Back to Archived Journals » International Journal of Clinical Transfusion Medicine » Volume 8
Convalescent Plasma Therapy: An Effective Therapeutic Option to Treat COVID-19? A Narrative Review
Authors Ray I , Sánchez DF , Robert CA , Robert MP
Received 3 July 2020
Accepted for publication 15 September 2020
Published 23 October 2020 Volume 2020:8 Pages 7—21
DOI https://doi.org/10.2147/IJCTM.S269691
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Cees Th. Smit Sibinga
Video abstract presented by Ishita Ray.
Views: 4685
Ishita Ray,1 Diana Fiorela Sánchez,2 Chris Andrea Robert,3 Mary Phyllis Robert4
1Department of Internal Medicine, Mahatma Gandhi Memorial Medical College, Indore, Madhya Pradesh, India; 2Department of Internal Medicine, Cayetano Heredia Peruvian University, Lima, Peru; 3Department of Obstetrics & Gynaecology, Sunrise Hospital, Pune, Maharashtra, India; 4Department of Obstetrics & Gynaecology, LLH Hospital, Abu Dhabi, United Arab Emirates
Correspondence: Ishita Ray
Madan Mahal Hospital, Prem Nagar, Nagpur Road, Jabalpur, Madhya Pradesh 482001, India
Tel +918959393344
Email [email protected]
Abstract: December 2019 marked the emergence of a new deadly strain of the coronavirus family which unleashed an unprecedented pandemic named the coronavirus disease 2019 outbreak (COVID-19) upending lives all over the world. We still do not have drugs or vaccines that specifically target this vile virus. Hence, the need to find alternative therapeutic options is to optimize recovery and reduce the viral load due to COVID-19. Convalescent plasma (CP) therapy is a potential therapeutic option being explored all over the world. In this study, we reviewed all relevant publications that mentioned the use of plasma therapy. Out of the 61 articles, eight publications specifically explored CP therapy in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patients and were studied in detail during this review. The plasma collected from convalescent COVID-19 patients contains neutralising antibodies which show potential benefit in reducing viral load and accelerating viral clearance with negative reverse transcriptase polymerase chain reaction (RT-PCR) test reports. The concentration of neutralising antibodies also increased post CP therapy. Improvement in clinical symptoms like fever, cough and dyspnoea was also reported. Radiological findings before and after CP therapy showed reduced ground glass opacities and resorption of pneumonia in several SARS-CoV-2 patients. Laboratory parameters also showed improvement as lymphocyte counts increased, and markers of inflammation like C-reactive protein (CRP), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) reduced after plasma therapy. There were no adverse reactions reported in any of the studies reviewed. However, potential adverse reactions cannot be ruled out like circulatory overload and anaphylaxis. Lack of large-scale clinical trials on CP therapy is a major shortcoming before this therapeutic modality starts being extensively used.
Keywords: convalescent plasma, sars-cov-2, coronavirus outbreak, COVID − 19, convalescent plasma therapy, neutralizing antibodies, plasma therapy
Introduction
A new coronavirus surfaced in Wuhan, China, in December 2019. This virus was named Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and is known to infect the respiratory tract in humans and leads to Coronavirus Disease 2019 (COVID-19). Studies have reported that SARS-CoV-2 is a beta coronavirus intricately linked to the Severe Acute Respiratory Syndrome (SARS) virus.1 COVID-19 has 2,074,529 cases confirmed and 139,378 deaths globally as of April 17, 2020.2
No specific treatment has been proven to be effective for SARS-CoV-2 infection to date.3 Complications of acute respiratory disease syndrome (ARDS), multiorgan failure including acute kidney injury and heart injury (viral myocarditis), as well as sepsis have been identified in critically ill COVID −19 patients.4 Hence, the need of the hour is to accelerate the process of finding effective therapeutic modalities to mitigate the outbreak caused by COVID-19.
Current treatment modalities like Chloroquine and Hydroxychloroquine, antiviral drugs like Lopinavir-Ritonavir, new drugs like Remdesivir, corticosteroids accompanied by antibacterial therapy have failed to bring down death rates and proven to be ineffective in critically ill patients. World Health Organization (WHO) advises against the use of corticosteroids.5 In a systematic review of corticosteroid use in patients with SARS, it was observed that they cause more harm than benefit.6 Lopinavir and Ritonavir were not observed to cause any outstanding benefit in patients.7 A recent paper reported that Remdesivir and Chloroquine have an inhibitory effect on the growth of SARS-CoV-2 in vitro.8 An early clinical trial conducted in COVID-19 Chinese patients showed that when compared to the control groups Chloroquine had a promising effect in viral clearance and significantly improved patient clinical condition.9,10 However, it seems to be an ineffective treatment option in a critically ill patient.
