Back to Journals » Infection and Drug Resistance » Volume 15
Continuous Vancomycin Infusion versus Intermittent Infusion in Critically Ill Patients
Authors Maluangnon C , Tongyoo S , Permpikul C
Received 29 October 2022
Accepted for publication 10 December 2022
Published 28 December 2022 Volume 2022:15 Pages 7751—7760
DOI https://doi.org/10.2147/IDR.S395385
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Chailat Maluangnon, Surat Tongyoo, Chairat Permpikul
Department of Medicine, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand
Correspondence: Chairat Permpikul, Department of Medicine, Faculty of Medicine, Siriraj Hospital, Mahidol University, 2, Wanglang Road, Siriraj, Bangkoknoi, Bangkok, 10700, Thailand, Tel +66 81 408 1676, Fax +66 2 419 8597, Email [email protected]
Background: Vancomycin is the best-choice medication for methicillin-resistant staphylococcal and enterococcal infections, which are major problems in intensive care units (ICUs). Intermittent infusion is standard for vancomycin, although delayed therapeutic target achievement and supra- and subtherapeutic levels are concerns. A recently proposed alternative with superior therapeutic target achievement is continuous infusion.
Objective: To compare the benefits of continuous (CVI) and intermittent (IVI) vancomycin infusion.
Methods: This quasi-experimental study used propensity score-matched historical controls and adult patients in medical and surgical ICUs for whom vancomycin was indicated. The experimental group received CVI for ≥ 48 hours. Data on patients receiving IVI between January 2018 and October 2020 were reviewed. Capability to achieve serum vancomycin therapeutic targets (48 and 96 hours), episodes of supra- and subtherapeutic levels, treatment success, mortality, and incidence of acute kidney injury (AKI) were analyzed before and after one-to-two propensity score matching.
Results: The CVI group had 31 patients, while the unmatched IVI group had 125. More CVI patients achieved the therapeutic target within 48 hours (54.8% vs 25.6%; P=0.002). CVI patients had a higher median number of supratherapeutic episodes (2 vs 1; P=0.007) but a lower median for subtherapeutic episodes (0 vs 1; P=0.003). Other outcomes demonstrated no differences. After propensity score matching, target achievement within 48 hours (54.8% vs 22.6%; P=0.002) and fewer subtherapeutic episodes (0 vs 1; P=0.014) remained significant.
Conclusion: CVI’s rapid therapeutic target achievement and fewer subtherapeutic episodes make it superior to IVI. No differences in treatment success, mortality, or AKI are evident.
Keywords: continuous infusion, intensive care units, pharmacokinetics, therapeutic drug monitoring, vancomycin
Plain Language Summary
One of the key success factors in treating sepsis is rapidly achieving the antibiotic therapeutic target. We conducted a study comparing the continuous vancomycin infusion to the conventional intermittent infusion in critically ill intensive care unit patients and found that the continuous infusion provided better target attainment with lower episodes of subtherapeutic level. These findings give clinicians more confidence in using this method and may lead to a better outcome.
Introduction
The nosocomial Gram-positive pathogens, methicillin-resistant Staphylococcus aureus (MRSA) and Enterococci, cause severe hospital-acquired infections. Vancomycin has long been considered the first-line agent despite the availability of many new antibiotics. Administration of this agent requires a thorough understanding of pharmacokinetics and pharmacodynamics so that appropriate dose adjustments can be made to achieve the therapeutic index.
The antimicrobial activity of vancomycin varies directly with the area under a concentration curve divided by the minimal inhibitory concentration (AUC/MIC).1–4 In human pharmacodynamic studies, values of more than 400 measured during 24 hours (AUC24/MIC) are associated with successful treatment outcomes.4–6 As a resistant organism might develop if the drug level is constantly low,1,6 vancomycin therapeutic drug monitoring (TDM) is crucial to treatment success.
