Back to Journals » Integrated Blood Pressure Control » Volume 9
Consumption of nattokinase is associated with reduced blood pressure and von Willebrand factor, a cardiovascular risk marker: results from a randomized, double-blind, placebo-controlled, multicenter North American clinical trial
Authors Jensen GS , Lenninger M, Ero MP, Benson KF
Received 30 October 2015
Accepted for publication 19 August 2016
Published 13 October 2016 Volume 2016:9 Pages 95—104
DOI https://doi.org/10.2147/IBPC.S99553
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Steven Atlas
Video abstract presented by Gitte S Jensen.
Views: 155618
Gitte S Jensen,1 Miki Lenninger,1 Michael P Ero,2 Kathleen F Benson,1
1NIS Labs, Klamath Falls, OR, 2Machaon Diagnostics, Inc., Oakland, CA, USA
Objective: The objective of this study is to evaluate the effects of consumption of nattokinase on hypertension in a North American hypertensive population with associated genetic, dietary, and lifestyle factors. This is in extension of, and contrast to, previous studies on Asian populations.
Materials and methods: A randomized, double-blind, placebo-controlled, parallel-arm clinical study was performed to evaluate nattokinase (NSK-SD), a fermented soy extract nattō from which vitamin K2 has been removed. Based on the results from previous studies on Asian populations, 79 subjects were enrolled upon screening for elevated blood pressure (BP; systolic BP ≥130 or diastolic BP ≥90 mmHg) who consumed placebo or 100 mg nattokinase/d for the 8-week study duration. Blood collections were performed at baseline and 8 weeks for testing plasma renin activity, von Willebrand factor (vWF), and platelet factor-4. Seventy-four people completed the study with good compliance.
Results: Consumption of nattokinase was associated with a reduction in both systolic and diastolic BP. The reduction in systolic BP was seen for both sexes but was more robust in males consuming nattokinase. The average reduction in diastolic BP in the nattokinase group from 87 mmHg to 84 mmHg was statistically significant when compared to that in the group consuming placebo, where the average diastolic BP remained constant at 87 mmHg (P<0.05), and reached a high level of significance for males consuming nattokinase, where the average diastolic BP dropped from 86 mmHg to 81 mmHg (P<0.006). A decrease in vWF was seen in the female population consuming nattokinase (P<0.1). In the subpopulation with low plasma renin activity levels at baseline (<0.29 ng/mL/h), an increase was seen for 66% of the people after 8-week consumption of nattokinase (P<0.1), in contrast to only 8% in the placebo group.
Conclusion: The data suggest that nattokinase consumption in a North American population is associated with beneficial changes to BP in a hypertensive population, indicating sex-specific mechanisms of action of nattokinase’s effect on vWF and hypertension.
Keywords: subtilisin NAT, systolic blood pressure, diastolic blood pressure, von Willebrand factor, plasma renin activity
Introduction
Hypertension is a chronic medical condition involving increased arterial blood pressure (BP) and is associated with increased risk of cardiovascular and renal disease, as well as type II diabetes.1 According to current guidelines from the WHO,2 EU,3and the US Preventive Service Task Force4,5 on diagnosis and treatment of elevated BP,6 the assessment of low, moderate, and high risk factors for cardiovascular events7 is not simply determined according to BP but also takes a number of confounding factors into account, such as sex, age, genetic predisposition to hypertension, estrogenic status, diabetes, smoking, and diet.8 Even though the awareness of hypertension, its treatment, and control has significantly increased over the past 10 years, many adults with known hypertension do not control their BP,9 and a high occurrence of uncontrolled hypertension remains a significant and costly health care challenge.10,11
Various pharmaceutical treatment options are commonly available for the treatment of hypertension. Antihypertensive drugs include thiazide-type diuretics, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, calcium channel blockers, and beta-adrenergic blockers. Multiple considerations must be made when using pharmaceutical-grade antihypertensive drugs, including adverse effects, contraindications, synergistic effects, and use in special populations.12 There is a growing interest in nonpharmaceutical sources of angiotensin-converting enzyme inhibitors,13,14 as well as food-based strategies for supporting cardiovascular function and specifically for reducing hypertension. Natural methods to reduce such inflammatory conditions are of interest, and dietary components of functional benefit for the hypertensive patient include essential fatty acids,15,16 where cardioprotective fatty acids found in oils from fish, flax, nuts, seeds, and algae have known anti-inflammatory activities.17