This warrants the need for better treatment options for COVID-19. One such option currently being explored and showing good results is convalescent plasma (CP) therapy. CP or immunoglobulins have been the last hope in critical patients who failed to improve despite treatment. Patients receiving convalescent plasma therapy have been studied to have early discharge from the hospital in addition to a lower mortality rate. The Spanish influenza (1918–1920 pandemic) was the first viral infection to show the potential effect of convalescent blood products in clinical studies.11 Cheng et al 2003 study of SARS patients in Hong Kong showed that patients receiving CP before day fourteen of infection had a higher discharge rate by day 22.12 Based on the genomic similarity between SARS and SARS-CoV-2 and the success of using convalescent plasma for SARS, it was examined as an option. The first of these studies done on SARS-CoV-2 patients was done by Shen et al; they found decreased or negative viral loads within 12 days after transfusion along with increased SARS-CoV-2 specific ELISA and neutralizing antibody titers. In addition, ARDS resolved in four of the five patients 12 days post plasma therapy.13 Another study done by Tan et al reported that one of their cases with long drawn out infection showed rapid improvement after infusion.14
In this article, we reviewed all published literature using CP therapy for the treatment of SARS-COV-2 and examined its effectiveness in treatment and its impact on the recovery period of infected patients.
Materials and Methods
Search Method and Strategy
The literature search was performed up to May 2020, using the keywords: COVID-19, coronavirus, convalescent plasma, convalescent plasma therapy, SARS-CoV-2, SARS, Middle Eastern Respiratory Syndrome (MERS), plasma infusion, and their combinations. The MeSH keywords used were Coronavirus, SARS Virus, Plasma, COVID-19, SARS-Plasma, Plasma-COVID-19, Coronavirus-Plasma. We searched on databases like PubMed, Google scholar, and Scielo. There were no constraints on the publication date. References were managed using Mendeley. The figure was created using Biorender.
Study Selection
All studies and study designs that describe CP therapy and its usage were included. We included articles with information on other infectious diseases too such as SARS, MERS, and Ebola Virus Disease (EVD) to derive comparative information. Also, we selected the reports of some cases in which CP was used. Finally, we selected 61 articles. Out of the 61 studies, eight were found that were pertaining to the use of CP in SARS-CoV-2. All patient data were included irrespective of gender, age, or location.
Ethical Issues
As patients were not directly involved, ethical approval was not requested.
Statistical Analysis
Statistical analysis was unnecessary, as this is a traditional review.
Results
The literature search produced 61 relevant publications in the PubMed database. Out of the 61, eight publications were pertaining to the use of CP in SARS-CoV-2 patients.
In a pilot study of 10 critical COVID-19 patients by Duan et al, a single dose of 200 mL of CP with neutralizing antibody titers above 1:640 was administered to ten critical COVID 19 patients, along with full standard supportive treatment and antiviral agents, which resulted in a rapid increase in neutralizing antibodies to 1:640 in five of the cases, whereas the other four cases maintained a high level (1:640) of antibodies culminating in the disappearance of viremia in seven days.15 A drastic decrease in viral load was observed in other studies too following plasma infusion.14,16 Improvement in oxygen saturation was observed in most patients after CP therapy, and there were mentions of critically ill patients being weaned from mechanical ventilation and put on high flow nasal cannula.15,16 Resorption of pneumonia and lung infiltrates was seen on Lung Computerized Tomography (CT) scan post plasma therapy.17 Fever subsided with normal body temperature measured within three days in four of five critically ill patients, the Sequential Organ Failure Assessment (SOFA) score decreased, and (PAO2)/(FIO2) increased after plasma therapy where (PAO2) stands for partial pressure of arterial oxygen and (FIO2) stands for percentage of inspired oxygen.13
Normalization of routine laboratory criteria and pulmonary function improvement was observed in all studies. Lymphocytopenia was found to be an important index for prognosis in COVID-19.15 Lymphocyte counts increased in most patients post plasma therapy, whereas most Inflammatory markers like C-reactive protein (CRP) and markers of liver dysfunction decreased.15,16
No adverse events during plasma infusion were recorded in any of the studies included in this review.15–18 However, adverse transfusion reactions like anaphylaxis, circulatory overload, and especially dangerous ones like Transfusion Related Acute Lung Injury (TRALI) should never be ruled out as a disadvantage of this therapy along with the risk of transmitting the pathogen during plasma transfusion.18,19 The effect of other concomitantly given therapeutic modalities like antiviral drugs and corticosteroids cannot be totally ruled out.15 The number of antibodies of the donor given to patients during plasma therapy is yet to be standardized.16 Antibody-dependent enhancement [ADE] of infection has also been quoted as a limiting factor for passive and active immunization schedules. It is known to account for the severity of COVID-19 cases.20 Lack of large-scale trials was a major limitation cited by most of the publications.13,17,21
