Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Continuous Intravenous versus Subcutaneous Administration of Insulin for Glycemic Variability in Acute Ischemic Stroke
Authors Du LZ, Liu PY, Ge CY, Li Y , Li YY, Tang MF, Chen JJ
Received 14 April 2022
Accepted for publication 23 June 2022
Published 1 July 2022 Volume 2022:18 Pages 1309—1314
DOI https://doi.org/10.2147/NDT.S370776
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Lin-Zhe Du, Pei-Yan Liu, Chen-Yan Ge, Yang Li, Yuan-Yuan Li, Mu-Fei Tang, Jin-Jin Chen
Department of Clinical Pharmacy, Nanjing First Hospital, Nanjing, People’s Republic of China
Correspondence: Jin-Jin Chen, Department of Clinical Pharmacy, Nanjing First Hospital, No. 68 Changle Road, Qinhuai District, Nanjing, 210006, People’s Republic of China, Tel +86-025-87726312, Email [email protected]
Background: Continuous intravenous infusion (IV) or subcutaneous injection (SC) of insulin was widely applied to control hyperglycemia after ischemic stroke. However, the impact of different administration modes on glycemic variability was unknown.
Methods: Consecutive stroke patients treated with intravenous thrombolysis were screened. Subjects who received insulin treatment were included and entered into the IV or SC group according to the respective administration mode. Blood glucose was closely monitored within the first 72 hours, and the target range of glucose was from 7.7 to 10.0 mmol/L for all patients. The variabilities of glucose, assessed using standard deviation of the mean, variable coefficient and range from the maximum to the minimum value, were compared between the two groups.
Results: A total of 130 patients were enrolled with 66 in the IV groups and 64 in the SC group. Compared with the SC group, the IV group had higher glycemic variability evaluated as either standard deviation (2.7 ± 0.7 mmol/L vs 2.2 ± 0.9 mmol/L, p = 0.002), variable coefficient (0.26 ± 0.06 vs 0.23 ± 0.08, p = 0.011) or range (10.0 ± 3.6 mmol/L vs 8.1 ± 3.1 mmol/L, p = 0.001). Multivariate logistic regression analyses found that continuous intravenous infusion was associated with higher level of the standard deviation (adjusted OR 3.01, 95% CI 1.29– 7.28, p = 0.011), variable coefficient (adjusted OR 5.97, 95% CI 2.55– 13.96, p < 0.001) and range (adjusted OR 6.08, 95% CI 2.63– 14.05, p < 0.001).
Conclusion: Continuous intravenous infusion of insulin was associated with higher glycemic variability than subcutaneous injection in acute stroke patients receiving thrombolysis.
Keywords: acute ischemic stroke, insulin, continuous intravenous infusion, subcutaneous injection, administration mode
Corrigendum for this paper has been published.
Introduction
Hyperglycemia is a common and essential therapeutic target in acute ischemic stroke with and without premorbid diabetes.1,2 Recent studies found the variability of glucose after stroke onset, or mentioned as glucose fluctuation, was an independent risk factor for poor outcomes as well as the glucose level,3–6 with underlying mechanisms including increased oxidative stress, aggravated inflammation, endothelial impairment and microcirculation dysfunction.6–8 It is also recommended to avoid glycemic excessive fluctuation or hypoglycemia in patients with acute ischemic stroke.9
Continuous intravenous infusion or subcutaneous injection of insulin was the main therapies to control post-stroke hyperglycemia at the acute phase.10,11 However, few evidences were reported on which mode of administration was better. In the “Stroke Hyperglycemia Insulin Network Effort (SHINE)” study, intensive treatment with continuous intravenous insulin infusion was compared with standard treatment with subcutaneous injection after ischemic stroke.12,13 After a 90-day follow-up, no significant difference in favorable outcome was found between the two administration strategies, while more hypoglycemia occurred in the intensive treatment group, implying a potential harm of continuous intravenous infusion for patients with acute stroke.13 Considering the higher risk of hypoglycemia, we hypothesized that continuous intravenous infusion of insulin might lead to more fluctuation in blood glucose than subcutaneous administration.
