Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Contributions of cardiovascular risk and smoking to chronic obstructive pulmonary disease (COPD)-related changes in brain structure and function
Authors Spilling CA , Bajaj MPK , Burrage DR , Ruickbie S, Thai NJ, Baker EH , Jones PW , Barrick TR, Dodd JW
Received 26 April 2019
Accepted for publication 10 July 2019
Published 21 August 2019 Volume 2019:14 Pages 1855—1866
DOI https://doi.org/10.2147/COPD.S213607
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Catherine A Spilling,1 Mohani-Preet K Bajaj,1 Daniel R Burrage,2 Sachelle Ruickbie,2 N Jade Thai,3 Emma H Baker,2 Paul W Jones,2 Thomas R Barrick,1 James W Dodd4
1Institute for Molecular and Clinical Sciences, St George’s University of London, London SW17 ORE, UK; 2Institute for Infection and Immunity, St George’s University of London, London SW17 ORE, UK; 3Clinical Research and Imaging Centre, University of Bristol, Bristol BS2 8DX, UK; 4Academic Respiratory Unit, University of Bristol, Bristol BS10 5NB, UK
Correspondence: James W Dodd
Academic Respiratory Unit, University of Bristol, Learning & Research Building Southmead Hospital, Bristol BS10 5NB, UK
Tel +44 117 414 1276
Email [email protected]
Background: Brain damage and cardiovascular disease are extra-pulmonary manifestations of chronic obstructive pulmonary disease (COPD). Cardiovascular risk factors and smoking are contributors to neurodegeneration. This study investigates whether there is a specific, COPD-related deterioration in brain structure and function independent of cardiovascular risk factors and smoking.
Materials and methods: Neuroimaging and clinical markers of brain structure (micro- and macro-) and function (cognitive function and mood) were compared between 27 stable COPD patients (age: 63.0±9.1 years, 59.3% male, forced expiratory volume in 1 second [FEV1]: 58.1±18.0% pred.) and 23 non-COPD controls with >10 pack years smoking (age: 66.6±7.5 years, 52.2% male, FEV1: 100.6±19.1% pred.). Clinical relationships and group interactions with brain structure were also tested. All statistical analyses included correction for cardiovascular risk factors, smoking, and aortic stiffness.
Results: COPD patients had significantly worse cognitive function (p=0.011), lower mood (p=0.046), and greater gray matter atrophy (p=0.020). In COPD patients, lower mood was associated with markers of white matter (WM) microstructural damage (p<0.001), and lower lung function (FEV1/forced vital capacity and FEV1) with markers of both WM macro (p=0.047) and microstructural damage (p=0.028).
Conclusion: COPD is associated with both structural (gray matter atrophy) and functional (worse cognitive function and mood) brain changes that cannot be explained by measures of cardiovascular risk, aortic stiffness, or smoking history alone. These results have important implications to guide the development of new interventions to prevent or delay progression of neuropsychiatric comorbidities in COPD. Relationships found between mood and microstructural abnormalities suggest that in COPD, anxiety, and depression may occur secondary to WM damage. This could be used to better understand disabling symptoms such as breathlessness, improve health status, and reduce hospital admissions.
Keywords: chronic lung disease, cigarette smoke, cognition, depression, MRI, neuroimaging
Introduction
Chronic obstructive pulmonary disease (COPD) is associated with a number of extra-pulmonary, manifestations including cardiovascular disease, diabetes, arthritis, osteoporosis, obesity, metabolic syndrome, muscle weakness, sleep disturbance, and anemia.1,2 These occur at a higher rate than in smokers and never-smokers,3 have a deleterious effect on patient outcomes and wellbeing4,5 and contribute substantially to the financial burden of the disease.6 However, the disease presentation is highly heterogeneous and it is currently unclear whether these comorbidities are pathogenically linked to the disease or reflect the co-existence of numerous age-related risk factors and conditions.7
Cognitive dysfunction, anxiety, and depression8–10 are key comorbidities of COPD where they are associated with greater disability,11,12 poorer medical compliance13 , increased risk of exacerbation,14 and mortality.11 However, their pathophysiology in relation to COPD remains poorly understood. Neuroimaging findings suggest that there are underlying changes to brain structure and function associated with both reduced lung function in the general population and with established COPD.15–32 The pattern of structural changes is consistent with cerebral small-vessel disease (SVD).33 However, the majority of these studies have failed to adequately control for individual differences in cardiovascular risk and smoking history (eg,17–23,25); factors known to accelerate age-related SVD.34 Consequently, it cannot be established whether this is a COPD-specific effect per se, or the cumulative effect of greater cardiovascular risk in COPD.
The objectives of this study are to determine whether COPD is associated with specific differences in brain structure (macro- and micro-) and function (cognitive function and mood) beyond those which can be attributed to cardiovascular risk factors or smoking. This will be achieved by cross-sectional comparison of a very well-characterized cohort of COPD patients with age-matched non-COPD control subjects with a history of smoking. Additionally, the relationships between clinical measures and brain structure and whether these relationships differ between groups will be investigated in order to establish whether the same disease processes are active in both COPD patients and non-COPD smokers.