Discussion
A novel pneumonia caused by a newly emerging deadly virus called SARS-CoV-2 first emerged in the province of Wuhan, China, in December 2019. It was declared the COVID-19 pandemic by the WHO.22–24 The most common symptoms are fever, cough, and fatigue. Other associated symptoms include sputum production, headache, haemoptysis, diarrhoea, dyspnoea, and lymphopenia.25 CT scan of the lung showed bilateral pulmonary involvement in 98% of cases, with the typical findings being multiple areas of consolidation and bilateral ground-glass opacity.26 Also, many reports suggested that person-to-person transmission is the most important route for spreading COVID-19 infection. Currently, contact tracing, extensive social distancing and quarantine measures are being encouraged to control the current outbreak of COVID-19 in different countries.25,27 The epidemic which was initially limited to China was declared a pandemic according to WHO on March 11, 2020.25 The COVID-19 pandemic has caused 25,842,652 cases worldwide and caused 858,629 deaths.28 The United States of America itself has seen 6,011,042 cases and 183,610 deaths, with New York being the hardest hit state as of September 03, 2020, with 32,972 deaths.28 The exponentially growing number of patients presenting to hospitals is burdening the current healthcare system like never. The case rate fatality is 2.2%.26 Management to date has been mainly supportive.29 Some drugs in the investigational stage, including Remdesivir and Lopinavir/Ritonavir, are being studied as potential therapeutic modalities.8,30 Remdesivir was reported to show promise as an antiviral therapy due to good results seen in a single patient in the USA; however, randomized controlled trials are ongoing to ascertain efficacy and safety.31 Remdesivir is also known to reduce the viral titer of mice infected with MERS-COV has been in the news lately.32 Interferon-alpha (IFN-alpha) at a dose of 5 million units twice a day through inhalation and ritonavir/lopinavir (400 mg/100 mg, twice a day, per oral) are recommended as antiviral therapy for the novel COVID-19 according to current guidelines.33 A study reported that all Chinese experts are recommending that all patients suffering from various stages of COVID-19 from most severe, moderate to mild pneumonia and without any comorbidities or contradictions to the usage of chloroquine, be treated with Chloroquine (500 mg) taken twice a day per orally for 10 days.34 Another study reports the synergistic effect when Azithromycin is also combined with Hydroxychloroquine.35 Corticosteroid like Dexamethasone as a treatment for COVID-19 lung injury is controversial due to complications and delayed effect.5,36 However, according to a trial conducted recently by Horby et al, treatment with dexamethasone at a dose of 6 mg once daily for up to 10 days reduces 28-day mortality in patients with COVID-19 who are receiving respiratory support. No benefit was found among patients who did not require oxygen.37
No effective vaccine or specific antiviral drugs are available; therefore, an alternative treatment method for COVID-19 is indispensable, especially among the critically ill.
CP collected from recovered patients has been an age-old therapeutic modality for various past epidemics. It has proved especially useful against viral infections.29 According to the Food and Drug Administration (FDA) of the United States, plasma from convalescent patients containing antibodies to the newly emergent coronavirus or the SARS-CoV-2 is being explored as a treatment modality for patients inflicted by the novel coronavirus. The Spanish influenza (pandemic of 1918–1920) was the first viral infection for which convalescent blood products were found to be potentially effective during clinical studies.11 A meta-analysis of 8 Spanish flu trials (1,703 of patients) reported decreased mortality after treatment with convalescent blood products.38 CP therapy has been studied in other outbreaks of respiratory infections, including the SARS-CoV-1 epidemic of 2003, the Influenza A Virus subtype H1N1 (influenza virus pandemic) between 2009–2010, and the MERS-CoV epidemic in 2012 (Table 1). It is important to study the safety and efficacy of COVID-19 CP therapy in clinical trials as it is still an upcoming treatment modality.12,29,43,44 Effective plasma containing the high-titer specific antibodies binding with and neutralising SARS-CoV-2 should prevent uninfected cells from entering, and activate potent effector mechanisms.45
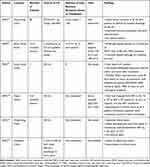 |
Table 1 Table of Historical Precedents of Plasma Therapy |
In this review, we have carefully gone through six articles, including clinical trials and case series exploring the potential of CP therapy in COVID-19 patients (Table 2).
 |  |  |  |
Table 2 Table of Articles Exploring Convalescent Plasma Therapy to Treat COVID-19 Patients |
Important Elements of Convalescent Plasma Therapy
A study done by Brown et al mentioned the following elements as an essential part of any CP therapy program:48
- The availability of donors who have recovered from the targeted disease. They are required to meet the eligibility criteria for plasma donation to ensure the safety of both the donor and the recipient.