The 2009 guidelines of the Infectious Disease Society of America (IDSA) recommended that vancomycin TDM use a “trough level” between 15 and 20 mg/L before the fourth dose in cases of severe MRSA infection. Within this range, the AUC24/MIC was believed to achieve a level >400 in most MRSA-infected patients, giving a minimum inhibitory concentration (MIC) of ≤1 mg/L. For nonsevere MRSA infections, the vancomycin trough level range could be lower, between 10 and 15 mg/L.6
Subsequently, evidence emerged that the trough level was not an acceptable therapeutic target. Even though the trough level might imply an AUC24/MIC of >400, it cannot express the exact value of AUC24/MIC, thereby risking an overdose. Therefore, the recent IDSA 2020 guidelines suggest using an AUC24/MIC of 400 to 600 as the TDM target for MRSA infections.7 In practice, however, using the AUC for TDM is problematic. This is because multiple vancomycin levels are needed for calculations or for use with Bayesian-derived AUC monitoring. Thus, TDM with trough level measurement remains the standard method.
In current medical practice, a loading dose, followed by intermittent vancomycin infusion (IVI), is the standard method of administering vancomycin. Delayed achievement and the risk of supratherapeutic or subtherapeutic levels are the major concerns of the IVI method, especially in critically ill patients with sepsis and alternating renal function. Recently, continuous vancomycin infusion (CVI) was introduced. It has been proposed as an alternative method that can more rapidly achieve the therapeutic target,8–11 provide a steadier drug level,8 and reduce renal toxicity.12,13 Additionally, TDM is less complicated, serum concentration measurements can be performed at any time in the steady state, and the AUC can be easily calculated with fewer samples and without complex statistics. However, there are concerns regarding phlebitis and compatibility with other drugs.14
Rapid achievement and regular maintenance of the therapeutic target are needed in critically ill patients while avoiding adverse drug reactions. Hence, we conducted a study that aimed to compare the capabilities of CVI and IVI in achieving the therapeutic target.
Materials and Methods
Study Setting and Population
This was a quasi-experimental study with a propensity score-matched historical control design. It enrolled adult patients admitted to medical and surgical intensive care units (ICUs) at Siriraj Hospital, Bangkok, Thailand, who were indicated for vancomycin. The inclusion criteria were as follows:
- an age of at least 18 years,
- creatinine clearance of more than 30 mL/min/1.73 m2,15
- had or was suspected of having a staphylococcal or enterococcal infection, and
- vancomycin was indicated and was anticipated for at least 48 hours.
The historical control group subjects were selected by reviewing medical records to identify patients who met the inclusion criteria and received IVI between January 2018 and October 2020. Regarding the prospective experimental CVI group, patients meeting the inclusion criteria between January 2021 and December 2021 were screened for enrollment.
Ethics Approval and Informed Consent
Written informed consent was obtained from every CVI patient prior to study participation. Before this research began, the Institutional Review Board of the Faculty of Medicine, Siriraj Hospital, Mahidol University, approved its protocol (Si027/2021). The study was conducted per the Code of Ethics of the World Medical Association (Declaration of Helsinki). It was registered with the Thai Clinical Trials Registry (TCTR20210122005).
Study Design and Definitions
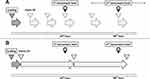
Immediately after the CVI patients were enrolled and their informed consent was obtained, a loading dose of 25 to 30 mg/kg (actual body weight) of vancomycin was administered at a maximum loading dose of 3000 mg. The maintenance dose of vancomycin was continuously infused per the study protocol (Figure 1). Other treatments (eg, other antibiotics, vasoactive agents, ventilatory management, or nutritional support) were decided by attending physicians according to the standard of care.
Continuous Vancomycin Infusion Protocol
Drug Preparation and Administration
In normal saline, vancomycin was diluted to a 10 mg/mL concentration. The drug was continuously infused over the next 24 hours with an infusion pump. The daily dose of CVI (mg/day) was calculated with the formula (17 × creatinine clearance) + 100.16 Creatinine clearance was determined using the Cockcroft-Gault equation.15
Vancomycin TDM, Adjustment, and Discharge
The serum vancomycin concentration was measured 24 hours after the commencement of the CVI. The therapeutic target was a concentration of 15 to 20 mg/L. The infusion dose remained unchanged if the measured serum concentration was within this range. However, the dose was adjusted by an increase or decrease of 480 mg/day if the concentration was < 15 mg/L or > 20 mg/L, respectively. The maximum daily infused dose was 3000 mg/day.