Hypertension is associated with inflammatory stress in the arterial walls18 and also with coagulopathy, often linked to a fibrinolytic deficit.19 Therefore, direct support of fibrinolysis and regulation of dysregulated coagulation biology are of interest. Nattokinase is an enzyme that is present in a traditional fermented Japanese food called nattō.20 Nattō has been consumed by Japanese for over a thousand years and is produced by fermentation of boiled soy beans by adding the bacterium Bacillus subtilis natto. Its name is misleading since nattokinase is not a kinase enzyme but a serine protease of the subtilisin family and exhibits a strong fibrinolytic activity,21,22 thrombolytic effects,23,24 and reduction of platelet aggregation and clotting.25
The traditional food nattō also contains vitamin K2, which is involved in carboxylation of certain proteins linked to hemostasis.26 An extract from nattō, from which vitamin K2 has been removed, has been studied for its effects on cardiovascular markers in animals and humans. The enzyme, when consumed, tolerates the conditions in the stomach and is transported across the gut into the blood plasma in an active form.27 A direct measurement of nattokinase in human serum peaked at 13 hours postconsumption.28 Peptides from nattokinase (subtilisin NAT) inhibited the angiotensin I (Ang-I)-converting enzyme.29 Spontaneously hypertensive rats fed a high-cholesterol diet showed beneficial impact of consumption of a culture filtrate from B subtilis natto on various biomarkers associated with the renin–angiotensin system.30
Previous randomized double-blind, placebo-controlled studies on the consumption of the fibrinolytic enzyme nattokinase have shown multiple effects on vascular health, including fibrinolytic effects.31,32 A previous clinical study on the effects of nattokinase on BP showed statistically significant reduction in both systolic and diastolic BP after 8 weeks of consumption of nattokinase, whereas the changes after consuming placebo were not significantly different from baseline.33
It is well documented that a Western diet and lifestyle are associated with a higher incidence of high BP and cardiovascular disease. The present study is, to the best of our knowledge, the first human clinical study performed outside of Asia to address the effects of nattokinase on BP in a study population adhering to a Western diet and lifestyle, with no previous dietary exposure to nattō. This study was comparable with a previous Asian study in the duration and number of study subjects but used a more stringent documentation of whether a subject had elevated BP (systolic BP ≥130 mmHg or diastolic BP ≥90 mmHg), by implementing a screening process that involved BP measurements on 3 separate days.
Materials and methods
Study design
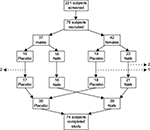
A multicenter, randomized, double-blind, placebo-controlled study design was used for this clinical study (NCT02886507). Subjects from the databases at the two study sites, representing people who have previously indicated interest in participating in clinical studies, were approached, and if interested in being considered for the present study, they were invited for screening. Two hundred and twenty-one people went through screening for hypertension, and 79 qualified for enrollment in the 8-week study upon signing written informed consent, as approved by the institutional review board of Sky Lakes Medical Center (FWA 2603; Figure 1). The subjects were randomized to receive either product or placebo for the 8-week study. The study was carried out during 2012–2013 at the following two centers: 1) 54 subjects were enrolled through NIS Labs in southern OR, USA, where study subjects live and work at an elevation of 1,200–1,500 m above sea level and 2) 25 subjects were enrolled through KGK Synergize, ON, Canada, where study subjects live and work at an elevation of 200–300 m above sea level.
The prescreening involved an interview to document sex, age, body mass index (BMI), medical/surgical history, diet/lifestyle, current health issues, medication, and supplement use. If subjects met the inclusion and exclusion criteria, they were scheduled for three successive appointments to measure their BP. Authorities agree that several readings, performed on different days, are needed to verify that someone has elevated BP.3,4 The three separate screening visits for BP measurements determined whether a person was eligible for the study.
Subjects who passed the prescreening interview and met the BP screening of repeat measures of elevated BP across all three screening visits were enrolled into the study upon providing written informed consent.