- Identification of potential donors among COVID-19 patients through a screening process. Patient recovery after CP therapy needs to be reported by conducting appropriate standardized viral nucleic acid and antibody screening.49
- Recently approved serological tests are required to detect SARS-CoV-2 in serum and virological tests to measure viral neutralization.50
- Identification of the desired level of antibody in CP donors, preferably with high neutralizing antibody titers.51 This is important as even among individuals who recover from SARS-CoV-2 one-third have low or undetectable neutralizing antibody titers.52
- Blood banking facilities with experience in plasmapheresis are needed for conducting plasma donations.
- A specific blood product needs to be identified for the therapy (eg, FFP, fresh plasma or lyophilized plasma) and standardized amounts of plasma need to be collected.
- Establishing a dosage schedule based on scientific research on SARS-CoV-2 antibodies.
- Establishing a registry for possible future donations.51
- Although, CP therapy can become an effective therapy for COVID-19. Blood products are important sources of HIV infection in low- and middle-income nations due to suboptimal healthcare infrastructures and less strict regulations.53
Plasma black market which exist in such countries led to an HIV epidemic in China in the 1990s which began with local pay for plasma schemes.54
FDA (The Food and Drug Administration) recommendations for use of Convalescent Plasma Therapy in the COVID −19 pandemic: Participation in clinical trials is a way for patients to obtain access to CP, it may not be readily available to all patients in need. Therefore, during the public health emergency of the COVID-19 pandemic till the safety of CP can be determined through clinical trials, FDA is also facilitating access to COVID-19 CP for use in patients with immediately life-threatening COVID-19 infections through the process of the patient’s physician requesting a single patient emergency. This allows the use of an investigational drug like CP for an individual patient by a licensed physician upon FDA authorization, if the applicable regulatory criteria are met.51
Advantages of Convalescent Plasma Therapy
Rise in Neutralizing Antibody Titers and Fall in Viral Titers
One dose of 200 mL of CP was used in a pilot study to investigate the practicality of the plasma therapy in ten critical COVID-19 patients (Figure 1). The plasma donors were recently recovered patients with a requirement that plasma would contain antibody titers neutralized above 1:640. The collected plasma was transfused to consenting patients, along with concomitant maximum supportive care and antiviral agents. When blood work was performed and post plasma transfusion analysed, favourable results were achieved in neutralizing antibodies, which increased rapidly to 1:640 in five cases. Compared to the above, the remaining four cases also showed encouraging results and were sustained at a high level (1:640), resulting in the seven-day disappearance of viremia.15 Neutralizing CP activity against SARS-CoV-2 was evaluated using a plaque reduction test using a newly isolated viral strain which is a classic test.22 Decrease in viral burden (viral particles per mL) was also observed in a case series involving two critically ill patients who received plasma therapy done by Ahn et al in Seoul (Korea).16 In a study done by Tan et al viral clearance was also observed with the viral tests of SARS-CoV-2 done by oropharyngeal swabs producing negative results after plasma infusion therapy making it a potential lifesaver for patients with COVID-19.14 It has been established through many studies on plasma therapy used in outbreaks in the past, that antibodies present in the convalescent plasma can suppress viruses.23 Moreover, studies done in vivo had outcomes favouring the benefits of plasma therapy as the neutralizing antibodies caused not only viral clearance but also accelerated infected cell clearance.55 Drastic falls in viral load were also observed in a study by Zhang et al, where the SARS-CoV-2 virus loads after initiation of CP transfusion significantly fell. The time between CP transfusion and obtaining a negative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) test results in the four patients included in the study ranged from three to 22 days.17 Shen et al also reported positive results of convalescent plasma therapy as Viral loads decreased and RT-PCR became negative within 12 days, and SARS-CoV-2-specific ELISA and neutralizing antibody concentrations increased following transfusion (from 40–60 before and 80–320 on the seventh day) in five critically ill patients with COVID-19.13 Ye et al reported clearance of SARS-CoV-2 in throat swab test post CP therapy.46
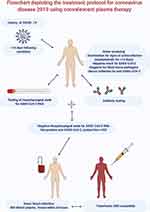 |
Figure 1 Flowchart depicting the treatment protocol for coronavirus disease 2019 using convalescent plasma therapy. |
Improvement in Clinical Symptoms