It is hypothesized that when compared to non-COPD smokers, COPD patients will show evidence of differences in brain structure and function that cannot be explained by cardiovascular risk factors and smoking. It is predicted that the pattern of differences in brain structure will be consistent with age-related SVD.
Materials and methods
Study participants
Twenty-seven stable COPD patients (defined as forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) <70% and/or clinical/radiological diagnosis of emphysema who had not experienced an exacerbation within the preceding 4 weeks) were recruited from inpatient and outpatient clinics at North Bristol NHS Trust, and 23 non-COPD smoker controls were recruited from the Bristol Primary Care Research North Hub between 2014 and 2015. All subjects provided written informed consent for participation in the study. Inclusion criteria required all subjects to be current or ex-smokers with greater than 10 pack years smoking history. Subjects were excluded from participation if their resting oxygen saturations (SaO2) were below 92% on room air, were on long-term oxygen therapy, they had known alpha-1-anti-trypsin deficiency or co-morbid neurological, cardiovascular, or psychiatric conditions likely to affect neuroimaging findings. Subjects with incidental findings on neuroimaging, visual, or hearing impairment that precluded neuropsychological assessment or contraindications for magnetic resonance imaging (MRI) were also excluded. For a full list of inclusion and exclusion criteria see Table S1. Groups were well matched for age, sex, educational attainment category (defined as, none, GCSE, A-level, degree or other), body mass index, blood pressure, and smoking status. However, COPD patients had smoked for a numerically greater number of pack years (see Table 1).
 |
Table 1 Demographic and clinical characteristics |
Ethics statement
Ethical approval was obtained from the NRES Committee South West – Frenchay (13/SW/0319). The study was conducted in accordance with the Declaration of Helsinki.
Procedure
The data used in this study formed part of a larger protocol, therefore, data acquisition took place across three study visits. Standard demographic information (age, sex, educational attainment category, smoking status, pack year history, body mass index) and clinical measures of disease severity (post-bronchodilator spirometry performed as per the recommendations of the American Thoracic Society and European Respiratory Society Consensus Statement,35 disease status (COPD Assessment Test, CAT36), SaO2 (by pulse oximetry), mood (Hospital Anxiety and Depression Scale, HADS)37 and cognitive function (Montreal Cognitive Assessment, MoCA)38 were collected on visit one. A brief description of the HADS and MoCA can be found in the Supplementary Material. Aortic stiffness (aortic pulse-wave velocity and central augmentation index measured using SphygmoCor system),39 blood pressure and earlobe capillary blood gasses (PO2, PCO2, and pH)40 were collected on visit two at the Respiratory Research Unit at the North Bristol Lung Centre. MRI scans were acquired on visit three at the Clinical Research Imaging Centre (CRICBristol), Bristol University.
Image acquisition and processing
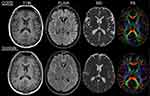
3-Tesla T1-weighted (T1W), fluid-attenuated inversion recovery (FLAIR) and diffusion tensor images (DTI) were acquired for all subjects allowing measures of tissue macrostructure and tissue microstructure to be calculated. Representative images for each group can be found in Figure 1. This procedure is summarized below. For detailed description of image acquisition and processing see the Supplementary Material.
Tissue macrostructure
Supratentorial gray matter, white matter, and cerebrospinal fluid (CSF) tissue volumes were calculated from the T1W images and white matter hyperintensities (WMHs) volumes from the T1W and FLAIR images using a semi-automated procedure described in Spilling et al 2017 and Lambert et al 2015.25,41 All volumes were normalized for head size (% of total intracranial volume, TIV).
Tissue microstructure
Fractional anisotropy (FA) (local tissue directionality) and mean diffusivity (MD) (local magnitude of diffusion) were calculated for each voxel of the DTI. The median and normalized peak height of the distribution of FA and MD values within the normal-appearing white matter (NAWM) (white matter excluding WMHs) were calculated.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics (IBM SPSS, version 24). Data residuals were checked for Gaussian distribution using Shapiro–Wilk’s tests, histograms, and quartile-quartile plots. Non-Gaussian data were log10-transformed (or reflected and log10-transformed). Group differences in clinical measures (including demographic information, disease severity, aortic stiffness, blood pressure, blood gases, mood, and cognitive function) and brain macrostructure (normalized gray matter, white matter, CSF, and WMH volumes) and microstructure (median FA, FA peak height, median MD, MD peak height) were tested using analysis of covariance and chi-squared tests. Relationships between clinical measures and brain structure, and group interactions with these measures were tested using multiple linear regression models. Post-hoc multiple linear regression models were used to probe significant interactions. Results were considered significant at p<0.05. Unless indicated otherwise, all statistical analyses were performed using models with demographic and cardiovascular risk factors entered as covariates of no interest (age, sex, smoking status, pack year history, mean arterial pressure, and aortic pulse-wave velocity). Additionally, educational attainment category was included in any model testing group differences or relationships with cognitive function.
Results
Between-group differences
Clinical measures
Group comparisons of clinical measures can be found in Table 1. As expected, COPD patients had significantly lower lung function (p<0.001) and worse disease status (p<0.001) than non-COPD smoker controls, with unstandardized regression coefficients indicating that COPD was associated with a decrease in FEV1 of 44% pred. and an increase in CAT score of 10. Following correction for cardiovascular risk, COPD patients had significantly worse mood (p=0.046) and following additional correction for educational attainment, worse cognitive function (p=0.011) than non-COPD smoker controls. Unstandardized regression coefficients indicated that COPD was associated with an increase in HADS – total score of 3 points and decrease in MoCA – total score of 2 points. There were no group differences in SaO2 or in aortic pulse-wave velocity and central augmentation index (see Table 1).
