figure 4 Correlation analysis by Spearman test between copeptin levels and apnea-hypopnea index (r=0.32; P<0.001). |
  | Figure 5 Correlation analysis by Spearman test between copeptin levels and desaturation index (r=0.23; P=0.012). |
  | Figure 6 Correlation analysis by the Spearman test between copeptin levels and arousal index (r=0.24; P=0.010). |
  | Figure 7 Correlation analysis by the Spearman test between copeptin levels and C-reactive protein level (r=0.26; P=0.011). |
Discussion
In the present study, we found an increased level of copeptin in patients with OSAS when compared with healthy controls. Multivariable logistic regression analysis revealed that copeptin levels were an independent predictor of severity of OSAS. Copeptin levels had a significant positive correlation with AHI, desaturation index, arousal index, and CRP level. Inflammation has been suggested as a potential mechanism for OSAS. High levels of various biomarkers of inflammation, such as tumor necrosis factor-alpha, interleukin-6, and CRP, have been proposed to be associated with OSAS.12,13 Copeptin has been reported to be elevated in coronary heart disease and central nervous system disorders, including acute ischemic stroke and subarachnoid hemorrhage caused by aneurysm.14–16 Higher copeptin levels are reported to be related to a poor outcome and to poor prognostic factors in patients with coronary heart disease or chronic stable heart failure.14,17 It is known that there is an increased risk of ischemic heart disease and neurological complications in patients with OSAS.18,19
We found that copeptin levels were significantly higher in OSAS patients than in controls, and this could be particularly important for prediction of the cardiac and neurological complications of OSAS. Intermittent obstruction of the upper airway and obstruction-induced hypoxia seen in OSAS patients is believed to increase cardiovascular risk by increasing sympathetic activation and oxidative stress. Oxidative stress and increased sympathetic activation, namely endogenous stress, lead to release of antidiuretic hormone.14,20,21 Recurrent episodes of upper airway obstruction followed by adequate ventilation lead to multiple hypoxia and reoxygenation periods. These repetitive episodes are proposed to play a role in hypoxia-reperfusion injury, which augments endogenous oxidative stress.22 Release of antidiuretic hormone, and its more stable metabolite copeptin, may be expected to increase in patients with OSAS and this endogenous stress. Given that patients with OSAS already have an increased risk of cardiovascular disease, copeptin might be a useful biomarker to define the cardiovascular risk level in patients who have not shown manifestations of cardiovascular disease.
In the present study, we found that copeptin levels were significantly higher in patients with an AHI ≥30 than in those with an AHI <30. We also found a significant positive correlation between copeptin levels, AHI, desaturation index, and arousal index. The risk of fatal and nonfatal cardiovascular complications is 2.8 and 3.5 higher, respectively, in OSAS patients having an AHI >30 when compared with the healthy population.23 Therefore, patients with severe OSAS are likely to have serious cardiovascular complications. Copeptin was found as an independent predictor of severe OSAS in this study, and high levels of copeptin may also be a useful biomarker of cardiovascular disease in patients with OSAS. Copeptin may be used to identify a need for early continuous positive airway pressure (CPAP) treatment and/or to evaluate the response to CPAP in patients with OSAS. From the point of view of oxidative stress and increased sympathetic activation, which leads to increased antidiuretic hormone and ultimately increased copeptin levels, treatment with CPAP may decrease copeptin levels in the subgroup with an AHI >30. However, this approach needs to be addressed in further studies.