CP therapy was unanimously observed to bring about significant improvement in clinical symptoms and oxyhaemoglobin saturation. Recuperation from most clinical symptoms of COVID-19 in all the ten patients, especially fever, cough, chest pain, and dyspnoea was observed within one to three days after CP transfusion by Duan et al Oxygen saturation greatly improved with two patients weaned off ventilators to high-flow nasal cannula (HFNC) therapy, and one patient was also weaned off HFNC.15 Ahn et al also reported improved oxygenation in patients following plasma infusion.16 Clinical improvement was also reported by Li et al and Ye et al.46,47 The four critically ill patients treated with plasma therapy in a study by Zhang et al showed drastic improvements post plasma infusion with patient one extubated with persistent absorption of consolidation along with a significant fall in viral load. Patient two showed improved oxygen saturation, and gradual resolution of interstitial pneumonia in lung CT scan after plasma therapy, and all drugs were discontinued except for the corticosteroid called methylprednisolone. The third patient was critically ill with complications of confirmed positive active cystorrhagia (bleeding in the bladder), pneumorrhagia (haemorrhage in the lungs), and GI bleeding. He also showed decreased viral loads and absorption in lung infiltrates on CT and was eventually stepped down from the ICU. The fourth patient also recovered from SARS-COV-2 and was discharged soon after CP therapy.17 In the Shen et al study, patients became afebrile within three days in four out of five critically ill patients, PAO2/FIO2 increased within 12 days (ranging from 172–276 before and 284–366 after plasma therapy), and the SOFA decreased. The SOFA score, which is a mortality predictor and is based on the degree of dysfunction in six organ systems (with a range of 0–24, a higher number of graver labs and a more critically ill patient), was used to evaluate improvement in the patient’s condition after plasma therapy.13
Amelioration of Radiological Findings
Radiological studies like CT scan of the lung done before and after plasma therapy showed remarkable improvements. According to Duan et al, post CP transfusion, all patients showed significant degrees of resorption of pulmonary lesions. Chest CT, which showed massive infiltration and ground-glass attenuation of the lung before plasma therapy, started to show a gradual but steady improvement in the status of lung lesions five days after plasma transfusion.15 Absorption of lung infiltrates, and resolution of consolidation was observed in other studies as well.16,17
Favourable Laboratory Findings Post Convalescent Plasma Therapy
Amelioration of conventional laboratory criteria and improvement of pulmonary function was observed in all studies. Lymphocytopenia was found to be an important prognosis index in COVID-19.23 Lymphocyte counts increased after CP transfusion (median: 0.65 × 109 per L [before] vs 0.76 × 109 per L [after]). Seven out of the ten patients in the study showed an improvement in lymphocyte counts according to Duan et al.15 There was a decrement of markers indicative of liver dysfunction and/or inflammation after CP therapy, for example, alanine aminotransferase (ALT) (median: 42.00 U/L [before] vs 34.30 U/L [after]), and aspartate aminotransferase (AST) (median: 38.10 U/L [before] vs 30.30 U/L [after]) and CRP (median: 55.98 mg/L [before] vs 18.13 mg/L [after]). An increase in oxygen saturation (SaO2) (median: 93.00% [before] vs 96.00% [after]) was observed indicating recovering lung function.15,33 Ahn et al observed on CP administration lymphocyte count immediately rose to normal level (from 0.52 × 103/µL [before] to 1.21 × 103/µL [after]) and then remained in the normal range. Decreased inflammatory markers were also observed.16 According to Duan et al, when patients receiving CP infusion were compared to a control group, it was observed that three cases were discharged early and seven cases near discharge in the plasma therapy group whereas in the control group the outcomes were three deaths, six stabilized cases and one case showing improvement (P < 0.001).15
Limitations of Convalescent Plasma Therapy
In the study by Duan et al, no unfavourable adverse effects except an evanescent facial red spot in one patient were observed during CP therapy.15 No significant adverse events were reported during plasma transfusion in the other studies included in Table 2 either except Li et al who reported two patients with adverse events within hours after transfusion that improved with supportive care.47 Plasma therapy itself has important complications like TRALI, circulatory overload, or anaphylaxis.18 These complications should always be a concern. The risk of Hepatitis B virus, Hepatitis C virus and HIV disease transmission through the donated plasma should be thoroughly investigated.56
The transmission of the potential pathogen is a major risk of CP therapy. Methylene blue photochemistry was used by Eikmann et al to inactivate the residual virus while maintaining the function of neutralizing antibodies. This method was found to be more efficient than ultraviolet C (UVC) light.19 No targeted virus was detected in the CP before transfusion. A serious complication of plasma therapy known as TRALI was reported during plasma therapy in an Ebola virus female patient by Mora Rillo et al in their study.57 An adverse event like TRALI would not be a concern in the general population receiving CP therapy but becomes a major concern in critically ill patients who have significant lung injury making them more susceptible to TRALI.58 Another disadvantage affiliated with CP therapy is antibody-dependent infection enhancement (ADE), especially when plasma is transfused with antibodies at sub neutralizing concentrations. This does not neutralize the infection right away as it was intended to function but rather leads to an enhancement of infection by suppressing the body’s innate antiviral systems and allow exponential intracellular growth of the virus.59 This infection enhancement was observed in experiments with SARS-CoV-2 infection in vitro.60 If occurring in COVID-19 patients, ADE may account for some severe outcomes occurring later during the natural course of the disease.61