The study population included healthy adults of both sexes, aged 18–85 years, and elevated BP was identified by systolic BP ≥130 mmHg or diastolic BP ≥90 mmHg, confirmed on three separate occasions. People were excluded from the study if they had consumed nattokinase-containing supplements within 60 days prior to enrollment; were currently on BP medication; had a history of cancer or chemotherapy within the last 12 months; significant active uncontrolled disease; were consuming more than an average of 2 standard alcoholic drinks/d (14 drinks/wk); were currently experiencing intense stressful events/life changes that would negatively affect compliance; were pregnant, nursing, or trying to become pregnant; were females not using effective contraception; or had food allergies related to ingredients in test product.
Dietary and lifestyle recommendations
At the initial study visit, all subjects in both the placebo and the nattokinase groups received dietary and lifestyle recommendations, as recommended by the American Heart Association.
Consumable product and placebo
Nattokinase NSK-SD® (subtilisin NAT) was encapsulated in veggie caps with 100 mg/capsule and standardized to at least 2,000 fibrinolytic units (FU) per each 100 mg daily dose. Matching placebo capsules were made with microcrystalline cellulose. Subjects were given 8 weeks supply of either a placebo or a nattokinase. Subjects were instructed to take one capsule daily in the morning and to return the bottles with any remaining capsules at the 8-week visit. The active product was well tolerated, and no adverse events were associated with its consumption. Compliance was evaluated by documenting capsule count in returned bottles and exceeded 80% for all study subjects (average compliance 96.4%).
Blood pressure
BP readings were performed on the right arm using an Omron 741 or 742 monitor, and subjects were scheduled at the same time of the day for each visit. All subjects adhered to the requirements of abstinence from exercise and nicotine and caffeine use for 1 hour prior to a study visit. Screening for hypertension was performed over three separate days. The last (third) screening BP reading became the baseline data for that subject. The BP reading at study exit was performed at the 8-week follow-up visit and involved a minimum of two readings, with a third reading if the initial two systolic readings were not within 5 mmHg of each other. Subjects were asked not to smoke, eat, or exercise, 1 hour before their appointment. At each study visit, BP was measured two to three times at a sitting position, where the first measurement was performed after 5 minutes rest, and with at least 3 minutes interval between each measurement.
Blood collection
On each of the two study visits (baseline and 8 weeks), a blood sample was drawn. The blood collection was performed after the person had been sitting for at least 15 minutes. Blood was drawn into EDTA, the vial was spun, and the plasma was harvested and banked at -80°C. After the end of the clinical phase of the study, the plasma samples were tested for plasma renin activity (PRA) at Machaon Diagnostics, Inc. (Oakland CA, USA). Additional blood was drawn into serum separator vials and allowed to coagulate for a minimum of 30 minutes before centrifugation and serum harvesting. Serum samples were banked at -80°C and used for testing for von Willebrand factor (vWF) and platelet factor-4 (PF4).
Plasma renin activity
Plasma samples were tested for PRA at Machaon Diagnostics, Inc.. Renin plays a central role in the hormone system that regulates BP and body fluid volumes.34,35 Renin acts to generate Ang-I from angiotensinogen, leading to an elevation of BP and increased sodium retention by the kidney. Assessment of PRA is currently performed by this specialized clinical laboratory to detect elevations that may be associated with elevated BP. PRA was measured in a competitive enzyme-linked immunosorbent assay that measures Ang-I. PRA was calculated by comparing the Ang-I generation that occurred after 37°C to the Ang-I generation that occurred after 0°C incubation after 180 minutes.
vWF and PF4
Serum samples were tested in duplicate for vWF and PF4 using a customized Luminex magnetic bead array (MILLIPLEX MAP Human Cardiovascular Disease [Acute Phase] Magnetic Bead Panel 3; EMD Millipore, Billerica, MA, USA), performed according to the manufacturer’s instructions.36 Briefly, serum samples were diluted 1:40,000, and 25 µL incubated for 2 hours with magnetic beads coated with the vWF versus PF4 capturing antibodies. Beads were then washed, and biotinylated detection antibodies were added for 1 hour. Following this, the beads were washed, and a streptavidin-phycoerythrin-labeled antibody was added for 30 minutes. Beads were then washed, and samples were analyzed on a MagPix instrument (Luminex Corporation, Austin, TX, USA) using the Xponent® 4.2 software (Luminex Corporation), where concentrations (mg/mL) were calculated based on the fit to the standard curve allowing conversion from mean fluorescence intensity to milligram per milliliter.