Methods
To ascertain this hypothesis, we performed a retrospective analysis based on a prospective stroke registry from the National Advanced Stroke Center of Nanjing First Hospital. Consecutive patients with ischemic stroke and hyperglycemia who received intravenous thrombolysis from January 2017 to February 2021 were screened for enrollment. The inclusion criteria were 1) received insulin via continuous intravenous infusion or subcutaneous injection within the first 72 hours after hospital admission; 2) had blood glucose monitored for 8 or more times each day within the first 72 hours. Patients entered into the continuous intravenous infusion group (IV group) or the subcutaneous injection group (SC group) according to the respective administration strategy. Baseline characteristics, medical history, treatment information, blood glucose measures and clinical outcomes were collected. Our study complies with the Declaration of Helsinki. Informed consents were obtained from all participants before enrollment, and this analysis was approved by the Ethics Committee of Nanjing First Hospital.
The treatment of insulin (Biosynthetic Human Insulin, Novolin R) via intravenous or subcutaneous was determined by the clinicians autonomously. The dose adaptions to the capillary glucose test were performed as needed every two hours in the IV group and every three hours in the SC group, respectively, according to a widely applied nomogram.14 The target range of glucose was from 7.7 to 10.0 mmol/L for all patients. The variability of blood glucose was assessed using standard deviation of the mean, variable coefficient and range from the maximum to the minimum value.
Statistical Analysis
Baseline characteristics and the glycemic variabilities were compared between the IV and SC groups. Data were presented as mean ± standard deviation or median (interquartile range) for continuous variables and numbers (percentage) for categorical variables. Metric and ordinal variables were analyzed by one-way ANOVA and Kruskal–Wallis test, respectively, while frequencies were compared using Fisher’s exact method. Univariate and multivariate logistic regression analyses were performed to study the independent impact of different administration strategies on the variabilities. The standard deviation, variable coefficient and range were transformed into binary variables by the cutoff points of average values. Variables with p <0.1 in univariate analysis were adjusted in multivariate models. Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) version 20.0 (SPSS Inc. Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
A total of 570 patients with acute ischemic stroke who received intravenous thrombolysis were screened and 143 cases had insulin therapy through continuous intravenous infusion or subcutaneous injection. After excluding patients who did not have enough glucose data or suspended insulin therapy for non-medical reasons, 130 participants entered into the final analysis (Figure 1). Of these included patients, 50% subjects had pre-stroke diabetes, and the average of HbA1c was 7.0%±1.6%. Among the participants, 66 (50.8%) patients entered into the IV group and 64 (49.2%) into the SC group. Patients in the IV group had higher HbA1c than in the SC group (7.4%±1.8% vs 6.5%±1.2%, p = 0.002), and other baseline characteristics were comparable between the two groups (Table 1).
 |
Table 1 Baseline Characteristics |
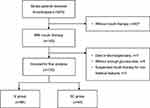 |
Figure 1 Flow chart of patient inclusion. |
No hypoglycemia was reported in either group. Within the first 72 hours, the means of maximum (16.3 ± 3.6 mmol/L vs 14.4 ± 2.8 mmol/L, p = 0.001) and average glucose (10.3 ± 1.7 mmol/L vs 9.6 ± 1.4 mmol/L, p = 0.029) were higher in the IV group than in the SC group, and no difference was found regarding the minimum glucose. Compared with the SC group, the IV group had higher glycemic variability evaluated as either standard deviation (2.7 ± 0.7 mmol/L vs 2.2 ± 0.9 mmol/L, p = 0.002), variable coefficient (0.26 ± 0.06 vs 0.23 ± 0.08, p=0.011), or range (10.0 ± 3.6 mmol/L vs 8.1 ± 3.1 mmol/L, p = 0.001) (Table 2). Univariate and multivariate logistic regression analyses of the glycemic variability are shown in Table 3. After adjusted for the co-variables, the IV group showed higher levels of the standard deviation (adjusted OR 3.01, 95% CI 1.29–7.28, p = 0.011), variable coefficient (adjusted OR 5.97, 95% CI 2.55–13.96, p <0.001) and range (adjusted OR 6.08, 95% CI 2.63–14.05, p < 0.001) than the SC group. The proportions of target glucose were 27% (19%–40%) in the IV group and 35% (21%–44%) in the SC group, respectively (p = 0.062).
 |
Table 2 Blood Glucose Variables and Clinical Outcomes Between Groups |
 |
Table 3 Univariate and Multiple Logistic Regression of Blood Glucose Variation |
There was no difference in clinical outcomes between the two groups, regarding modified Rankin Scale (mRS) score at 90 days [1(0–3) vs 1(0–3), p = 0.666], proportion of a mRS score of 0 to 2 (60.6% vs 68.8%, p = 0.363) and symptomatic intracranial hemorrhage within 24 hours (6.1% vs 4.7%, p = 0.517) (Table 2).