The serum vancomycin concentration was measured every additional 24 hours, with the daily dose adjusted similarly. Once the concentration was within the target range on 2 successive days, we deemed that it had reached a steady-state concentration. Repeat measurements were subsequently made every 5 days. Nevertheless, additional tests were still possible, depending on the attending physicians’ judgment. After 48 hours of CVI, attending physicians were encouraged to continue CVI, but the decision depended on them. Vancomycin was discharged once there were no longer any indications supporting its continued use or for other appropriate reasons.
Safety Monitoring
Complete blood count and serum creatinine were obtained on days 2 and 7 to monitor acute kidney injury (AKI) and drug-induced cytopenia. If vancomycin was administered via a peripheral vein, thrombophlebitis was monitored every 8 hours. Each patient was assessed for tinnitus and alteration of hearing upon completion of vancomycin treatment by the attending physician. In the event of these symptoms, an otolaryngologist consultation was made.
Intermittent Vancomycin Infusion Protocol (Figure 1)
Our institution’s protocol for IVI consisted of a 25 to 30 mg/kg (actual body weight) loading dose and a 15 to 20 mg/kg maintenance dose every 8 to 12 hours. TDM was made by measuring the vancomycin trough level 30 minutes before the fourth dose. The attending physician adjusted the dose to keep the trough level between 15 and 20 mg/L.
The medical records of patients in the medical and surgical ICUs who received vancomycin between January 2018 and October 2020 were reviewed. One of the investigators recorded the serum vancomycin levels intended for trough level measurement.
Measurement of Outcomes
The primary outcome was the capability to achieve a therapeutic target within 48 hours. This was defined as achieving a steady-state concentration of serum vancomycin either at 24 or 48 hours for the CVI group, or as reaching the trough level for the IVI group (15–20 mg/L) within 48 hours. The other outcomes of interest were achieving the vancomycin therapeutic target within 96 hours of the commencement of infusion and the median number of subtherapeutic and supratherapeutic episodes during this period.
Treatment success, mortality rate, AKI rate, and other adverse drug reactions were assessed in patients who received vancomycin as a definite treatment, defined by culture positivity for pathogens susceptible to vancomycin and with the drug given for at least 96 hours. Treatment success was defined as the resolution of the infection, indicated by clinical defervescence, improvement in abnormal signs and symptoms related to the infection, or disappearance of the pathogen. Mortality was assessed on day 14 after enrollment and before hospital discharge. If the patient was discharged before day 14, mortality was assessed on the discharge date. AKI was defined as a serum creatinine increase of ≥0.3 mg/dL or ≥1.5 times from baseline.17
Statistical Analysis
The sample size calculation was based on data from clinical trials.18 A literature review revealed that 41% of patients using the CVI method reached a serum vancomycin level within the therapeutic target range compared with 11% of patients using the IVI method.18 With a 95% confidence interval (Z=1.96) and a power of 90% (β=0.1), the sample size was calculated using the Z-test for 2 independent proportions (without continuity correction). At least 31 subjects needed to be prospectively enrolled in the CVI group. We planned to use one-to-two propensity score matching for the patients in the IVI group; thus, 62 subjects needed to be retrospectively reviewed in the IVI group.
Continuous variables were compared using the independent samples t-test for data with a normal distribution or the Mann–Whitney U-test for nonnormally distributed data. As appropriate, the categorical variables were compared using Pearson’s χ2 test or Fisher’s exact test. Probability (P) values <0.05 were considered statistically significant.
All potential clinical parameters underwent univariate analysis to determine the primary outcome’s predictive factors. The clinical parameters showing an association with the primary outcome, indicated by a P value of < 0.1 in the univariate analysis, were entered into a multivariable logistic regression model.
To balance the baseline characteristics that might interfere with the achievement of the primary outcome, propensity score matching was performed using a logistic regression model. Factors that might relate to the primary outcome, together with the method of infusion, were used. One-to-two patient baseline matching was planned using the nearest-neighbor method while balancing the key characters. All analyses were performed using PASW Statistics for Windows, version 18.0 (SPSS Inc, Chicago, IL, USA).