Analysis
The number of subjects was determined based on a previously published placebo-controlled study in an Asian population, where 86 subjects were enrolled and 73 completed the 8-week study.33 Of the 79 subjects enrolled into the North American study described here, data from 74 people were analyzed. The remaining five people were removed from analysis for the following reasons: two people dropped out, two people required medication that may affect BP, and data from one person was not retrievable.
Average and standard deviation for each data set were calculated using Microsoft Excel. Statistical analysis of clinical data was performed as “between-group” analysis using the two-tailed, unpaired t-test. Statistical significance of changes from baseline to later assessments was evaluated by between-treatment analysis using “within-subject” analysis using the two-tailed paired t-test. Subgroup analysis was performed for the group that had lower than normal renin activity at study start, and sex-specific subgroup analysis was performed. A trend was defined as P<0.1. Statistical significance was defined as P<0.05, and a high level of significance as P<0.01.
Results
Study enrollment, compliance, and completion
Seventy-nine healthy subjects of both sexes were enrolled after institutional review board approval and written informed consent. The screening led to the exclusion of a large number of subjects who despite initial high BP readings did not classify as true hypertensive upon the strict repeat-screening criteria. People who passed screening were enrolled into the study (Figure 1).
Table 1 shows the number, sex, average age, and average BMI of the 79 volunteers who enrolled in the study. The randomization led to an even distribution between females and males in the two groups. There were no significant differences between age and BMI between the females and males in the placebo versus active product group.
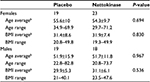  | Table 1 Demographics of the study populationa Notes: aSeventy-four subjects were analyzed. bData are given as average ± standard deviation. Abbreviation: BMI, body mass index. |
Systolic and diastolic BP
Subjects consuming nattokinase showed a decrease in systolic BP; however, the decrease was not significantly different from the placebo group (Figure 2). When evaluating the reduction in systolic BP within the group consuming nattokinase, the decrease from 144±1.5 mmHg to 140±2.1 mmHg reached a statistical trend (P<0.1). Comparing the diastolic BP between the two groups showed a significantly lower average diastolic BP at 8 weeks in the nattokinase group (84±1.9 mmHg) when compared to the placebo group (87±1.7 mmHg; P<0.04). The reduction in diastolic BP was highly significant for males consuming nattokinase, where the average baseline diastolic BP at 86 mmHg dropped to 81±2.5 mmHg after 8 weeks of consuming nattokinase, in contrast to the placebo group where the diastolic BP remained constant at 88±2.6 mmHg (P<0.006).
Platelet factor-4
Serum levels of PF4 (CXCL4) did not show differences between the nattokinase and placebo group (data not shown).
von Willebrand factor
The average level of vWF was reduced by 15% in the nattokinase group, whereas no consistent change was seen for the subjects consuming placebo for 8 weeks (Figure 3). The difference in vWF levels at 8 weeks between the two groups showed a statistical trend (P<0.09). This decrease in vWF levels was sex specific, as it was seen for the group of females consuming nattokinase (26% reduction, P<0.09) but not in the male group consuming nattokinase.
Plasma renin activity
The average PRA did not change in either group and did not reach statistical significance when comparing the nattokinase and the placebo groups. However, this is due to the observation that the effects were dependent on whether subjects started the study with low or high PRA. Consumption of nattokinase was associated with beneficial changes in PRA. Twenty-two people (30% of the study population) started the study with baseline PRA levels <0.29 ng/mL/h used in this analysis to differentiate between relatively low, normal, and elevated levels. The data showed that 66% of the study subjects in the nattokinase group who started the study with PRA levels below the normal range showed improvement to normal levels during the 8 weeks. This is in contrast to only one person (8%) of the study subjects in the placebo group who started the study with PRA below normal range and normalized during the 8-week study.
The association between nattokinase consumption and normalization of PRA was seen for both male and female study subjects (Figure 4). For the subgroup with <0.29 ng/mL/h values at baseline, the females in the nattokinase group showed a mild increase, whereas the males consuming nattokinase showed a robust fivefold increase that reached a statistical trend when compared to placebo (P<0.1). The average levels at 8 weeks were within normal range for both sexes (0.31 ng/mL/h for females and 1.18 ng/mL/h for males).
In addition, one male study subject in the nattokinase group started the study with very high PRA levels at 12.36 ng/mL/h. Even though this person’s PRA level did not fully normalize during the 8-week study, the level was reduced by ~50%, to 6.63 ng/mL/h at 8 weeks.