Brain structure
Following correction for cardiovascular risk, COPD patients were found to have significantly lower normalized gray matter volume than non-COPD smoker controls (p=0.020). Unstandardized regression coefficients indicated that the presence of COPD was associated with 1.1% decrement in normalized gray matter volume. No other significant differences were found for measures of brain macro- or microstructure (see Table 2).
 |
Table 2 Group differences in brain structure |
Clinical relationships and group interactions
Lung function, disease status, and blood gases
Multiple linear regression models assessing the main effect and group interactions of lung function (FEV1 and FEV1/FVC) on brain structure showed that the FEV1 model explained 37% of the variance in median MD within the NAWM (r2=0.37, p=0.029) and that the interaction between group and FEV1 significantly predicted median MD (p=0.032). Post-hoc analysis indicated that for COPD patients lower FEV1 was related to greater median MD (p=0.028), but not for non-COPD smoker controls (p=0.133). Similarly, multiple linear regression models assessing the main effects and group interactions of FEV1/FVC explained 43% of the variance in normalized white matter volume (r2=0.43, p=0.005) with the group interaction significantly predicting normalized white matter volume (p=0.039). Post-hoc analysis indicated that lower FEV1/FVC was related to lower normalized white matter volume in COPD patients (p=0.047) but not in non-COPD smoker controls (p=0.461). These interactions are shown in Figure 2A, B. No relationships were found between CAT and SaO2 and brain structure.
Aortic stiffness
Multiple linear regression models found no significant main effects or group interactions of aortic stiffness (aortic pulse-wave velocity and central augmentation index) on brain structure.
Cognitive function
Multiple linear regression models assessing the main effect and group interactions of MoCA – total score did not find any significant relationships with brain structure.
Mood
The multiple linear regression model assessing the main effects and group interactions of mood (HADS – total score) on brain structure explained 38% of the variance in median FA (r2=0.38, p=0.022), 34% of the variance in median MD (r2=0.34, p=0.048) and 57% of the variance in MD peak height (r2=0.57, p<0.001). It showed that there were significant main effects of greater HADS – total score on lower median FA (p=0.009), lower MD peak height (p=0.005) and higher median MD (p=0.038) and group by total HADS score interactions for median FA (p=0.011), median MD (p=0.012), and MD peak height (p=0.020). Post-hoc analysis indicated that for COPD patients higher HADS – total score was related to lower median FA (p<0.001), lower MD peak height (p<0.001), and higher median MD (p<0.001) whereas there were no relationships for non-COPD smoker controls: median FA: p=0.616; MD peak height: p=0.125 and median MD: p=0.334. Graphs showing these interactions can be found in Figure 2C, D.
Discussion
This study was designed to test whether COPD is an additional independent risk factor for deterioration in brain structure and function beyond that attributable to age, traditional vascular risk factors, and smoking. COPD patients showed evidence of greater cerebral atrophy, lower cognitive function, and worse mood. Additionally, exploratory tests of group interactions showed that lower lung function (FEV1 and FEV1/FVC) was associated with a deterioration in white matter macro- and microstructure, and worse mood state was associated with a deterioration in white matter microstructure in COPD patients but not in non-COPD smokers.
Previous studies have shown that COPD and reduced lung function are associated with a number of neuroimaging features of small-vessel cerebrovascular disease, including small subcortical infarcts, WMHs, white matter microstructural abnormalities, cerebral microbleeds, and cerebral atrophy.15–29 Consequently, it has been suggested that COPD-related deteriorations in brain structure and function occur secondary to SVD15,42 either due to the high prevalence of age-related vascular risk factors or because COPD is itself a risk factor. Our study could not replicate these white matter findings15,17,19,22,25 and we suggest that they are likely to have been caused by group differences in cardiovascular risk and smoking exposure. However, in our study, COPD patients did have greater cerebral atrophy, lower cognitive function and worse mood state than non-COPD smokers and these could not be explained by differences in cardiovascular risk factors or smoking. The 1.1% decrement in normalized gray matter volume associated with COPD in this study is greater than the annual rate of gray matter volume and brain volume decline reported in normal aging (ranging approximately between 0.3% and 0.8%) eg,43–45 and SVD (0.9%).46 It is also similar in magnitude to the decrease in gray matter volume cross-sectionally associated with diabetes mellitus when compared to normal controls eg, 1.2%.47 Consistent with Cleutjens et al,48 no relationships were found between brain structure and the lower cognitive function in COPD patients. However, significant relationships were found between reduced lung function and greater macro- and microstructural white matter abnormalities in the COPD patients which suggests that other mechanisms are contributing to neurodegeneration and impaired cognition and mood in COPD.
