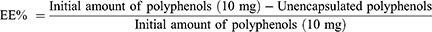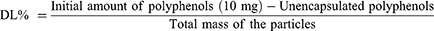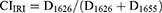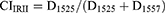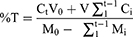Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Controlled-Release Wedelia trilobata L. Flower Extract Loaded Fibroin Microparticles as Potential Anti-Aging Preparations for Cosmetic Trade Commercialization
Authors Pham DT, Huynh QC, Lieu R, Nguyen VB, Tran VD , Thuy BTP
Received 19 January 2023
Accepted for publication 13 April 2023
Published 26 April 2023 Volume 2023:16 Pages 1109—1121
DOI https://doi.org/10.2147/CCID.S405464
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Duy Toan Pham,1 Quoc Cuong Huynh,1 Ruby Lieu,2 Viet Bach Nguyen,3 Van De Tran,4 Bui Thi Phuong Thuy5
1Department of Chemistry, College of Natural Sciences, Can Tho University, Can Tho, 900000, Vietnam; 2Faculty of Commerce, Van Lang University, Ho Chi Minh City, Vietnam; 3Faculty of Chemical Engineering, College of Engineering, Can Tho University, Can Tho, 900000, Vietnam; 4Department of Health Organization and Management, Can Tho University of Medicine and Pharmacy, Can Tho, 900000, Vietnam; 5Faculty of Fundamental Sciences, Van Lang University, Ho Chi Minh City, Vietnam
Correspondence: Bui Thi Phuong Thuy, Faculty of Fundamental Sciences, Van Lang University, 69/68 Dang Thuy Tram Street, Ward 13, Binh Thanh District, Ho Chi Minh City, Vietnam, Email [email protected]
Background: Wedelia trilobata L. (WT), a common herbal plant in Vietnam, is popularly used as a strong antioxidant in Vietnamese folk medicine. However, limited studies have reported the application of WT flower in cosmeceutical area.
Purpose: This study explored the potentials of WT loaded fibroin microparticles (FMPs-WT) as a novel anti-aging cosmeceutical product.
Methods: The WT flower was firstly extracted by maceration with methanol, ethanol 60%, and ethanol 96%, and its chemical compositions and total polyphenol content were investigated. Then, the FMPs-WT were developed by desolvation method and physicochemically characterized. Finally, the product antioxidant activities were in-vitro determined using DPPH assay.
Results: The optimal WT extract was the ethanol 60% extract, which contains polyphenols, alkaloids, flavonoids, saponins, glycosides, and organic acids; with a total polyphenol content of 46.47 ± 2.32 mg GAE/g plant powder. The FMPs-WT were successfully formulated, with a distinct silk-II polymorph; varied sizes of 0.592 to 9.820 μm, depending on the fibroin concentrations and the WT extraction solvent; high entrapment efficiencies of > 65%; and sustained-release patterns of polyphenol in pH 7.4 for > 6 h. Regarding the antioxidant activity, the pure WT flower extracts possessed high scavenging actions with IC50 of 7.98 ± 0.40 μg/mL, comparable with the standard ascorbic acid (IC50 = 4.23 ± 0.21 μg/mL). Moreover, the FMPs-WT could retain the extract antioxidant capacity, and exert the effects in a timely manner, corresponding to its release profile.
Conclusion: The FMPs-WT could be further investigated to become a potential anti-aging cosmeceutical product in the market.
Keywords: Wedelia trilobata L., silk, fibroin, microparticles, antioxidant, DPPH
Introduction
Aging is a natural phenomenon resulting from the gradual decline of biological and physiological functions as people age.1–3 The aging process is partly due to the accumulation of molecular damage in the cells. Aging can be caused by various factors, including internal factors such as inflammation, hormone deficiency, and physiological disorders, and external factors such as tobacco, alcohol, and unhealthy foods. Among them, one of the most important factors that accelerates aging process is cellular oxidative stress caused by the reactive oxygen species (ROS).4 Thus, to delay the aging progression and prolong the human life span, much effort has been made on the anti-aging compounds (ie, compounds that interfere and hinder the aging biological processes).5 However, synthetic and semi-synthetic anti-aging drugs, besides the desired therapeutic actions, possess numerous side effects affecting human health.6 Therefore, compounds from natural sources have gained increasing interest,7,8 especially polyphenols due to their high antioxidant activities.