Discussion
The goal of the study was to document the efficacy of nattokinase in a North American population with associated diet, lifestyle, and stressors. Beneficial changes were seen on systolic and diastolic BP in the group consuming nattokinase, when compared to placebo. In the study reported here, the reduction in both systolic and diastolic BP was most robust in the male population. A reduction in systolic BP was also observed for males in the placebo group but this did not reach statistical significance. An important factor in controlling the relationship between BP and sodium levels is the renin–angiotensin system. It has been suggested that androgens may increase BP via the renin–angiotensin system, possibly also associated with production of vasoconstrictor substances and reduction in nitric oxide availability.37 This may be a contributing factor to help explain why males and postmenopausal females are at relative higher risk for cardiovascular disease.
PRA measures the ability of plasma renin to generate Ang-I in a temperature-dependent manner. The renin–angiotensin system plays a role in the regulation of BP, and elevated levels of PRA have been associated with essential hypertension.38 However, some people with essential hypertension have abnormally low PRA, and this has been associated with elevated calcitriol and parathyroid hormone levels.39 Favorable changes in PRA levels were seen in the group consuming nattokinase. This improvement was seen for both sexes. The changes in PRA associated with nattokinase consumption in a North American hypertensive population did not reach statistical significance, in contrast to the previous study performed in South Korea and published by Kim et al.33 In the North American study population reported here, 30% of the study subjects had low PRA levels at baseline. This is in contrast to the PRA reported in the previous Korean study, where the average PRA at baseline was higher than the average PRA at baseline for the North American study.
The observed sex differences in BP normalization by nattokinase, where females showed a moderate change in BP while consuming nattokinase, are likely related to the complex effects of estrogen on BP in the female population. The sex differences were not reported or discussed in the previous study; and therefore, it is not known whether similar sex differences were observed in the study reported by Kim et al.33
Renin–angiotensin and nitric oxide are the two main counter-regulators modulating BP as well as sodium reabsorption and excretion.40,41 Estrogen is known to affect the renin–angiotensin system in complex manners, depending on whether the source of estrogen is endogenous or from an external source. External sources include hormonal birth control (oral, transdermal, and other), perimenopausal hormone replacement therapy, dietary plant-based phytoestrogens, and exposure to environmental xenoestrogenic pollutants such as organochlorines.
vWF is a mediator of platelet adhesion and is released by damaged endothelium and increases risk of thrombus formation. The sex-specific decrease in vWF seen in the female subgroup consuming nattokinase is of interest, in light of the known association between elevated vWF and the risk for ischemic stroke.42,43 This suggests that health improvements in females consuming nattokinase potentially involve different mechanisms than those in males. Also, the known relationship between vWF and the ABO blood type44 could potentially help explain differences between study results in different ethnic groups, as vWF is higher in subjects with a blood type different from type O, and blood type should be tracked in future studies on nattokinase and hypertension.
The coagulation and inflammation systems are interlinked, and fibrin degradation products (when fibrin is cleaved by plasmin) elicit a number of well-defined proinflammatory effects.45
Conclusion
The lowering of diastolic BP in males generated the most statistically significant and clinically important results in this study. The overall data suggest that the oral consumption of nattokinase supports healthy BP in both sexes, in a non-Asian population. In addition, the reduction in elevated vWF in the female subgroup suggests a possible reduction of risk for stroke. This is important, since previous studies were conducted in Asia where different genetic, dietary, and lifestyle factors contribute to hypertension and potentially affect which treatment strategies may help reduce hypertension and associated biomarkers. Limitations of the current study included the small number of subjects, as well as the broad age and BMI range, menstrual status at study visits, menopausal status for inclusion in study, and types of birth control in the female population. Future work should include studies on female populations to evaluate age-related and hormonal differences in the impact of nattokinase consumption, such as comparison of premenopausal and postmenopausal females, with and without hormone replacement therapy. Studies should also include populations with metabolic dysfunction such as diabetes and should also extend to include evaluation of cardiovascular risk factors in the morbidly obese of both sexes, and populations consuming a high level of saturated fat, with an in-depth testing of correlations between blood glucose, insulin, lipids, and kidney function.
Acknowledgments
The study was conducted at NIS Labs, an independent contract research laboratory specializing in natural products research, with a second site at KGK Synergize, London, ON, Canada. The study was sponsored by JBSL-USA, the distributor of the nattokinase product tested in this study.