Results
In all, 156 patients were enrolled (experimental CVI group, 31 patients; historical IVI group, 125 patients; Figure 2). Almost half of the patients in both groups were female (48.4% vs 47.2%; P=0.906), with a non significantly higher mean age in the CVI group (62 ± 12 vs 52 ± 20 years; P=0.190). The CVI patients also had a higher mean weight (67.4 ± 17.4 vs 57.7 ± 12.4 kg; P<0.001), body mass index (26.26 ± 6.52 vs 22.24 ± 4.71 kg/m2; P<0.001), and proportion with diabetes mellitus (58.1% vs 20.0%; P<0.001). Vancomycin was prescribed as the definite treatment for a higher proportion of patients in the IVI group than in the CVI group (41.6% vs 22.6%, respectively; P<0.001). The other baseline demographic and clinical characteristics of the 2 groups were similar (Table 1). For definite treatment, the leading sources of infection were catheter-related (CVI group, 28.6%; IVI group, 25.0%; P=1.000) and the bloodstream (CVI group, 28.6; IVI group, 21.2%; P=0.643). Most infections were caused by enterococci (CVI group, 42.9%; IVI group, 26.9%; P=0.399) and coagulase-negative staphylococci (CVI group, 28.6%; IVI group, 15.4%; P=0.338; Table 2).
 |
Table 1 Baseline Demographic and Clinical Characteristics |
 |
Table 2 Definite Sources of Infection and Causative Organisms |
Eleven parameters potentially affected the achievement of the vancomycin therapeutic level within 48 hours. The univariate analysis identified 3 of these factors as having a P value <0.1, and they were enrolled in our multivariate analysis model. Only CVI was determined to be an independent factor associated with achieving the therapeutic level within 48 hours (Table 3).
 |
Table 3 Independent Variables Related to Primary Outcome (Logistic Regression) |
Regarding the primary outcome, 17 of the 31 patients (54.8%) in the CVI group achieved the therapeutic target within 48 hours, whereas 32 of the 125 IVI patients (25.6%) did, with a significant difference (odds ratio [OR]: 3.53; 95% CI: 1.56–7.96; P=0.002). However, the achievement of the vancomycin therapeutic level within 96 hours by the 2 groups was not significantly different (18/31 [58.1%] vs 62/125 [49.6%] patients; OR: 1.14; 95% CI: 0.64–3.12; P=0.399). During the study period, the median incidence of subtherapeutic episodes in the CVI group was significantly lower than that in the IVI group (median 0 [IQR 0–1] vs 1 [0–1]; P=0.003). Conversely, the median for supratherapeutic episodes was higher in the CVI group than in the IVI group (2 [IQR 1–2] vs 1 [0–2]; P=0.007). The therapeutic success rate, the 14-day mortality rate, and the in-hospital mortality rate of the 2 groups did not differ (Table 4).
 |
Table 4 Outcomes |
Interestingly, although the vancomycin loading doses of the CVI and IVI groups were not different (25.91 ± 1.29 vs 26.12 ± 3.31 mg/kg; P=0.573), the maintenance dose for the CVI group was significantly lower (24.20 ± 9.96 vs 34.89 ± 8.37 mg/kg/day; P<0.001). The AKI rate was lower for the CVI patients (3/31 [9.7%] vs 26/125 [20.8%]; OR: 0.41; 95% CI: 0.12–1.45; P=0.154), but without significance.
For the matched cohort, propensity score matching resulted in 31 patients in the CVI group and 62 in the IVI group. There was no significant difference in any baseline clinical parameter (Table 1). The matching model revealed that a significantly higher proportion of CVI patients achieved the therapeutic target within 48 hours than IVI patients (17/31 [54.8%] vs 14/62 [22.6%]; OR: 4.16; 95% CI: 1.65–10.49; P=0.002). In addition, there were significantly fewer subtherapeutic episodes among patients receiving CVI (median 0 [IQR 0–1] vs 1 [0–1]; P=0.014). However, the 2 groups had no significant difference in their median numbers of episodes of supratherapeutic concentrations. Additionally, the occurrence of AKI in the groups did not differ significantly (Table 4).
Discussion
In this study, the CVI patients received an initial dose adjustment according to their renal function, followed by daily adjustments. In the IVI group, weight-based dose adjustments were rendered at the attending physicians’ discretion to achieve the therapeutic target within 48 hours. The analysis found that CVI was superior to IVI in critically ill patients. A higher proportion of patients in the CVI group achieved the therapeutic target within 48 hours, and there were fewer subtherapeutic episodes among the CVI patients than the IVI patients.