For that reason, Wedelia trilobata L. (WT), commonly known as “sài đất ba thùy” in Vietnamese, could be a potential plant for anti-aging due to its rich source of polyphenols. WT is an ethnopharmacological herb belonging to the Asteraceae family, native to Mexico and Central America.9 Currently, WT is found all over the world, including Vietnam. In Vietnam, WT has long been used as a folk medicinal plant for common cold, inflammation, and wound treatments. Pharmacological reports show that WT, especially its flower, possesses numerous therapeutic actions of antioxidant,10,11 analgesic,12 anti-inflammatory,13 antibacterial,14 wound healing,15 anticancer,16 liver protection,17 anti-diabetes, and fertility enhancement.18,19 Specifically, for anti-aging issue, WT-flower-extract strong antioxidant capacity has been confirmed, which is mainly due to its abundance of polyphenol compounds.9,11,14 However, because of its structures with lots of unsaturated bonds, most WT polyphenol compounds are unstable, readily interact with various chemicals, and easily being oxidized when exposed to light, temperature, pH, water, and enzymes.20 Therefore, to enhance the WT polyphenols stability, increase and prolong their activities, it is crucial to protect them from physicochemical damage prior to applications.
One potential approach is the encapsulation of WT flower extract in a biomaterial-based drug delivery system that is both safe for humans, effective in protection against external influences, and controlled releases of active ingredients.21 To this end, microparticles fabricated from silk fibroin are an interesting system. Fibroin, a protein commonly extracted from Bombyx mori silk, possesses favorable properties of high stability, non-toxicity, high biocompatibility, and biodegradability.22–27 Moreover, fibroin has been approved by the US FDA as a healthcare material,28 thus, the incorporation of WT flower extract in the fibroin microparticles (FMPs) can be applied as functional foods or dietary supplements for anti-aging purposes. Over the years, fibroin has been widely used in regenerative medicine, as well as in controlled drug delivery systems for carrying antioxidants.29,30 Nevertheless, most studies focus on only one pure antioxidant such as curcumin,31,32 resveratrol,33 and quercetin.34 To the best of our knowledge, limited studies have explored the potentials of FMPs in delivering plant extracts,21,28 and no report has mentioned about the application of FMPs for anti-aging purpose.
Therefore, from the above limitations, to fill in the literature gaps and to develop new anti-aging cosmeceutical products, this study formulated the FMPs containing various extracts of WT flowers (ie, ethanol 96%, ethanol 60%, and methanol extracts), determined the products physicochemical properties, and investigated their antioxidant effect.
Materials and Methods
Materials
WT flowers were collected in September 2021 in Can Tho, Vietnam, and identified according to the Vietnamese herb-species analysis system by a botanical expert, and the voucher specimens were kept at the College of Natural Sciences, Can Tho University. Silkworm cocoons (variety Bombyx mori M45) were collected in Truc Ninh district, Nam Dinh province. After harvesting, the cocoons were cleaned, dried, packed, and transported at normal temperature and humidity of 60–80% to the laboratory.
The chemicals used in this study were ascorbic acid (99%, Merck, Germany), gallic acid (Xilong, China), Folin-Ciocalteu (Merck, Germany), 2,2-Diphenyl-1-picrylhydrazyl (DPPH, 95%, Merck, Germany), Na2CO3 99.8% (Xilong, China). All other utilized compounds/solvents (ie, ethanol 96%, methanol, HCl, and NaOH) were of reagent grades or higher.
Plant Collection and Extraction
Fresh WT flowers, after being collected, were cleaned with water, dried naturally, ground into fine powder, and stored at 4°C. For the extraction, a simple maceration process was utilized. Briefly, 30 g of dry plant powder was soaked in methanol, ethanol 60%, or ethanol 96% with a raw materials/solvent ratio of 1:60 (w/v) for 72 h. Then, the samples were sonicated at 50°C for 60 min in triplicate, with the same raw materials/solvent ratio. The extracts were then filtered thrice and the solutions were evaporated using the rotavapor at low pressure and 45°C. The extraction efficiency was calculated as the final extract weights divided by the initial plant powder weight (30 g). In this study, three (3) WT extracts were employed, namely the methanol, ethanol 60%, and ethanol 96% extract.
Plant Chemical Composition Determinations
The main chemical constituents (alkaloid, glycoside, flavonoid, steroid, saponin, and organic acid) of the three WT flower extracts were determined based on the standard protocols summarized in Figure 1. Additionally, the total polyphenol content in each extract was spectroscopic quantified utilizing the well-known Folin-Ciocalteu reaction, following the manufacturer’s procedure.35,36 Accordingly, 2.5 mL of 10% Folin-Ciocalteu reagent was added to 0.5 mL of the extract, and the mixture was incubated in the dark for 3–8 min at 25°C. Then, 2 mL of 10% Na2CO3 solution was added to the mixture, followed by another 1-h incubation. Finally, the UV–Vis spectroscopic absorbance of the products was measured at a wavelength of 765 nm. The total polyphenol content in the extract was calculated based on the calibration curves of the standard gallic acid and was expressed as mg gallic acid equivalent/dry powder weight (mg GAE/g DPW).