ADE may affect safety and efficacy of passive and active immunisation schedules. ADE has been proposed to account for the severity also of COVID-19 cases initially observed in China compared with other regions of the world.20
It needs to be noted that in most of the studies in Table 2 apart from CP transfusion, the patients were also receiving other modes of standard care like antiviral drugs (Lopinavir/Ritonavir or Remdesivir), conventional therapy for viral infections, oxygen therapy, mechanical ventilation, anti-inflammatory medicine despite the uncertainty around the efficacy of such drugs. It cannot be denied that there is the possibility that these concomitantly delivered antiviral agents could contribute to the recovery of patients rather than plasma therapy or synergize with the beneficial effect of CP. Hence, there is a potential risk of attributing certain benefits to plasma therapy, which may have been caused by some other treatment modality altogether or due to the combined effect of plasma therapy along with another drug. Moreover, some patients were also given corticosteroid therapy, which can decrease inflammation and reduce the activity of the immune system hence delaying the effect of other treatment modalities and even slow down virus clearance.15
Secondly, it was noted that the average time between the onset of symptoms and CP transfusion was 16.5 days. During this period, from the onset of infection to getting a dose of CP, the kinetics of COVID-19 viremia during the natural history of the disease remains a mystery. This justifies the need to conduct further research to study the association between SARS-CoV-2 RNA reduction and plasma therapy, as well as find out the quintessential concentration of neutralizing antibodies to treat efficiently and prevent antibody-mediated infection enhancement lastly, we need to establish a treatment protocol for CP therapy. Third, the dynamic changes in cytokine levels during treatment need to be investigated as it interferes with the treatment. Moreover, the optimal dose at which CP therapy is provided is not yet standardized.15
Ahn et al reported the following limitations of their study. The scarcity of large-scale trials and the failure to standardize antibody concentrations.16 A study by Xu et al concluded its limitations were restricted sample sizes and limited period of observation. Thus, treatment of the current outbreak of SARS-CoV-2 infection, particularly in critical illness, via CP, should be carefully considered until well-designed clinical trials are conducted.21 In the study done by Zhang et al, the relative contributions of standard supportive care, other investigational therapies like Remdesivir, and the patient’s immune response on survival could not be fixated on. They also propose conducting more clinical trials before its safety and benefits are determined.17 Shen et al also cited a limited sample size of only five patients to be a limitation. They moreover emphasized that whether CP therapy will lower case fatality rates for sure remains unknown now.13
Conclusions
In this study, we carefully reviewed an upcoming treatment modality for COVID-19 patients in the form of convalescent plasma therapy. By all counts, and with proven results, we inferred from the studies that plasma therapy shows immense promise in neutralizing antibody titers and culminating in drastic fall in viral loads, especially in critically ill patients. The clinical symptoms and radiological findings were observed to improve immensely after initiating plasma therapy. Laboratory parameters like lymphocyte count increased, and inflammatory markers (eg, CRP, AST, ALT) showed a downward trend after plasma infusion. No adverse events during transfusion were reported by any of the reviewed articles. However, the lack of large-scale randomized control trials, failure to standardize the number of antibodies administered to each patient, antibody-dependent enhancement of infection and inability to rule out the interference caused by other concomitantly administered therapeutic modalities like antiviral drugs and corticosteroids were cited as limitations. Moreover, it is impossible to rule out the adverse transfusion reactions and risk of transmitting the pathogen itself while administering plasma therapy. Hence, we can conclude that CP therapy is a promising therapeutic option during the COVID-19 pandemic, and there is a need to explore this treatment modality thoroughly by investing in large-scale clinical trials.
Take Home Points
- Convalescent Plasma (CP) therapy is an upcoming therapeutic modality for COVID-19 due to the absence of specific antiviral drugs and vaccines.
- CP is collected from recently recovered COVID-19 patients.
- CP therapy shows great results in reducing viral loads and increasing neutralizing antibodies.
- CP therapy also results in improved clinical symptoms like fever, cough, chest pain, and dyspnea and radiological findings like reduction in ground glass attenuation of lung in Chest CT.
- Increase in Lymphocyte counts and reduction in inflammatory markers was also observed post CP therapy.
- Lack of large-scale clinical trials, a small sample size and a failure to standardize the dose of neutralizing antibodies given to the patients were cited as major drawbacks in addition to adverse transfusion reactions.