The generalized decrease in gray matter volume found in this study has not been shown previously in COPD, although other studies have reported localized reductions in gray matter density, cortical thickness, and hippocampal volume19–24 notably in regions associated with dyspnea and fear of physical activity.19,20,24 We used a segmentation technique optimized for use in elderly cohorts with WMHs,25,41 and use of this technique may have improved sensitivity to detecting subtle gray matter alterations not detectable in other studies. Alternatively, the inconsistency in gray matter findings may reflect heterogeneity between COPD cohorts.3
Like other chronic diseases, COPD is associated with a higher prevalence of anxiety and depression than the general population,49 with reported rates ranging from 7% to 50% and from 10% to 57%, respectively.10 Using the HADS, the present study found some degree of anxiety or depression in 39% and 22% of COPD patients, respectively, with COPD patients having significantly lower overall mood than non-COPD smokers. Group interactions were found such that worse mood was associated with greater white matter microstructural abnormalities in COPD patients but not in non-COPD smokers. Similar cross-sectional relationships have been reported in normal elderly and SVD cohorts where white matter alterations (both in terms of white matter microstructural change and severity of WMHs) were associated with higher incidence of depressive disorder, greater disability, and lower depression remission rates.50–54 A recent meta-analysis found that multiple markers of both cerebral (WMHs, microbleeds, and microinfarctions) and peripheral forms (plasma markers of endothelial dysfunction) of microvascular dysfunction were associated with increased odds of incident late-life depression.55 Furthermore, longitudinal studies have suggested that markers of SVD may precede the onset of depressive symptom.54,55 In these circumstances, it has been hypothesized that depression results from localized disruption of frontostriatal white matter networks involved in affective regulation.56 This is consistent with functional MRI findings in COPD, which show that COPD patients have an enhanced neural responses in gray matter regions involved in emotional processing and memory (including the medial prefrontal cortex,31 anterior cingulate cortex,31 amygdala30, and hippocampus30), to anticipation of dyspnea30 and dyspnea-related word-cues.31 These enhanced responses are associated with worse perception of dyspnea,30 greater anxiety30, and depression31 and worse disease status (health-related quality of life and exercise tolerance). Furthermore, it has been demonstrated to respond to pulmonary rehabilitation.32 However, recent studies suggest that frontostriatal network disruption leads to apathy rather than depression in SVD.57,58
Advantages and limitations
This study benefits from a well-defined stable COPD cohort and successful recruitment of a non-COPD cohort with a history of smoking, allowing the effects of smoking and COPD to be differentiated. The sample size is comparable to other similar MRI studies of the brain in COPD,18–20,22,23,25 however, modest sample size may limit the generalisability of these results in COPD patients with other levels of disease severity. The secondary analyses testing for clinical relationships and group interaction with brain measures were exploratory. The covariates were grouped into pre-defined clinically meaningful domains to reduce multiple comparisons and linear regression models were adjusted for demographic and cardiovascular risk factors, however, we are unable to completely exclude the possibility of type-I statistical error due to multiple comparisons. This study used the HADS to evaluate participants’ overall mood. This questionnaire was originally developed as a clinical screening tool with two separate sub-scales measuring anxiety and depression37 meaning that the present study has extended its use beyond its original intent. The HADS has known limitations in terms of the stability of this underlying factor structure (particularly in disease)59 and ceiling effects on individual items.60 A number of other authors have supported the validity of using the total HADS score (as in the present study) as a measure of overall psychological distress (eg,61–63). Serum cholesterol, a vascular risk factor, was unavailable for this dataset and could not be controlled for in statistical analyses.
Conclusion
COPD is associated with a specific pattern of structural and functional brain abnormalities that could not be explained by conventional measures of cardiovascular risk, smoking history or aortic stiffness. Worse lung function is associated with deterioration in white matter macro- and microstructure, and deterioration in white matter microstructure is associated with lower mood. This suggests that mechanisms other than cardiovascular risk and smoking contribute to brain changes in COPD. Cognitive dysfunction, anxiety, and depression are key comorbidities of COPD and are associated with greater disability risk of exacerbation and mortality. COPD-related anxiety and depression may occur secondary to white matter damage. These findings have important implications for the prevention and management of neuropsychiatric comorbidities in COPD.
Abbreviations
ANCOVA(s), analysis of covariance; BET, brain extraction tool; BMI, body mass index; CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; CRIC, Clinical Research Imaging Centre; CSF, cerebrospinal fluid; DTI, diffusion tensor imaging; FA, fractional anisotropy; FEV1, forced expiratory volume in 1 Second; FLAIR, fluid attenuated inversion recovery; FVC, forced vital capacity; HADS, Hospital Anxiety and Depression Scale; IQR, interquartile range; MD, mean diffusivity; MoCA, Montreal Cognitive Assessment Test; MRI, magnetic resonance imaging; NAWM, normal-appearing white matter; PO2, partial pressure of oxygen; PCO2, partial pressure of carbon dioxide; SD, standard deviation; SaO2, oxygen saturation; SVD, small vessel disease; TE, echo time; TI, inversion time; TIV, total intracranial volume; TR, repetition time; T1W, T1-weighted; WMHs, white matter hyperintensities.
Acknowledgment
This study was funded by the British Lung Foundation. The funders had no role in the study design, data collection, analysis, interpretation or writing of the report.