 |
Figure 1 Wedelia trilobata L. chemical constituents’ determination protocols. |
Silk Fibroin Extraction
Fibroin was extracted from Bombyx mori silkworm cocoons by microwave-assisted heat extraction method.37 Briefly, ten grams (10 g) of the cocoons were degummed with 0.5% Na2CO3 solution at 100°C for 1 h, followed by washing with distilled water and drying naturally. The degummed silk were then dissolved in CaCl2:H2O:Ca (NO3)2:EtOH mixture (30:45:5:20 w/w/w/w) and heated in a microwave oven (900 W) for 2 min. The silk solution was dialyzed with distilled water using a cellulose membrane filter (10,000 MWCO) at room temperature for 3–5 days. After dialysis, the solution was centrifuged at 10,000 rpm, 4°C for 30 min to remove impurities. Finally, the silk fibroin solution was lyophilized at −55°C and 10−4 Torr and the freeze-dried fibroin was cryopreserved for use in the following experiments.
Preparation of FMPs
The FMPs were prepared in two forms, the blank FMPs (FMPs with no WT extract) and the FMPs-WT (FMPs contain the WT flower extracts), using a simple desolvation method.24,37 For each form, a total of nine (9) formulas were prepared, corresponding to the fibroin concentrations of 1%, 2%, and 3%, and the extraction solvents of methanol, ethanol 60%, and ethanol 96%.
For the blank FMPs, the fibroin powder with the weights of 0.05 g/0.10 g/0.15 g was dissolved in 5 mL of distilled water to form the fibroin solution at concentrations of 1%/2%/3%. Then, 1 mL of the solvent (methanol, ethanol 60%, ethanol 96%) was slowly added to 2 mL of the fibroin solution and the mixture was shaken at 4°C for 24 h. Then, the spontaneously formed particles were collected by centrifugation at 6000 rpm for 40 min. The FMPs were washed with distilled water by centrifugation. All particles were freshly prepared prior to experiments.
For the FMPs-WT, the preparation process was performed similarly to the blank FMPs. However, 1 mL of the solvent was replaced with 1 mL of the WT flower extract solution, which comprises a polyphenol content of 10 mg GAE/g DPW.
Physico-Chemical Characterizations of FMPs
The blank FMPs and FMPs-WT were physicochemically characterized in terms of particle size, morphology, drug entrapment efficiency (EE%) and loading capacity (DL%), structure and crystallinity, and drug release profile.
For the size and morphology, the average particle size and particle size distribution (polydispersity index—PI) were determined by dynamic light scattering method using a MicroTrac S3500 analyzer. For this, 5 mL of the FMPs and FMPs-WT dispersions in distilled water were subjected to the sample holder, and the measurements were made at 25°C at a fixed angle of 90°.24 The particles morphology was observed under a scanning electron microscope in a nitrogenic atmosphere. The FMPs and FMPs-WT dispersions were dropped onto the carbon-coated copper grid, dried naturally, coated with platinum, and subjected to the instrument sample holder. The operating procedure was then followed the manufacturer’s guideline.
To determine the EE% and DL% of the WT flower extract total polyphenol that was encapsulated into the FMPs, an indirect method was employed.38 Briefly, after the FMPs-WT were formulated, the particles were centrifuged, and the un-encapsulated polyphenols presented in the supernatant were reacted with Folin-Ciocalteu reagent following the procedure described in Plant Chemical Composition Determinations. Then, the products were UV-Vis spectroscopic measured at a wavelength of 765 nm, and the amount of un-encapsulated polyphenols was calculated using the gallic acid standard curve. Finally, the EE% and DL% were determined using (Equations 1 and 2), respectively.
Regarding the particles structures and crystallinity, Fourier-transform infrared (FTIR) spectroscopy was utilized to determine the FMP structures, interactions between fibroin and WT polyphenols, and the crystallinity indexes (CI) of the systems. For this, the lyophilized fibroin powder, the blank FMPs, the FMPs-WT, and the WT extracts were subjected to FTIR measurements (Jasco, Japan, frequency ranges from 4000 to 400 cm−1) using the KBr pelleting technique. The analysis was performed under dry air filtration with an encoder of 256 interference images collected at a resolution of 4.0 cm−1. To evaluate the particle crystallinity, the fibroin amide-I and amide-II peak intensities were determined and the CI was calculated based on (Equations 3 and 4).
where CIIRI and CIIRII are CI values of amide-I and -II peaks, respectively; D1626 and D1525 are the intensities of the fibroin crystalline fractions at amides I and II, respectively, while D1655 and D1557 are the intensities of the fibroin amorphous fractions at amides I and II, respectively.24
In terms of the drug release profiles, the ability to release polyphenols of the FMPs-WT was performed by shaking method. To this end, FMPs-WT were dispersed in 50 mL of phosphate buffer (0.1 M, pH 7.4), and shaken at 200 rpm for 4 h. At each time point of 30, 60, 90, 120, 150, 180, 210, and 240 min, 1 mL of the sample was withdrawn and the same amount of buffer was added. Then, the withdrawn samples were centrifuged at 18,000 rpm for 2 min, and the polyphenol content in the supernatant was reacted with Folin-Ciocalteu reagent following the procedure described in Plant Chemical Composition Determinations, and UV–Vis spectroscopic determined at 765 nm. The amount of release polyphenols was determined based on the gallic acid calibration curve in phosphate buffer pH 7.4 (range: 2–10 µg/mL, y = 0.0985x + 0.0363, R2 = 0.9989). Finally, the polyphenol cumulative-release percentage (%T) was calculated by equation (5).