The finding that a higher proportion of CVI patients achieved the therapeutic target at 48 hours with fewer subtherapeutic episodes is paralleled in previous studies. However, these works were retrospective, and uniform intervention in the treatment arm was lacking.10,11,19 Although a few randomized controlled trials have been conducted, the IVI groups in these studies did not receive loading doses. The absence of a loading dose raises the concern that the better target attainment of the CVI group might be influenced by the initial loading.8,9 Our propensity-score matching design and adequate loading dose for both groups minimized our concerns about many previous studies.
In our work, the total loading doses of the CVI and IVI groups were similar (25.91 vs 26.12 mg/kg). However, the daily maintenance doses were significantly lower in the CVI group (24.20 vs 33.31 mg/kg/day). Pea and Viale demonstrated this finding using the same loading and daily maintenance dosage, but their maintenance dosage was separated into CVI, IVI twice daily, and IVI 4 times daily. Pea and Viale’s pharmacokinetic model revealed that CVI had a lower peak and a higher trough level than IVI.20 This finding revealed another way the CVI method is superior to the IVI method.
We found no difference in mortality or AKI when focusing on the clinical outcomes. Although the incidence of AKI in the CVI group was half that in the IVI group, it did not reach statistical significance. Many studies have shown less renal toxicity in CVI patients.11,13,19,21 The absence of a difference in mortality or AKI may have arisen because vancomycin was prescribed for three-quarters of our CVI cases as empirical treatment, with a median duration of 3 days. This period might have been too short to assess the effects, making it a limitation of our study. It is also challenging to document that AKI results solely from administering vancomycin. This is because each sepsis patient suffers many renal insults, such as sepsis itself, concomitant nephrotoxic medication, and inappropriate volume status.8 Interpretations of the causes of AKI must therefore be made very carefully. The only rare adverse event of concern in our study was thrombocytopenia, which developed in 1 of the CVI patients (3%). This very low level is consistent with the literature. Last, most of our patients received CVI via a central vein, with only a few patients infused via a peripheral vein. No phlebitis related to the infusions was observed.
There are some notable limitations of our study. It was a single-center study, was unrandomized, and had a historical control group. Moreover, the investigation was powered for pharmacokinetics and was not intended to measure clinical outcomes. We cannot conclude any clinical benefits since more than half of the patients used vancomycin as an empirical treatment. Last, our study targeted pharmacokinetic outcomes on serum concentration. In contrast, the latest recommendation proposed the use of AUC24/MIC.7 A multicenter randomized controlled trial targeting AUC24 and comparing the CVI and IVI methods is needed to determine CVI’s pharmacological and clinical benefits.
Conclusion
In conclusion, the present investigation confirmed that CVI appears beneficial in reaching the therapeutic target faster than IVI. It is also less likely to produce subtherapeutic concentrations without increasing the risk of treatment failure, mortality, and AKI. CVI should be considered an alternative infusion method in critically ill patients.
Data Sharing Statement
The dataset supporting this study’s findings will be available from the corresponding author 1 year after publication for a period of 1 year upon a reasonable request.
Acknowledgments
The authors express their gratitude to Dr Visanu Thamlikitkul and Dr Pornpan Koomanachai for their invaluable study design and methodological advice and gratefully acknowledge Khemajira Karaketklang for assistance with the statistical analyses. The authors are also indebted to Mr David Park for the English-language editing of this paper.
Funding
This research project was supported by the Siriraj Research Fund (grant number [IO] R016431031), Faculty of Medicine Siriraj Hospital, Mahidol University. The study funders were not involved in the study design, recruitment, management, analysis, interpretation of data, writing of the report, or the decision to submit the report for publication.
Disclosure
The authors have no conflicts of interest to disclose.