Author contributions
JWD and PWJ designed the study, JWD recruited the participants and acquired the clinical data. JWD and NJT acquired the MRI. CAS performed the analysis and drafted the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
PWJ is employed as a Global Medical Expert for GlaxoSmithKline. JWD reports grants from the British Lung Foundation, during the conduct of the study and has received personal fees and travel support unrelated to the content of this manuscript from Chiesi, Boehringer Ingelheim & NAPP pharmaceutical. DRB reports grants from National Institute for Health Research, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Cavaillès A, Brinchault-Rabin G, Dixmier A, et al. Comorbidities of COPD. Eur Respir Rev Off J Eur Respir Soc 2013;22(130):454–475. doi: 10.1183/09059180.00008612
2. Laforest L, Roche N, Devouassoux G, et al. Frequency of comorbidities in chronic obstructive pulmonary disease, and impact on all-cause mortality: a population-based cohort study. Respir Med 2016;117:33–39. doi:10.1016/j.rmed.2016.06.015
3. Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 2010;11(1):122. doi:10.1186/1465-9921-11-62
4. Mannino DM, Thorn D, Swensen A, Holguin F Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J 2008;32(4):962–969. doi:10.1183/09031936.00012408
5. Sin DD, Anthonisen NR, Soriano JB, Agusti AG Mortality in COPD: role of comorbidities. Eur Respir J 2006;28(6):1245–1257. doi:10.1183/09031936.00133805
6. Huber MB, Wacker ME, Vogelmeier CF, Leidl R Excess costs of comorbidities in chronic obstructive pulmonary disease: a systematic review. PLoS One 2015;10(4). doi:10.1371/journal.pone.0123292
7. Miller J, Edwards LD, Agustí A, et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med 2013;107(9):1376–1384. doi:10.1016/j.rmed.2013.05.001
8. Yohannes AM, Alexopoulos GS Depression and anxiety in patients with COPD. Eur Respir Rev 2014;23(133):345–349. doi:10.1183/09059180.00007813
9. Dodd JW, Getov SV, Jones PW Cognitive function in COPD. Eur Respir J 2010;35(4):913–922. doi:10.1183/09031936.00125109
10. Pumar MI, Gray CR, Walsh JR, Yang IA, Rolls TA, Ward DL Anxiety and depression-Important psychological comorbidities of COPD. J Thorac Dis 2014;6(11):1615–1631.
11. Chang SS, Chen S, McAvay GJ, Tinetti ME Effect of coexisting chronic obstructive pulmonary cisease and cognitive impairment on health outcomes in older adults. J Am Geriatr Soc 2012;60(10):1839–1846. doi:10.1111/j.1532-5415.2012.04171.x
12. Kim H, Kunik ME, Molinari VA, et al. Functional impairment in COPD patients: the impact of anxiety and depression. Psychosomatics 2000;41(6):465–471. doi:10.1176/appi.psy.41.6.465
13. Incalzi RA Verbal memory impairment in copd: its mechanisms and clinical relevance. Chest J 1997;112(6):1506. doi:10.1378/chest.112.6.1506
14. Laurin C, Moullec G, Bacon SL, Lavoie KL Impact of anxiety and depression on chronic obstructive pulmonary disease exacerbation risk. Am J Respir Crit Care Med 2012;185(9):918–923. doi:10.1164/rccm.201105-0939PP
15. van Dijk EJ. Arterial oxygen saturation, COPD, and cerebral small vessel disease. J Neurol Neurosurg Psychiatry 2004;75(5):733–736. doi:10.1136/jnnp.2003.022012
16. Lahousse L, Vernooij MW, Darweesh SKL, et al. Chronic obstructive pulmonary disease and cerebral microbleeds. The Rotterdam study. Am J Respir Crit Care Med 2013;188(7):783–788. doi:10.1164/rccm.201303-0455OC
17. Ryu CW, Jahng GH, Choi CW, et al. Microstructural change of the brain in chronic obstructive pulmonary disease. COPD J Chronic Obstr Pulm Dis.2013;10(3):357–366. doi:10.3109/15412555.2012.752808
18. Zhang H, Wang X, Lin J, et al. Reduced regional gray matter volume in patients with chronic obstructive pulmonary disease: a voxel-based morphometry study. Am J Neuroradiol 2013;34(2):334–339. doi:10.3174/ajnr.A3235
19. Zhang H, Wang X, Lin J, et al. Grey and white matter abnormalities in chronic obstructive pulmonary disease: a case-control study. BMJ Open 2012;2(2):e000844–e000844. doi:10.1136/bmjopen-2012-000844
20. Esser RW, Stoeckel MC, Kirsten A, et al. Structural brain changes in patients with COPD. Chest 2016;149(2):426–434. doi:10.1378/chest.15-0027
21. Wang C, Ding Y, Shen B, et al. Altered gray matter volume in stable chronic obstructive pulmonary disease with subclinical cognitive impairment: an exploratory study. Neurotox Res 2017;31(4):453–463. doi:10.1007/s12640-016-9690-9
22. Dodd JW, Chung AW, Broek MD, van Den BTR, Charlton RA, Jones PW Brain structure and function in chronic obstructive pulmonary disease: a multimodal cranial magnetic resonance imaging study. Am J Respir Crit Care Med 2012;186(3):240–245. doi:10.1164/rccm.201202-0355OC
23. Chen J, Lin I-T, Zhang H, et al. Reduced cortical thickness, surface area in patients with chronic obstructive pulmonary disease: a surface-based morphometry and neuropsychological study. Brain Imaging Behav 2015; 10(2)464–476. doi:10.1007/s11682-015-9403-7