In which: Ct, Ci: concentration of polyphenols released at time point t and i.
Antioxidant Activity Evaluations
The antioxidant activities of the WT extracts, the blank FMPs, and the FMPs-WT were in-vitro assessed by the free radical scavenging DPPH assay.14 For the WT extracts, 0.5 mL of the ethanolic solution of DPPH 0.1 mM was added to 1.5 mL of the extracts at different concentrations (3, 6, 9, 12, 15, 18 µg/mL). The reaction mixture was incubated in the dark for 30 min. Then, the absorbance values of the DPPH was UV-Vis spectroscopic measured at 517 nm. Ascorbic acid was used as a positive/reference control. The IC50 values of the WT extracts (ie, the minimum concentration to scavenge 50% of DPPH free radicals) were then determined.
For the blank FMPs and the FMPs-WT, the free radical scavenging efficiency was determined at different time points (30, 90, and 180 min) to investigate their antioxidant activities over time. The freeze-dried FMP samples, at polyphenol amounts similar to the WT extract IC50, were weighed and re-dispersed in distilled water. Then, 0.5 mL of the ethanolic solution of DPPH 0.1 mM was added to 1.5 mL of the dispersions. The mixtures were shaken at 200 rpm in the dark for 30, 90, and 180 min. Finally, the reaction mixtures were centrifuged at 2000 rpm for 2 min, and measured the DPPH absorbance values at 517 nm, similar to the WT extract procedure. The DPPH scanning efficiency percentage was calculated using the (Equation 6).
where A0 and A1 are the absorbance values of the DPPH without and with the presence of test/reference samples.
Statistical Analysis
All experiments were conducted in triplicate, and the data were reported in terms of mean ± SD (standard deviation). Student’s t-test and one-way analysis of variance (ANOVA) were utilized for statistical purposes, where necessary, with a p value of <0.05 for significant comparisons.
Results and Discussions
This study developed and evaluated the antioxidant properties of WT loaded FMPs as a potential anti-aging cosmeceutical product. To this end, we first extracted the WT flower using three different solvents of methanol, ethanol 60%, and ethanol 96%, and determined their chemical profiles and total polyphenol content. Then, the blank FMPs and FMPs-WT were formulated at different fibroin concentrations of 1%, 2%, and 3%, followed by physicochemical characterizations. Finally, the products were investigated for their antioxidant activities in in-vitro settings using the DPPH assay.
Plant Extraction and Chemical Composition Determinations
From 30 g of dry WT powder (water content of 7.89 ± 0.47%) extracted with different solvents, the ethanol 60% yielded the highest extraction efficiency of 9.05 ± 0.81%, followed by methanol (8.10 ± 0.75%) and ethanol 96% (7.85 ± 0.72%). All three WT flower extracts were yellow-brown color, solid and has a characteristic scent.
The chemical compositions in all three extracts showed positive results for various compounds, namely alkaloid, flavonoid, auron, flavonol, saponin, glycoside, and organic acids (Table 1), partly in agreement with previous studies.12,13,39 The differences in the compounds in our extracts and the other studies’ extracts could be due to the differences between the extraction methods and partly due to the influence of geographical, climatic, and soil conditions in each region.
 |
Table 1 Chemical Compositions in the Methanol, Ethanol 60%, and Ethanol 96% Extract of Wedelia trilobata L. Flower. (+) Presence; (-) Absence |
The polyphenol content in each extract was determined based on the gallic acid standard curve in methanol (y = 0.0984x + 0.0787 (R² = 0.9925)), ethanol 60% (y = 0.1023x + 0.0574 (R² = 0.9992)), and ethanol 96% (y = 0.1100x + 0.0436 (R² = 0.9994)). Correspondingly, the polyphenol content in the methanol, ethanol 60%, and ethanol 96% extracts was 33.47 ± 1.67, 46.47 ± 2.32, and 24.45 ± 1.22 mg GAE/g DPW. These results were consistent with the extraction efficiency data, as a solvent with higher extraction efficiency (ethanol 60%) yielded a higher polyphenol content.