Copeptin levels also showed a significant positive correlation (r=0.26; P=0.011) with CRP, which is associated with inflammation, in our OSAS patients. Inflammation has an important role in the pathophysiology of OSAS, particularly in the presence of cardiovascular disease.24 Inflammatory biomarkers, including CRP, interleukin-6, and tumor necrosis factor-alpha, increase with OSAS.25–27 Levels of CRP and interleukin-6 and spontaneous production of interleukin-6 by monocytes are also decreased by CPAP in OSAS patients.12
There is only one study in the literature that found lower copeptin levels in patients with OSAS,28 although there is an increased risk for neurological and cardiac diseases in OSAS patients and copeptin has been found elevated in these diseases.14–16 In this study, receiver operating characteristic curve analysis was used to determine sensitivity and specificity of copeptin levels for the diagnosis of OSAS, with an area under the curve of 0.71 (95% CI 0.60–0.82, P<0.01) for a cut-off value below 0.5 ng/mL, copeptin had 67% sensitivity and 69% specificity for diagnosing OSAS.28 Another biomarker known as omentin-1 has also been found to be significantly decreased in OSAS patients compared with healthy control controls. In one study, multivariate logistic regression analysis showed that serum omentin-1 levels were inversely associated with the presence of OSAS.29 Other potential biomarkers identified in OSAS patients include P-selectin, adiponectin, leptin, and cystatin-C.3,30–32
In the present study, it was found that the optimal cut-off value for copeptin in predicting severe OSAS was 2,044 pg/mL. The area under the curve for copeptin in the prediction of severe OSAS was 0.736 (95% CI 0.647–0.825, P<0.001) with copeptin levels >2,044 pg/mL, and the specificity and sensitivity for severe OSAS were 58% (95% CI 0.45–0.69) and 84% (95% CI 0.69–0.93), respectively. Therefore, copeptin may be used to identify patients with severe OSAS, and may be particularly important for prediction of future cardiovascular complications.
The main limitation of our study is its small control group. Although the study included an acceptable number of OSAS patients from the point of view of power of the study, it could be considered that the number of controls was too small. A second limitation could be the lack of investigation for a circadian rhythm in copeptin levels. However, in contrast with cortisol, copeptin levels have not demonstrated any consistent circadian rhythm.33
Conclusion
Copeptin is a potential candidate marker for identifying patients at high risk of early cardiovascular complications from OSAS. However, the benefit of such biomarker-driven strategies still has to be confirmed by large randomized controlled trials. Increased copeptin levels could be related to the prognosis of OSAS. Copeptin has modest sensitivity (84%) for discriminating patients with severe OSAS who are candidates for severe cardiovascular complications. Therefore, use of copeptin as a biomarker to predict the severity of OSAS and its complications, particularly cardiovascular events, may be recommended after further studies addressing the role of copeptin as a biomarker in patients with OSAS.
Acknowledgment
This research was supported by a grant from Recep Tayyip Erdoğan University.
Disclosure
The authors report no conflicts of interest in this work.
References
[No authors listed]. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. | ||
Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906–1914. | ||
De Luca Canto G, Pacheco-Pereira C, Aydinoz S, Major PW, Flores-Mir C, Gozal D. Biomarkers associated with obstructive sleep apnea: a scoping review. Sleep Med Rev. 2014;23C:28–45. | ||
Voors AA, von Haehling S, Anker SD, et al. C-terminal provasopressin (copeptin) is a strong prognostic marker in patients with heart failure after an acute myocardial infarction: results from the OPTIMAAL Study. Eur Heart J. 2009;30:1187–1194. | ||
Khan SQ, Dhillon OS, O’Brien RJ, et al. C-terminal provasopressin (copeptin) as a novel and prognostic marker in acute myocardial infarction: Leicester Acute Myocardial Infarction Peptide (LAMP) study. Circulation. 2007;115:2103–2110. | ||
Müller B, Morgenthaler N, Stolz D, et al. Circulating levels of copeptin, a novel biomarker, in lower respiratory tract infections. Eur J Clin Invest. 2007;37:145–152. | ||