References
1. Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006;42(Supplement_1):S35–S39. doi:10.1086/491712
2. DiMondi VP, Rafferty K. Review of continuous-infusion vancomycin. Ann Pharmacother. 2013;47(2):219–227. doi:10.1345/aph.1R420
3. Zamoner W, Prado IRS, Balbi AL, Ponce D. Vancomycin dosing, monitoring and toxicity: critical review of the clinical practice. Clin Exp Pharmacol Physiol. 2019;46:292–301. doi:10.1111/1440-1681.13066
4. Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43(13):925–942. doi:10.2165/00003088-200443130-00005
5. Song KH, Kim HB, Kim HS, et al. Impact of area under the concentration-time curve to minimum inhibitory concentration ratio on vancomycin treatment outcomes in methicillin-resistant Staphylococcus aureus bacteraemia. Int J Antimicrob Agents. 2015;46(6):689–695. doi:10.1016/j.ijantimicag.2015.09.010
6. Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49(3):325–327. doi:10.1086/600877
7. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835–864. doi:10.1093/ajhp/zxaa036
8. Wysocki M, Delatour F, Faurisson F, et al. Continuous versus intermittent infusion of vancomycin in severe Staphylococcal infections: prospective multicenter randomized study. Antimicrob Agents Chemother. 2001;45(9):2460–2467. doi:10.1128/aac.45.9.2460-2467.2001
9. Schmelzer TM, Christmas AB, Norton HJ, Heniford BT, Sing RF. Vancomycin intermittent dosing versus continuous infusion for treatment of ventilator-associated pneumonia in trauma patients. Am Surg. 2013;79(11):1185–1190. doi:10.1177/000313481307901123
10. Hong LT, Goolsby TA, Sherman DS, et al. Continuous infusion vs intermittent vancomycin in neurosurgical intensive care unit patients. J Crit Care. 2015;30(5):1153e1–6. doi:10.1016/j.jcrc.2015.06.012
11. Bissell BD, Riggi G, Morrison C. Evaluation of continuous infusion vancomycin administration in a critically ill trauma population. J Intensive Care Med. 2020;35(6):570–575. doi:10.1177/0885066618768749
12. Flannery AH, Bissell BD, Bastin MT, Morris PE, Neyra JA. Continuous versus intermittent infusion of vancomycin and the risk of acute kidney injury in critically ill adults: a systematic review and meta-analysis. Crit Care Med. 2020;48(6):912–918. doi:10.1097/CCM.0000000000004326
13. Hao JJ, Chen H, Zhou JX. Continuous versus intermittent infusion of vancomycin in adult patients: a systematic review and meta-analysis. Int J Antimicrob Agents. 2016;47(1):28–35. doi:10.1016/j.ijantimicag.2015.10.019
14. Raverdy V, Ampe E, Hecq JD, Tulkens PM. Stability and compatibility of vancomycin for administration by continuous infusion. J Antimicrob Chemother. 2013;68(5):1179–1182. doi:10.1093/jac/dks510
15. Cockcroft DW, Gault H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi:10.1159/000180580
16. Waineo MF, Kuhn TC, Brown DL. The pharmacokinetic/pharmacodynamic rationale for administering vancomycin via continuous infusion. J Clin Pharm Ther. 2015;40(3):259–265. doi:10.1111/jcpt.12270
17. Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi:10.1186/cc5713
18. Tafelski S, Nachtigall I, Troeger U, et al. Observational clinical study on the effects of different dosing regimens on vancomycin target levels in critically ill patients: continuous versus intermittent application. J Infect Public Health. 2015;8(4):355–363. doi:10.1016/j.jiph.2015.01.011
19. Saugel B, Nowack MC, Hapfelmeier A, et al. Continuous intravenous administration of vancomycin in medical intensive care unit patients. J Crit Care. 2013;28(1):9–13. doi:10.1016/j.jcrc.2012.02.003
20. Pea F, Viale P. Should the currently recommended twice-daily dosing still be considered the most appropriate regimen for treating MRSA ventilator-associated pneumonia with vancomycin? Clin Pharmacokinet. 2008;47(3):147–152. doi:10.2165/00003088-200847030-00001
21. Cataldo MA, Tacconelli E, Grilli E, Pea F, Petrosillo N. Continuous versus intermittent infusion of vancomycin for the treatment of Gram-positive infections: systematic review and meta-analysis. J Antimicrob Chemother. 2012;67(1):17–24. doi:10.1093/jac/dkr442
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.