24. Li J, Fei G-H The unique alterations of hippocampus and cognitive impairment in chronic obstructive pulmonary disease. Respir Res 2013;14(140):1–9. doi:10.1186/1465-9921-14-19
25. Spilling CA, Jones PW, Dodd JW, Barrick TR. White matter lesions characterise brain involvement in moderate to severe chronic obstructive pulmonary disease, but cerebral atrophy does not. BMC Pulm Med 2017;17(1):92. doi:10.1186/s12890-017-0500-9
26. Liao D, Higgins M, Bryan NR, et al. Lower pulmonary function and cerebral subclinical abnormalities detected by MRI: the atherosclerosis risk in communities study. Chest 1999;116(1):150–156. doi:10.1378/chest.116.1.150
27. Murray AD, Staff RT, Shenkin SD, Deary IJ, Starr JM, Whalley LJ Brain white matter hyperintensities: relative importance of vascular risk factors in nondemented elderly people. Radiology 2005;237(1):251–257. doi:10.1148/radiol.2371041496
28. Taki Y, Kinomura S, Ebihara S, et al. Correlation between pulmonary function and brain volume in healthy elderly subjects. Neuroradiology 2013;55(6):689–695. doi:10.1007/s00234-013-1157-6
29. Sachdev PS, Anstey KJ, Parslow RA, et al. Pulmonary function, cognitive impairment and brain atrophy in a middle-aged community sample. Dement Geriatr Cogn Disord 2006; 21 (5–6): 300–308. doi:10.1159/000091438
30. Esser RW, Stoeckel MC, Kirsten A, et al. Brain activation during perception and anticipation of dyspnea in chronic obstructive pulmonary disease. Front Physiol 2017 cited 2019 April 4];8. Available from: http://journal.frontiersin.org/article/10.3389/fphys.2017.00617/full
31. Herigstad M, Faull OK, Hayen A, et al. Treating breathlessness via the brain: changes in brain activity over a course of pulmonary rehabilitation. Eur Respir J 2017;50(3):1701029. doi:10.1183/13993003.00711-2017
32. Herigstad M, Hayen A, Evans E, et al. Dyspnea-related cues engage the prefrontal cortex: evidence from functional brain imaging in COPD. Chest J 2015; 148(4):953 doi:10.1378/chest.15-0416
33. Wardlaw JM, Smith C, Dichgans M Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging Lancet Neurol 2013;12(5):483–497. doi:10.1016/S1474-4422(13)70060-7
34. Pantoni L Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010;9(7):689–701. doi:10.1016/S1474-4422(10)70104-6
35. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
36. Jones PW, Harding G, Berry P, Wiklund I, Chen W-H, Kline Leidy N Development and first validation of the COPD assessment test. Eur Respir J 2009;34(3):648–654. doi:10.1183/09031936.00102509
37. Zigmond AS, Snaith RP The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67(6):361–370. doi:10.1111/acp.1983.67.issue-6
38. Nasreddine ZS, Phillips NA, Bédirian V, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53(4):695–699. doi:10.1111/j.1532-5415.2005.53221.x
39. Pauca AL, O’Rourke MF, Kon ND Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertens Dallas Tex 1979 2001;38(4):932–937.
40. Lilienthal JL, Riley RL On the determination of arterial oxygen saturations from samples of “capillary” blood. J Clin Invest 1944;23(6):904–906. doi:10.1172/JCI101565
41. Lambert C, Sam Narean J, Benjamin P, Zeestraten E, Barrick TR, Markus HS Characterising the grey matter correlates of leukoaraiosis in cerebral small vessel disease. NeuroImage Clin 2015;9:194–205. doi:10.1016/j.nicl.2015.07.002
42. Lahousse L, Tiemeier H, Ikram MA, Brusselle GG Chronic obstructive pulmonary disease and cerebrovascular disease: a comprehensive review. Respir Med 2015;109(11):1371–1380. doi:10.1016/j.rmed.2015.07.014
43. Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol 2003;60:989–994. doi:10.1001/archneur.60.7.989
44. Taki Y, Thyreau B, Kinomura S, et al. Correlations among brain gray matter volumes, age, gender, and hemisphere in healthy individuals. PLoS One 2011;6:e22734. doi:10.1371/journal.pone.0022734
45. Aljondi R, Szoeke C, Steward C, Yates P, Desmond P A decade of changes in brain volume and cognition. Brain Imaging Behav 2019;13:554–563. doi:10.1007/s11682-018-9887-z
46. Nitkunan A, Lanfranconi S, Charlton RA, Barrick TR, Markus HS Brain atrophy and cerebral small vessel disease: a prospective follow-up study. Stroke 2011;42:133–138. doi:10.1161/STROKEAHA.110.594267
47. Kooistra M, Geerlings MI, Mali WPTM, Vincken KL, van der Graaf Y, Biessels GJ Diabetes mellitus and progression of vascular brain lesions and brain atrophy in patients with symptomatic atherosclerotic disease. The SMART-MR study. J Neurol Sci 2013;332:69–74. doi:10.1016/j.jns.2013.06.019
48. Cleutjens FAHM, Ponds RWHM, Spruit MA, et al. The relationship between cerebral small vessel disease, hippocampal volume and cognitive functioning in patients with COPD: an MRI study. Front Aging Neurosci 2017;9. doi:10.3389/fnagi.2017.00077
49. Hynninen KMJ, Breitve MH, Wiborg AB, Pallesen S, Nordhus IH Psychological characteristics of patients with chronic obstructive pulmonary disease: a review. J Psychosom Res 2005;59(6):429–443. doi:10.1016/j.jpsychores.2005.04.007