Physico-Chemical Characterizations of FMPs
The mean particle sizes of the FMPs and FMPs-WT are reported in Table 2. In general, the formulations possess different sizes, ranging from 0.592 to 9.820 µm, depending on the fibroin concentrations and the WT extraction solvent. Additionally, all formulas had a PI value of <0.3, indicating the system's narrow size distributions. From the results, the FMPs particle sizes followed the solvent order of methanol > ethanol 96% > ethanol 60%. This re-confirms the effects of different desolvating solvents on the fibroin micro-/nanoparticle formations, sizes, and shapes.40 Moreover, an increase in the fibroin concentrations, from 1% to 3%, yielded proportionally larger particles. This phenomenon could be explained by a fibroin structure that contains both hydrophobic (75% w/w) and hydrophilic segments (25% w/w). This dominant hydrophobic segment, characterized by a high content of repeat units of Glycine-Alanine, renders fibroin crystalline process in aqueous solution. Additionally, when increasing the fibroin concentration, the interactions between the microcrystals and the free fibroin molecules, as well as between the microparticles themselves correspondingly increase. Therefore, in a limited space, it is easier for FMPs at higher fibroin concentrations to agglomerate and form larger-sized particles.
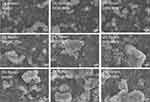
The particles morphologies were further investigated using SEM technique (Figure 2). The micrographs illustrate that in all nine formulas, the FMPs-WT were spherical in shape, and mostly stay in aggregate forms (ie, the particles link together to form long and complex chains). Moreover, when the fibroin concentrations increased from 1% to 3%, the bigger particle clumps with larger sizes were observed, which confirms the DLS results. Additionally, the formulating solvents (methanol, ethanol 60%, and ethanol 96%) did not significantly affect the particles shapes.
In terms of the EE% and DL%, the polyphenol entrapment efficiencies into the FMPs, at all formulas, were relatively high (>65%) (Table 3). This indicates that the polyphenol compounds in the WT extract strongly interact with the structure of silk fibroin, which will be further confirmed in the FTIR spectra. Moreover, among three extracts, the EE% of the ethanol 60% extract were the highest, suggesting that the polyphenol compounds in this extract interact better with the fibroin molecules than the compounds in the methanol and ethanol 96% extracts. Interestingly, higher fibroin contents resulted in higher EE% and lower DL% values. This could be explained by the saturated loading capacity of the FMPs. When the fibroin amount increases, the saturated loading capacity increases, thus, the EE% increases. However, the increase in fibroin amount produced more FMPs, yet the loaded polyphenol compounds reached their saturation levels, consequently decreases the DL% of the whole systems.
Regarding the FMPs structures, FTIR spectroscopy was employed. To this end, the spectra of all blank FMPs and FMPs-WT show specific silk-II (ie, crystalline-dominant silk structure) peaks at 1626 cm−1 (amide I), 1525 cm−1 (amide II), and 1234 cm−1 (amide III) (Figure 3).37 On the other hand, the raw fibroin powder shows specific silk-I (ie, amorphous-dominant silk structure) peaks. Moreover, the characterized peaks of the WT extracts of 2912 cm−1 and 2830 cm−1 appeared in the FMPs-WT formulas but did not appear in the blank FMPs. These two phenomena indicate that the FMPs formulation processes were complete41 and the WT extracts were successfully loaded into the particles. Additionally, the fact that other WT extract signals did not show in the FMPs-WT spectra suggests that the extract compounds were mostly encapsulated in the particles in molecular dispersion states.26 Furthermore, from the FTIR data, the CI of the microparticle systems and the raw fibroin powder were calculated based on our previous study37 (Table 4) and followed the order of CIfibroin powder < CIFMPs-WT < CIBlank FMPs. The CI values are used to assess the particle crystalline/amorphous state, the higher the values, the higher the crystallinity of the material.37 Hence, the results re-confirm that the fibroin in FMPs has been changed to silk-II crystalline structure, rather than the silk-I amorphous structure of the fibroin powder; and the WT extract was encapsulated in the FMPs in molecular dispersion amorphous form, which reduces the overall CI of the systems.
The release profiles of the total polyphenol content from FMPs-WT were investigated in phosphate buffer (0.1 M, pH 7.4) (Figure 4). The maximum polyphenol releases of the FMPs-WT were different, depending on their fibroin concentrations and extraction solvents. Specifically, the FMPs-WTMeOH, the FMPs-WTEtOH 60%, and the FMPs-WTEtOH 96% possesses a maximum polyphenol release of 26.94%, 23.67%, 17.38%; of 26.77%, 23.18%, 19.87%; and of 32.98%, 28.3%, 20.62%, corresponding to the fibroin concentration of 1%, 2%, and 3%, respectively. Such differences could be explained based on the composition of the polyphenol compounds that were encapsulated into the FMPs, which had different interactions with the fibroin molecules, making them released differently. Moreover, all nine formulas show a 2-phases-release pattern of polyphenol, with a burst release in the first 30 min, followed by a sustained release for the remaining time. There were two reasons for this, (1) as previously discussed, the polyphenol was encapsulated in the FMPs in molecular dispersion form, thus, some polyphenols are easily dissolved out into the medium, making the burst release phase; (2) some polyphenols possess significant hydrophobic interactions with fibroin silk-II β-sheet structure,9,30,32 which contribute to the gradual release phase. Conclusively, this 2-phases release pattern is beneficial for FMPs to deliver the WT extracts, since the burst release phase gives adequate drug amount for therapeutic action, and the slow-release phase protects the polyphenols from degradation by environmental agents, as well as controls their therapeutic effects.