Discussions
In this study, we found that continuous intravenous infusion of insulin for hyperglycemia was associated with higher glycemic variability than subcutaneous injection in patients with acute stroke. Despite closer monitoring and adaption, patients in the IV group seemed to had lower proportion of target glucose than in the SC group.
The underlying mechanisms of different variabilities in two administration routes were not entirely clear. The primary explanation might be the different absorption rates of insulin.10 In the SC group, insulin is injected into subcutaneous tissue and enters into circulation through blood and lymph capillaries. Subcutaneous tissue consists of fat lobules and extracellular matrix, which constitutes a physiological barrier to insulin delivery and keeps a steady increase in insulin concentration in circulation.15–17 In the IV group, insulin is directly injected into the venous system, which might lead to a steep change in plasma concentration, especially at the time points of start, stop and dose adjustment. Another possible explanation is that the IV strategy needed more doses of insulin than the SC group under the same glycemic level according to the nomogram.14 A larger dose might lead to greater change in blood glucose.
By comparing the glycemic variabilities of different insulin therapies, our findings were in accordance with the SHINE study in regard to revealing harmful effects of continuous insulin infusion on patients with acute ischemic stroke. Unlike the SHINE study which was stopped early for high risk of hypoglycemia, no patients in our cohorts experienced such adverse event, and the average blood glucose was similar in the two groups. This might be due to different glycemic targets, which were 4.4–7.2 mmol/L and 4.4–9.9 mmol/L in the intensive and standard groups of the SHINE study, respectively,13 and 7.7 to 10.0 mmol/L in both groups of ours. Furthermore, our objective was to compare the two administration strategies, instead of exploring the relative effect of “intensive glycemic control” over “standard control”.
Our study added evidences of different effects of continuous intravenous and subcutaneous injection of insulin on the glycemic variability. However, some limitations should be mentioned. First, as a single-center and hospital-based retrospective study with a small sample size, the selection bias must exist. We only enrolled patients who had records of blood glucose for 72 hours, and patients died or be discharged early would had been excluded. Second, as a case–control study, the stroke severities and HbA1c levels were not balanced and patients in the IV groups tended to be severer and had a higher glucose level before stroke, which possibly impacted our results. Although we used regression analysis to adjust the confounding factors, the statistical power might be weakened. Third, we chose patients receiving intravenous thrombolysis as participants because glycemic control could be initiated quickly and within the first few hours after stroke onset in these patients.1 Whereas, the interactive effect of thrombolytic agents on glycemic variability was not known, and it was still unclear whether insulin administration modes would affect glycemic variability in patients who did not perform thrombolysis. Fourth, the glycemic variability was calculated based on capillary glucose at different time points. However, continuous glucose monitoring might provide more and accurate measurements. Further studies with prospective design and large sample size are needed.
Conclusion
Continuous intravenous infusion of insulin for glycemic control might be associated with higher glycemic variability than subcutaneous administration in patients who had acute stroke and received thrombolysis. For controlling hyperglycemia smoothly and avoiding high fluctuation, the mode of subcutaneous injection might be preferred to continuous intravenous infusion in clinical practice.
Funding
This study was funded by the Jiangsu Pharmaceutical Association (A201908).
Disclosure
The authors report no conflicts of interests in this work.