50. Alexopoulos GS, Kiosses DN, Choi SJ, Murphy CF, Lim KO Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am J Psychiatry 2002;159(11):1929–1932. doi:10.1176/appi.ajp.159.11.1929
51. Teodorczuk A, Firbank MJ, Pantoni L, et al. Relationship between baseline white-matter changes and development of late-life depressive symptoms: 3-year results from the LADIS study. Psychol Med 2010;40(4):603–610. doi:10.1017/S0033291709990857
52. Emsell L, Adamson C, De Winter F-L, et al. Corpus callosum macro and microstructure in late-life depression. J Affect Disord 2017;222:63–70. doi:10.1016/j.jad.2017.06.063
53. Alexopoulos GS, Murphy CF, Gunning-Dixon FM, et al. Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry 2008;165(2):238–244. doi:10.1176/appi.ajp.2007.07050744
54. Qiu WQ, Himali JJ, Wolf PA, DeCarli DC, Beiser A, Au R Effects of white matter integrity and brain volumes on late life depression in the Framingham Heart Study. Int J Geriatr Psychiatry 2017;32(2):214–221. doi:10.1002/gps.v32.2
55. van Agtmaal MJM, Houben AJHM, Pouwer F, Stehouwer CDA, Schram MT Association of microvascular dysfunction with late-life depression: a systematic review and meta-analysis. JAMA Psychiatry 2017;74(7):729–739. doi:10.1001/jamapsychiatry.2017.0984
56. Brookes RL, Herbert V, Lawrence AJ, Morris RG, Markus HS Depression in small-vessel disease relates to white matter ultrastructural damage, not disability. Neurology 2014;83(16):1417–1423. doi:10.1212/WNL.0000000000000882
57. Hollocks MJ, Lawrence AJ, Brookes RL, et al. Differential relationships between apathy and depression with white matter microstructural changes and functional outcomes. Brain 2015;138(12):3803–3815. doi:10.1093/brain/awu353
58. Lohner V, Brookes RL, Hollocks MJ, Morris RG, Markus HS Apathy, but not depression, is associated with executive dysfunction in cerebral small vessel disease. PLoS One 2017;12(5). doi:10.1371/journal.pone.0176943
59. Martin CR What does the Hospital Anxiety and Depression Scale (HADS) really measure in liaison psychiatry settings? Curr Psychiatry Rev. 2004. [cited 2019 April 15]. Available from: http://www.eurekaselect.com/79829/article.
60. Djukanovic I, Carlsson J, Årestedt K Is the Hospital Anxiety and Depression Scale (HADS) a valid measure in a general population 65–80 years old? A psychometric evaluation study. Health Qual Life Outcomes 2017;15. doi:10.1186/s12955-017-0759-9
61. Crawford JR, Henry JD, Crombie C, Taylor EP Normative data for the HADS from a large non-clinical sample. Br J Clin Psychol 2001;40(4):429–434.
62. Pallant JF, Tennant A An introduction to the Rasch measurement model: an example using the Hospital Anxiety and Depression Scale (HADS). Br J Clin Psychol 2007;46(1):1–18. doi:10.1348/014466506X158996
63. Martin CR, Tweed AE, Metcalfe MS A psychometric evaluation of the Hospital Anxiety and Depression Scale in patients diagnosed with end-stage renal disease. Br J Clin Psychol 2004;43(Pt 1):51–64. doi:10.1348/014466504772812968
Supplementary materials
Inclusion/Exclusion criteria
 |
Table S1 Inclusion and exclusion criteria |
Clinical measures
Hospital Anxiety and Depression Scale (HADS)
The HADS is a 14-item self-report questionnaire comprising two 7-item subscales measuring anxiety and depression. It was originally developed as a clinical screening tool for use in a general medical outpatient setting and so explicitly excludes items that might be confounded by somatic aspects of illness or serious mental disorders.1
Montreal Cognitive Assessment (MoCA)
The MoCA is a brief 30-item cognitive assessment tool designed to be sensitive to mild cognitive impairment in individuals presenting with subjective cognitive complaints.2 The MoCA has previously been applied to chronic obstructive pulmonary disease (COPD) cohorts where it has been shown to be sufficiently sensitive to detect mild cognitive impairment in COPD patients with moderate-severe disease.3
Image acquisition
All images were acquired with a 3-Tesla Siemens Magnetron Skyra MRI scanner equipped with a 32-channel head coil with a maximum gradient strength of 45 mT/m. Sagittal T1-weighted 3D volume (T1W) images were acquired using a magnetization prepared rapid gradient echo sequence (TE=2.25 ms, TR=1800 ms, TI=800 ms, flip angle 9°, 169 contiguous sagittal slices with a 0.9 mm3 isotropic voxel dimension and field-of-view of 225 mm×240 mm×180 mm). Axial fluid-attenuated inversion recovery (FLAIR) was acquired using an inversion recovery sequence (TE-126 ms, TR=11,000 ms, TI=2690 ms, flip angle=150°, with 60 contiguous slices, voxel dimension of 0.7 mm×0.7 mm×3 mm and field-of-view of 201.25 mm×230 mm×180 mm). Diffusion tensor images (DTI) were acquired using an echo-planar imaging sequence with opposite phase-encode polarities (TE=76 ms, TR=6000 ms, flip angle =90°, 55 contiguous axial slices with a voxel dimension of 2.0 mm×2.0 mm×2.5 mm and field-of-view of 192 mm×192 mm×137.5 mm). For each phase-encode polarity, 8 volumes were acquired without diffusion sensitization and 60 with non-collinear diffusion gradients applied.