Funding
No financial support was received for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Novel Coronavirus Pneumonia Emergency Responde Epidemiology Team. The epidemiological characteristics of an outbreak of2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):113–122.
2. World Health Organization (WHO). Coronavirus disease 2019 (COVID-19) situation report - 88. 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200417-sitrep-88-covid-191b6cccd94f8b4f219377bff55719a6ed.pdf?sfvrsn=ebe78315_6.
3. Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19.. Lancet Infect Dis. 2020;20:398–400.
4. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi:10.1016/S2213-2600(20)30079-5
5. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. The Lancet. 2020;395:473–475. doi:10.1016/S0140-6736(20)30317-2
6. Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:9. doi:10.1371/journal.pmed.0030343
7. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382(19):1787–1799. doi:10.1056/NEJMoa2001282
8. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi:10.1038/s41422-020-0282-0
9. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi:10.5582/bst.2020.01047
10. Chinese Clinical Trial Registry. Chinese Clinical Trial Registry. 2020. Available from: http://www.chictr.org.cn/.
11. Mcguire LW, Redden WR. Treatment of influenza pneumonia by the use of convalescent human serum: preliminary report. J Am Med Assoc. 1918;71(16):1311. doi:10.1001/jama.1918.26020420013013e
12. Cheng Y, Wong R, Soo YOY, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2004;24(1):44–46. doi:10.1007/s10096-004-1271-9
13. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA - J Am Med Assoc. 2020;323(29):1582–1589. doi:10.1001/jama.2020.4783
14. Tan L, Kang X, Zhang B, et al. A special case of COVID-19 with long duration of viral shedding for 49 days. medRxiv. 2020. doi:10.1101/2020.03.22.20040071
15. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci. 2020;117(17):9490–9496. doi:10.1073/pnas.2004168117
16. Ahn JY, Sohn Y, Lee SH, et al. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci. 2020;35(14):e149. doi:10.3346/jkms.2020.35.e149
17. Zhang B, Liu S, Tan T, et al. Treatment with convalescent plasma for critically Ill patients with SARS-CoV-2 infection. Chest. 2020;S0012-3692(20):30571–30577.
18. Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012;52:65S–79S. doi:10.1111/j.1537-2995.2012.03663.x
19. Eickmann M, Gravemann U, Handke W, et al. Inactivation of Ebola virus and Middle East respiratory syndrome coronavirus in platelet concentrates and plasma by ultraviolet C light and methylene blue plus visible light, respectively. Transfusion. 2018;58(9):2202–2207. doi:10.1111/trf.14652
20. Tetro JA. Is COVID-19 receiving ADE from other coronaviruses? Microbes Infect. 2020;22(2):72–73. doi:10.1016/j.micinf.2020.02.006
21. Xu Y, Xu Z, Liu X, et al. Clinical findings in critically ill patients infected with SARS-CoV-2 in Guangdong Province, China: a multi-center, retrospective, observational study. medRxiv. 2020 doi:10.1101/2020.03.03.20030668
22. Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi:10.1038/s41586-020-2012-7
23. Chen N, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7
24. World Health Organization (WHO). Coronavirus. 2020. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
25. Rothan HA, Byrareddy SN. The epidemeology and pathogenesis of coronavirus (Covid-19) outbreak. J Autoimmun. 2020;109:102433. doi:10.1016/j.jaut.2020.102433
26. Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur J Clin Invest. 2020;50(3):e13209. doi:10.1111/eci.13209
27. Hellewell J, Abbott S, Gimma A, et al. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Heal. 2020;8(4):e488–96. doi:10.1016/S2214-109X(20)30074-7
28. World Health Organization (WHO). Novel Coronavirus (2019-nCoV) situation reports. 2020. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/.
29. Sullivan HC, Roback JD. Convalescent plasma: therapeutic hope or hopeless strategy in the SARS-CoV-2 pandemic. Transfus Med Rev. 2020;S0887-7963(20):30025.
30. Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends. 2020;14(1):69–71. doi:10.5582/bst.2020.01020
31. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi:10.1056/NEJMoa2001191
32. Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11(1):1–14. doi:10.1038/s41467-019-13940-6
33. National Health Commission of the People’s Republic of China. NHC. Notice on printing and distributing the diagnosis and treatment plan of pneumonia with new coronavirus infection. 2020. Available from: http://www.nhc.gov.cn/yzygj/.
34. Multicenter, Pneumonia. collaboration group of D of S and T of GP and HC of GP for chloroquine in the treatment of novel coronavirus. [Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia]. Chinese J Tuberc Respir Dis. 2020;43(3):185–188. Chinese.