Antioxidant Activity Evaluations
The antioxidant activity of the WT flower extracts, the blank FMPs, and the FMPs-WT was determined based on their ability to reduce the spectral absorbance of the DPPH solution. Compared to the standard ascorbic acid with an IC50 of 4.23 ± 0.21 µg/mL, the methanol extract, the ethanol-60% extract, and the ethanol-96% extract of the WT flower possessed moderate antioxidant activities, with IC50 of 8.67 ± 0.43 µg/mL, 7.98 ± 0.40 µg/mL, and 10.70 ± 0.54 µg/mL, respectively. These data were in correspondence with the total polyphenol content of the three extracts (ie, the polyphenol content in ethanol-60% extract > methanol extract > ethanol-96% extract), suggesting that these polyphenols play an important role in the WT antioxidant activity. Our WT extract IC50 was much lower than that of previous studies, which show that the IC50 of WT flower methanol extract ranged from 19.072 µg/mL to 90 µg/mL.11,15 These differences might be due to geographical variations, climate, and other environmental factors affecting the chemical composition of the plant.
Regarding the FMPs antioxidant activities (Table 5), the blank FMPs could scavenge a small amount of DPPH, possibly because of the tyrosine residues presented in the structure of fibroin.28 Moreover, all FMPs-WT formulas demonstrate high antioxidant activity, indicating that the WT polyphenols actions were preserved when the extracts were encapsulated into the FMPs. Interestingly, the FMPs-WT could scavenge the free radicals in a time-dependent manner, as the longer the reaction time, the higher the scavenging percentages. These results corresponded with the release profiles of the polyphenol compounds from the particles. Since the FMPs-WT possessed a sustained-release pattern, their antioxidant effects were also prolonged. This fact shows much potential of the FMPs in delivering the WT extracts as a cosmeceutical product for anti-aging action since the particles could protect the polyphenols, as well as extend their action sustainably.
Conclusions
The present study developed and fully evaluated the FMPs incorporating WT flower extract as a novel anti-aging product. The WT flower methanol, ethanol 60%, and ethanol 96% extracts showed high antioxidant activities of 8.67 ± 0.43 µg/mL, 7.98 ± 0.40 µg/mL, and 10.70 ± 0.54 µg/mL, respectively. Furthermore, the polyphenol compounds in these extracts were successfully loaded into the FMPs in molecular dispersion amorphous forms, with a high entrapment efficiency of >65%, and a sustain-release profile of >6 h. In addition, the particles could retain the extract antioxidant capacity and exert the effects in a timely manner, corresponding to their release profile. Conclusively, the controlled-release FMPs-WT could both protect polyphenols in the WT extract from being degraded by environmental factors and control the release profile of these compounds, which make the particles a potential application for anti-aging purpose.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author Bui Thi Phuong Thuy.
Acknowledgments
This research is funded by the Vietnam Ministry of Education and Training under grant number B2022-TCT-04. The authors would like to thank Can Tho University, Van Lang University, and Can Tho University of Medicine and Pharmacy for their helpful support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research is funded by the Vietnam Ministry of Education and Training under grant number B2022-TCT-04.
Disclosure
The authors report no conflicts of interest in this work.
References
1. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi:10.1016/j.cell.2013.05.039
2. Ferreira MS, Magalhães MC, Oliveira R, Sousa-Lobo JM, Almeida IF. Trends in the use of botanicals in anti-aging cosmetics. Molecules. 2021;26(12):3584. doi:10.3390/molecules26123584
3. Ok S-C. Insights into the anti-aging prevention and diagnostic medicine and healthcare. Diagnostics. 2022;12(4):819. doi:10.3390/diagnostics12040819
4. Liguori I, Russo G, Curcio F, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757. doi:10.2147/CIA.S158513
5. Wang J, Cao B, Zhao H, Feng J. Emerging roles of ganoderma lucidum in anti-aging. Aging Dis. 2017;8(6):691–707. doi:10.14336/AD.2017.0410
6. Engel N, Mahlknecht U. Aging and anti-aging: unexpected side effects of everyday medication through sirtuin1 modulation. Int J Mol Med. 2008;21:223–232. doi:10.3892/ijmm.21.2.223
7. Nguyen PH, Tran V, Pham DT, Dao TNP, Dewey RS. Use of and attitudes towards herbal medicine during the COVID-19 pandemic: a cross-sectional study in Vietnam. Eur J Integr Med. 2021;44:101328. doi:10.1016/J.EUJIM.2021.101328
8. Tran VD, Pham DT, Cao TTN, et al. Perspectives on COVID-19 prevention and treatment using herbal medicine in Vietnam: a cross-sectional study. Ann Ig. 2021;34(5):515–531. doi:10.7416/AI.2021.2484
9. Balekar N, Nakpheng T, Srichana T. Wedelia trilobata L.: a phytochemical and pharmacological review. Chiang Mai J Sci. 2014;41(3):590–605.