Image processing
Tissue macrostructure
The T1W images were re-sampled to 1 mm3 isotropic. The FLAIR was affine-registered to the T1W images using Advanced Normalisation Tools4 and a semi-automatic procedure used to segment the T1W images into supra-tentorial gray matter, white matter and cerebrospinal fluid tissue probability maps. White matter hyperintensities (WMHs) were segmented using the combined image intensities from the T1W and FLAIR images, then binarised at a manually determined threshold (i.e., dichotomized so that 1=WMH and 0=non-WMH). This process is described in full in Spilling et al, 2017 and Lambert et al, 2015.5,6 Tissue volumes were quantified by integrating the values within each tissue segmentation and normalizing for head size – calculated as a percentage of total intracranial volume (gray matter + white matter + cerebrospinal fluid). Additionally, these tissue segmentations were used to define regions of normal-appearing white matter (NAWM) on the DTI (see below).
Tissue microstructure
The DTI data were corrected for movement artifacts, eddy-current distortions, and susceptibility-induced local gradients using FSL’s (FMRIB Software Library, version 5.0.6) “eddy”.7 The diffusion tensor model was fitted at every voxel within the DTI using FSL’s (FMRIB Software Library, version 5.0.6) “dtifit”,8 the skull removed using FSL’s (FMRIB Software Library, version 5.0.6) Brain Extraction Toolbox9 and mean diffusivity (MD) and fractional anisotropy (FA) maps calculated from the DTI, indicating the local magnitude and directionality of diffusion, respectively.
T1W images were aligned to the DTI data using the boundary-based registration procedure implemented in FSL’s (FMRIB Software Library, version 5.0.6) “epi-reg” script.10 This transformation was applied to the T1W tissue segmentations and binary WMH map (using trilinear interpolation) to align them with the DTI. The WMH map was re-binarised at 0.5 creating a WMH mask. These segmentations were used to define the probability of the DTI voxels belonging to each tissue-type. Voxels were considered to belong to the supra-tentorial NAWM where the probability of belonging to the white matter was higher than for any other tissue-type providing that they were not included within the WMH mask.
Normalized histograms (i.e. probability density functions) of FA and MD values within the NAWM were constructed using 100 equal bins ranging in value from 0 to 1 for FA and 0 to 2×10−4 mm2/s for MD. The median and peak height values were used to characterize the distribution of these histograms.
References
1. Zigmond AS, Snaith RP The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/acp.1983.67.issue-6
2. Nasreddine ZS, Phillips NA, Bédirian V, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi:10.1111/j.1532-5415.2005.53221.x
3. Villenueve S, Pepin V, Rahayel S, et al. Mild cognitive impairment in moderate to severe COPD: a preliminary study. Chest. 2012;146(6):1516–1523. doi:10.1378/chest.11-3035
4. Avants BB, Tustison N, Song G Advanced normalization tools (ANTS). Insight J. 2009. [cited 2014 March 12]. Available from: ftp://ftp3.ie.freebsd.org/pub/sourceforge/a/project/ad/advants/Documentation/ants.pdf.
5. Spilling CA, Jones PW, Dodd JW, Barrick TR White matter lesions characterise brain involvement in moderate to severe chronic obstructive pulmonary disease, but cerebral atrophy does not. BMC Pulm Med. 2017. [cited 2018 August 14];17(1). doi:10.1186/s12890-017-0500-9
6. Lambert C, Sam Narean J, Benjamin P, Zeestraten E, Barrick TR, Markus HS Characterising the grey matter correlates of leukoaraiosis in cerebral small vessel disease. NeuroImage Clin. 2015;9:194–205. doi:10.1016/j.nicl.2015.07.002
7. Andersson JLR, Sotiropoulos SN An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–1078. doi:10.1016/j.neuroimage.2015.10.019
8. Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL NeuroImage 2012;62(2):782–790. doi:10.1016/j.neuroimage.2011.09.015
9. Smith SM Fast robust automated brain extraction. Hum Brain Mapp 2002;17(3):143–155. doi:10.1002/(ISSN)1097-0193
10. Greve DN, Fischl B Accurate and robust brain image alignment using boundary-based registration. NeuroImage 2009;48(1):63–72. doi:10.1016/j.neuroimage.2009.06.060
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.