Stolz D, Christ-Crain M, Morgenthaler NG, et al. Copeptin, C-reactive protein, and procalcitonin as prognostic biomarkers in acute exacerbation of COPD. Chest. 2007;131:1058–1067. | ||
Potocki M, Breidthardt T, Mueller A, et al. Copeptin and risk stratification in patients with acute dyspnea. Crit Care. 2010;14:213–221. | ||
Lin Q, Fu F, Chen H, Zhu B. Copeptin in the assessment of acute lung injury and cardiogenic pulmonary edema. Respir Med. 2012;106:1268–1277. | ||
Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–545. | ||
[No authors listed]. EEG arousals: Scoring rules and examples: a preliminary report from the sleep disorders ATLAS Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–184. | ||
Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–1134. | ||
Calvin AD, Albuquerque FN, Lopez-Jimenez F, Somers VK. Obstructive sleep apnea, inflammation, and the metabolic syndrome. Metab Syndr Relat Disord. 2009;7:271–278. | ||
Potocki M, Reichlin T, Thalmann S, et al. Diagnostic and prognostic impact of copeptin and high-sensitivity cardiac troponin T in patients with pre-existing coronary artery disease and suspected acute myocardial infarction. Heart. 2010;98:558–565. | ||
Katan M, Fluri F, Morgenthaler NG, et al. Copeptin: a novel, independent prognostic marker in patients with ischemic stroke. Ann Neurol. 2009;66:799–808. | ||
Zhu XD, Chen JS, Zhou F, Liu QC, Chen G, Zhang JM. Detection of copeptin in peripheral blood of patients with aneurysmal subarachnoid hemorrhage. Crit Care. 2011;15:R288. | ||
Tentzeris I, Jarai R, Farhan S, et al. Complementary role of copeptin and high-sensitivity troponin in predicting outcome in patients with stable chronic heart failure. Eur J Heart Fail. 2011;13:726–733. | ||
Cifçi N, Uyar M, Elbek O, Süyür H, Ekinci E. Impact of CPAP treatment on cardiac biomarkers and pro-BNP in obstructive sleep apnea syndrome. Sleep Breath. 2010;14:241–244. | ||
Oktay B, Akbal E, Firat H, Ardic S, Akdemir R, Kizilgun M. Evaluation of the relationship between heart type fatty acid binding protein levels and the risk of cardiac damage in patients with obstructive sleep apnea syndrome. Sleep Breath. 2008;12:223–228. | ||
Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52:112–119. | ||
Enhörning S, Wang TJ, Nilsson PM, et al. Plasma copeptin and the risk of diabetes mellitus. Circulation. 2010;121:2102–2108. | ||
Archontogeorgis K, Nena E, Papanas N, Steiropoulos P. Biomarkers to improve diagnosis and monitoring of obstructive sleep apnea syndrome: current status and future perspectives. Pulm Med. 2014;2014:930535. | ||
Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. | ||
Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Thorax. 2009;64:631–636. | ||
Lui MM, Lam JC, Mak HK, et al. C-reactive protein is associated with obstructive sleep apnea independent of visceral obesity. Chest. 2009;135:950–956. | ||
Minoguchi K, Tazaki T, Yokoe T, et al. Elevated production of tumor necrosis factor-alpha by monocytes in patients with obstructive sleep apnea syndrome. Chest. 2004;126:1473–1479. | ||
Mehra R, Storfer-Isser A, Kirchner HL, et al. Soluble interleukin 6 receptor: a novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med. 2006;166:1725–1731. | ||
Ozben S, Guvenc TS, Huseyinoglu N, et al. Low serum copeptin levels in patients with obstructive sleep apnea. Sleep Breath. 2013;17:1187–1192. | ||
Wang Q, Feng X, Zhou C, Li P, Kang J. Decreased levels of serum omentin-1 in patients with obstructive sleep apnoea syndrome. Ann Clin Biochem. 2013;50:230–235. | ||
Cofta S, Wysocka E, Dziegielewska-Gesiak S, et al. Plasma selectins in patients with obstructive sleep apnea. Adv Exp Med Biol. 2013;756:113–119. | ||
Tauman R, Serpero LD, Capdevila OS, et al. Adipokines in children with sleep disordered breathing. Sleep. 2007;30:443–449. | ||
Zhang XB, Lin QC, Deng CS, Chen GP, Cai ZM, Chen H. Elevated serum cystatin C in severe OSA younger men without complications. Sleep Breath. 2013;17:235–241. | ||
Darzy KH, Dixit KC, Shalet SM, Morgenthaler NG, Brabant G. Circadian secretion pattern of copeptin, the C-terminal vasopressin precursor fragment. Clin Chem. 2010;56:1190–1191. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