10. Mardina V, Ilyas S, Harmawan T, Halimatussakdiah H, Tanjung M. Antioxidant and cytotoxic activities of the ethyl acetate extract of Sphagneticola trilobata (L.) J.F. Pruski on MCF-7 breast cancer cell. J Adv Pharm Technol Res. 2020;11(3):123–127. doi:10.4103/japtr.JAPTR_31_20
11. Mardina V, Mastura H, Sufriadi E. Flower of sphagneticola trilobata (L.) J.F Pruski from Aceh, Indonesia: antioxidant and cytotoxic activity on HeLa cells. IOP Conf Ser Mater Sci Eng. 2020;1007(1):012182. doi:10.1088/1757-899X/1007/1/012182
12. Sureshkumar S, Bhama S, Kumar TS, Mjn C, Rajesh R. Analgesic activities of the medicinal plants of Wedelia trilobata, Wedelia biflora and Eclipta alba in standard experimental animal models. Biosci Biotechnol Res Asia. 2007;4(1):201–206.
13. Govindappa M, Sravya N, Poojashri MN, et al. Antimicrobial, antioxidant and in vitro anti-inflammatory activity and phytochemical screening of water extract of Wedelia trilobata (L.) Hitchc. J Med Plants Res. 2011;5:5718–5729.
14. Chethan J, Sampath Kumara KK, Niranjana SR, Prakash HS. Evaluation of antioxidant and antibacterial activities of methanolic flower extract of Wedelia trilobata (L.) Hitch. African J Biotechnol. 2012;11(41):9829–9834. doi:10.5897/ajb11.3729
15. Balekar N, Katkam NG, Nakpheng T, Jehtae K, Srichana T. Evaluation of the wound healing potential of Wedelia trilobata (L.) leaves. J Ethnopharmacol. 2012;141(3):817–824. doi:10.1016/j.jep.2012.03.019
16. Mardina V, Ilyas S, Halimatussakdiah H, Harmawan T, Tanjung M, Yusof F. Anticancer, antioxidant, and antibacterial activities of the methanolic extract from Sphagneticola trilobata (L.) J. F Pruski Leaves. J Adv Pharm Technol Res. 2021;12(3):222–226. doi:10.4103/japtr.JAPTR_131_21
17. Upadhyay K, Gupta NK, Dixit VK. Development and characterization of phyto-vesicles of wedelolactone for hepatoprotective activity. Drug Dev Ind Pharm. 2012;38(9):1152–1158. doi:10.3109/03639045.2011.643892
18. Kade I, Barbosa N, Ibukun E, Igbakin AP, Nogueira C, Rocha JB. Aqueous extracts of Sphagneticola trilobata attenuates streptozotocin-induced hyperglycaemia in rat models by modulating oxidative stress parameters. Biol Med. 2010;2:1–13.
19. Lans C. Ethnomedicines used in Trinidad and Tobago for reproductive problems. J Ethnobiol Ethnomed. 2007;3:13. doi:10.1186/1746-4269-3-13
20. Elia R, Guo J, Budijono S, et al. Encapsulation of volatile compounds in silk microparticles. J Coatings Technol Res. 2015;12(4):793–799. doi:10.1007/s11998-015-9668-1
21. Bayraktar O, Köse MD, Baspinar Y. Development of olive leaf extract loaded fibroin microparticles by spray drying. Drug Discovery. 2019;13:39–45.