References
1. Jiang Y, Liu N, Han J, et al. Diabetes mellitus/poststroke hyperglycemia: a detrimental factor for tPA thrombolytic stroke therapy. Transl Stroke Res. 2021;12:416–427. doi:10.1007/s12975-020-00872-3
2. Shi Z, Guo S, Pan J, Xu C, Geng Y, Zheng S. Increased postoperative fasting glucose is associated with unfavorable outcomes in patients treated with mechanical thrombectomy treatment. Front Neurol. 2021;12:668363. doi:10.3389/fneur.2021.668363
3. Hui J, Zhang J, Mao X, et al. The initial glycemic variability is associated with early neurological deterioration in diabetic patients with acute ischemic stroke. Neurol Sci. 2018;39:1571–1577. doi:10.1007/s10072-018-3463-6
4. Fuentes B, Pastor-Yborra S, Gutiérrez-Zúñiga R, et al. Glycemic variability: prognostic impact on acute ischemic stroke and the impact of corrective treatment for hyperglycemia. The GLIAS-III translational study. J Transl Med. 2020;18:414. doi:10.1186/s12967-020-02586-4
5. Peng X, Ge J, Wang C, et al. Longitudinal average glucose levels and variance and risk of stroke: a Chinese cohort study. Int J Hypertens. 2020;2020:8953058. doi:10.1155/2020/8953058
6. Kim TJ, Lee JS, Park SH, Ko SB. Short-term glycemic variability and hemorrhagic transformation after successful endovascular thrombectomy. Transl Stroke Res. 2021;12:968–975. doi:10.1007/s12975-021-00895-4
7. Camara-Lemarroy CR. Glucose and stroke: what about glycemic variability? J Neurol Sci. 2017;373:242–243. doi:10.1016/j.jns.2017.01.015
8. Lee DY, Han K, Park S, et al. Glucose variability and the risks of stroke, myocardial infarction, and all-cause mortality in individuals with diabetes: retrospective cohort study. Cardiovasc Diabetol. 2020;19:144. doi:10.1186/s12933-020-01134-0
9. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 Update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi:10.1161/STR.0000000000000211
10. Tumminia A, Crimi S, Sciacca L, et al. Efficacy of real-time continuous glucose monitoring on glycaemic control and glucose variability in type 1 diabetic patients treated with either insulin pumps or multiple insulin injection therapy: a randomized controlled crossover trial. Diabetes Metab Res Rev. 2015;31:61–68. doi:10.1002/dmrr.2557
11. Cerecedo-Lopez CD, Cantu-Aldana A, Patel NJ, Aziz-Sultan MA, Frerichs KU, Du R. Insulin in the management of acute ischemic stroke: a systematic review and meta-analysis. World Neurosurg. 2020;136:e514–e534. doi:10.1016/j.wneu.2020.01.056
12. Bruno A, Durkalski VL, Hall CE, et al. The Stroke Hyperglycemia Insulin Network Effort (SHINE) trial protocol: a randomized, blinded, efficacy trial of standard vs. intensive hyperglycemia management in acute stroke. Int j Stroke. 2014;9:246–251. doi:10.1111/ijs.12045
13. Johnston KC, Bruno A, Pauls Q, et al. Intensive vs standard treatment of hyperglycemia and functional outcome in patients with acute ischemic stroke: the shine randomized clinical trial. JAMA. 2019;322:326–335. doi:10.1001/jama.2019.9346
14. Rosso C, Corvol JC, Pires C, et al. Intensive versus subcutaneous insulin in patients with hyperacute stroke: results from the randomized INSULINFARCT trial. Stroke. 2012;43:2343–2349. doi:10.1161/STROKEAHA.112.657122
15. Famulla S, Hövelmann U, Fischer A, et al. Insulin injection into lipohypertrophic tissue: blunted and more variable insulin absorption and action and impaired postprandial glucose control. Diabetes Care. 2016;39:1486–1492. doi:10.2337/dc16-0610
16. Gradel AKJ, Porsgaard T, Lykkesfeldt J, Seested T, Gram-Nielsen S. Factors affecting the absorption of subcutaneously administered insulin: effect on variability. J Diabetes Res. 2018;2018:1205121. doi:10.1155/2018/1205121
17. Faggionato E, Schiavon M, Dalla Man C. Knockout of Arabidopsis thaliana VEP1, encoding a PRISE (progesterone 5β-reductase/iridoid synthase-like enzyme), leads to metabolic changes in response to exogenous methyl vinyl ketone (MVK). Metabolites. 2021;12:11. doi:10.3390/metabo12010011
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