35. Gautret P, Lagier J-C, Parola P, Hoang VT, Al E. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med Infect Dis. 2020;34:101663. doi:10.1016/j.tmaid.2020.101663
36. Zhao JP, Hu Y, Du RH, et al. [Expert consensus on the use of corticosteroid in patients with 2019-nCoV pneumonia]. Chinese J Tuberc Respir Dis J Tuberc Respir Dis. 2020;43(3):183–184. Chinese.
37. Horby PW, Lim W; Recovery Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 — preliminary report. N Engl J Med. 2020.
38. Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? In: Annals of Internal Medicine. Vol. 145. American College of Physicians; 2006: 599–609. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16940336.
39. Yeh KM, Chiueh TS, Siu LK, et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005;56(5):919–922. doi:10.1093/jac/dki346
40. Wong VWS, Dai D, Wu AKL, Sung JJY. Treatment of severe acute respiratory syndrome with convalescent plasma. Hong Kong Med J. 2003;9(3):199–201.
41. Kong L. Severe acute respiratory syndrome (SARS). Transfus Apher Sci. 2003;29(1):101. doi:10.1016/S1473-0502(03)00109-5
42. Ko JH, Seok H, Cho SY, et al. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir Ther. 2018;23(7):617–622. doi:10.3851/IMP3243
43. U.S.Food & Drug. Investigational COVID-19 Convalescent Plasma- Emergency INDs. 2020. Available from: https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma.
44. Chun S, Chung CR, Ha YE, et al. Possible transfusion-related acute lung injury following convalescent plasma transfusion in a patient with middle east respiratory syndrome. Ann Lab Med. 2016;36(4):393–395. doi:10.3343/alm.2016.36.4.393
45. Cunningham AC, Goh HP, Koh D. Treatment of COVID-19: old tricks for new challenges. Crit Care. 2020;24(1):91.
46. Ye M, Fu D, Ren Y, et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. 2020;92(10):1890–1901. doi:10.1002/jmv.25882
47. Li L, Li L, Zhang W, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA - J Am Med Assoc. 2020;324(5):460. doi:10.1001/jama.2020.10044
48. Brown BL, McCullough J. Treatment for emerging viruses: convalescent plasma and COVID-19. Transfus Apher Sci. 2020;59(3):102790. doi:10.1016/j.transci.2020.102790
49. Liu S, Liu X, Zhao S, et al. An exceptional peroxide birefringent material resulting from d–π interactions. Angew Chemie - Int Ed. 2020;59(24):9414–9417. doi:10.1002/anie.202002828
50. Okba NMA, Widjaja I, Li W, et al. Serologic detection of Middle East respiratory syndrome coronavirus functional antibodies. Emerg Infect Dis. 2020;26(5):1024–1027. doi:10.3201/eid2605.190921
51. U.S.Food & Drug. Recommendations for Investigational COVID-19 Convalescent Plasma. 2020. Available from: https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma.
52. Wu F, Wang A, Liu M, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. SSRN Electron J. 2020.
53. Ferreira LMR, Mostajo-Radji MA. Plasma-based COVID-19 treatments in low- and middle-income nations pose a high risk of an HIV epidemic. Npj Vaccines. 2020;5:58. doi:10.1038/s41541-020-0209-2
54. McLaughlin K. China’s AIDS history explains part of the “CRISPR babies” story. STAT. 2020. Available from: https://www.statnews.com/2018/12/14/china-aids-history-crispr-babies/.
55. Lu CL, Murakowski DK, Bournazos S, et al. Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science. 2016;352(6288):1001–1004. doi:10.1126/science.aaf1279
56. Pawar AY, Hiray AP, Sonawane DD, Bhambar RS, Derle DV, Ahire YS. Convalescent plasma: a possible treatment protocol for COVID-19 patients suffering from diabetes or underlying liver diseases. Diabetes Metab Syndr Clin Res Rev. 2020;14(4):665–669. doi:10.1016/j.dsx.2020.05.023
57. Mora-Rillo M, Arsuaga M, Ramírez-Olivencia G, et al. Acute respiratory distress syndrome after convalescent plasma use: treatment of a patient with Ebola virus disease contracted in Madrid, Spain. Lancet Respir Med. 2015;3(7):554–562. doi:10.1016/S2213-2600(15)00180-0
58. Benson AB, Moss M, Silliman CC. Transfusion-related acute lung injury (TRALI): a clinical review with emphasis on the critically ill. Br J Haematol. 2009;147(4):431–443.
59. Halstead SB. Dengue antibody-dependent enhancement: knowns and unknowns. Microbiol Spectr. 2014;2:6.
60. Wang SF, Tseng SP, et al. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem Biophys Res Commun. 2014;451(2):208–214. doi:10.1016/j.bbrc.2014.07.090
61. Negro F. Is antibody-dependent enhancement playing a role in COVID-19 pathogenesis? Swiss Med Wkly. 2020;150:1516.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