22. Pham DT, Phewchan P, Navesit K, Chokamonsirikun A, Khemwong T, Tiyaboonchai W. Development of metronidazole-loaded in situ thermosensitive hydrogel for periodontitis treatment. Turkish J Pharm Sci. 2021;18(4):510. doi:10.4274/TJPS.GALENOS.2020.09623
23. Pham DT, Tiyaboonchai W. Fibroin-coated poly (ethylenimine)-docusate nanoparticles as a novel drug delivery system. Curr Sci. 2021;121(6):775–780. doi:10.18520/CS/V121/I6/775-780
24. Pham DT, Saelim N, Tiyaboonchai W. Alpha mangostin loaded crosslinked silk fibroin-based nanoparticles for cancer chemotherapy. Colloids Surfaces B Biointerfaces. 2019;181:705–713. doi:10.1016/j.colsurfb.2019.06.011
25. Pham DT, Thao NTP, Thuy BTP, De TV, Nguyen TQC, Nguyen NNT. Silk fibroin hydrogel containing Sesbania sesban L. extract for rheumatoid arthritis treatment. Drug Deliv. 2022;29(1):882–888. doi:10.1080/10717544.2022.2050848
26. Pham DT, Tiyaboonchai W. Fibroin nanoparticles: a promising drug delivery system. Drug Deliv. 2020;27(1):431–448. doi:10.1080/10717544.2020.1736208
27. Pham DT, Saelim N, Cornu R, Béduneau A, Tiyaboonchai W. Crosslinked fibroin nanoparticles: investigations on biostability, cytotoxicity, and cellular internalization. Pharmaceuticals. 2020;13(5):86. doi:10.3390/ph13050086
28. Hcini K, Lozano-Pérez AA, Luis Cenis J, Quílez M, José Jordán M. Extraction and encapsulation of phenolic compounds of tunisian rosemary (Rosmarinus officinalis L.) extracts in silk fibroin nanoparticles. Plants. 2021;10(11):2312. doi:10.3390/plants10112312
29. Omenetto FG, Kaplan DL. New opportunities for an ancient material. Science. 2010;329(5991):528–531. doi:10.1126/science.1188936
30. Altman GH, Diaz F, Jakuba C, et al. Silk-based biomaterials. Biomaterials. 2003;24(3):401–416. doi:10.1016/S0142-9612(02)00353-8
31. Montalbán MG, Coburn JM, Lozano-Pérez AA, Cenis JL, Víllora G, Kaplan DL. Production of curcumin-loaded silk fibroin nanoparticles for cancer therapy. Nanomater. 2018;8(2):126. doi:10.3390/nano8020126
32. Crivelli B, Bari E, Perteghella S, et al. Silk fibroin nanoparticles for celecoxib and curcumin delivery: ROS-scavenging and anti-inflammatory activities in an in vitro model of osteoarthritis. Eur J Pharm Biopharm Off J Arbeitsgemeinschaft fur Pharm Verfahrenstechnik eV. 2019;137:37–45. doi:10.1016/j.ejpb.2019.02.008
33. Lozano-Pérez AA, Rodriguez-Nogales A, Ortiz-Cullera V, et al. Silk fibroin nanoparticles constitute a vector for controlled release of resveratrol in an experimental model of inflammatory bowel disease in rats. Int J Nanomedicine. 2014;9:4507–4520. doi:10.2147/IJN.S68526
34. Lozano-Pérez AA, Rivero HC, Del Carmen Pérez Hernández M, et al. Silk fibroin nanoparticles: efficient vehicles for the natural antioxidant quercetin. Int J Pharm. 2017;518(1):11–19. doi:10.1016/j.ijpharm.2016.12.046
35. Blainski A, Lopes GC, de Mello JCP. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from limonium Brasiliense L. Molecules. 2013;18:6852–6865. doi:10.3390/molecules18066852
36. Tailor CS, Goyal A. Antioxidant activity by DPPH radical scavenging method of ageratum conyzoides Linn. Leaves. Am J Ethnomed. 2014;1:244–249.
37. Pham DT, Saelim N, Tiyaboonchai W. Crosslinked fibroin nanoparticles using EDC or PEI for drug delivery: physicochemical properties, crystallinity and structure. J Mater Sci. 2018;53(20):14087–14103. doi:10.1007/s10853-018-2635-3
38. Pham DT, Tetyczka C, Hartl S, et al. Comprehensive investigations of fibroin and poly (ethylenimine) functionalized fibroin nanoparticles for ulcerative colitis treatment. J Drug Deliv Sci Technol. 2019:101484. doi:10.1016/j.jddst.2019.101484
39. Mizokami SS, Arakawa NS, Ambrosio SR, et al. Kaurenoic acid from sphagneticola trilobata inhibits inflammatory pain: effect on cytokine production and activation of the NO–cyclic GMP–protein kinase G–ATP-sensitive potassium channel signaling pathway. J Nat Prod. 2012;75(5):896–904. doi:10.1021/np200989t
40. Buga MR, Zaharia C, Stancu I-C, Vasile E, Trusca R, Cincu C. Natural silk fibroin micro- and nanoparticles with potential uses in drug delivery systems. UPB Sci Bull Ser B. 2013;75:43–52.
41. Pham DT, Saelim N, Tiyaboonchai W. Paclitaxel loaded EDC-crosslinked fibroin nanoparticles: a potential approach for colon cancer treatment. Drug Deliv Transl Res. 2019;10(2):413–424. doi:10.1007/s13346-019-00682-7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.