Disclosure
GSJ, ML, and KFB are employed at NIS Labs, an independent contract research organization. MPE is employed at Machaon Diagnostics Inc., an independent laboratory specializing in blood clotting diagnostics. None of the authors have any financial interest in the subject matter. The authors report no other conflicts of interest in this work.
References
Kim MJ, Lim NK, Choi SJ, Park HY. Hypertension is an independent risk factor for type 2 diabetes: the Korean genome and epidemiology study. Hypertens Res. 2015;38(11):783–789. | ||
Whitworth JA; World Health Organization, International Society of Hypertension Writing Group. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21(11):1983–1992. | ||
Mancia G, Fagard R, Narkiewicz K, et al; Task Force for the Management of Arterial Hypertension of the European Society of Hypertension and the European Society of Cardiology. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press. 2014;23(1):3–16. | ||
U.S. Preventive Services Task Force. Screening for high blood pressure: U.S. Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2007;147:783. | ||
Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162(3):192–204. | ||
Krause T, Lovibond K, Caulfield M, McCormack T, Williams B; Guideline Development Group. Management of hypertension: summary of NICE guidance. BMJ. 2011;343:d4891. | ||
Clark CE, Taylor RS, Shore AC, Ukoumunne OC, Campbell JL. Association of a difference in systolic blood pressure between arms with vascular disease and mortality: a systematic review and meta-analysis. Lancet. 2012;379(9819):905–914. | ||
Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D. Call to action on use and reimbursement for home blood pressure monitoring: executive summary: a joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52(1):1–9. | ||
Yoon S, Ostchega Y, Louis T. Recent Trends in the Prevalence of High Blood Pressure and Its Treatment and Control, 1999–2008. Hyattsville, MD: National Center for Health Statistics; 2010. [NCHS data brief, no 48]. | ||
Fryar CD, Hirsch R, Eberhardt MS, et al. Hypertension, High Serum Total Cholesterol, and Diabetes: Racial and Ethnic Prevalence Differences in U.S. Adults, 1999–2006. Hyattsville, MD: National Center for Health Statistics; 2010. [NCHS data brief, no 36]. | ||
Gillespie C, Kuklina EV, Briss PA, Blair NA, Hong Y. Vital signs: prevalence, treatment, and control of hypertension – United States, 1999–2002 and 2005–2008. MMWR Morb Mortal Wkly Rep. 2011;60(04):103–108. | ||
Esteras R, Perez-Gomez MV, Rodriguez-Osorio L, Ortiz A, Fernandez-Fernandez B. Combination use of medicines from two classes of renin–angiotensin system blocking agents: risk of hyperkalemia, hypotension, and impaired renal function. Ther Adv Drug Saf. 2015;6(4):166–176. | ||
Asher GN, Viera AJ, Weaver MA, Dominik R, Caughey M, Hinderliter AL. Effect of hawthorn standardized extract on flow mediated dilation in prehypertensive and mildly hypertensive adults: a randomized, controlled cross-over trial. BMC Complement Altern Med. 2012;12:26. | ||
Yarnell EL. Botanical medicines used for kidney disease in the United States. Iran J Kidney Dis. 2012;6(6):407–418. | ||
Kaur N, Chugh V, Gupta AK. Essential fatty acids as functional components of foods-a review. J Food Sci Technol. 2014;51(10):2289–2303. | ||
Jain AP, Aggarwal KK, Zhang PY. Omega-3 fatty acids and cardiovascular disease. Eur Rev Med Pharmacol Sci. 2015;19(3):441–445. | ||
Ellulu MS, Khaza’ai H, Abed Y, Rahmat A, Ismail P, Ranneh Y. Role of fish oil in human health and possible mechanism to reduce the inflammation. Inflammopharmacology. 2015;23(2–3):79–89. | ||
Alexander RW. Hypertension and the pathogenesis of atherosclerosis. Oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension. 1995;25:155–161. | ||
Altman R, Scazziota A, Rouvier J, et al. Coagulation and fibrinolytic parameters in patients with pulmonary hypertension. Clin Cardiol. 1996;19(7):549–554. | ||
Yanagisawa Y, Chatake T, Chiba-Kamoshida K, et al. Purification, crystallization and preliminary X-ray diffraction experiment of nattokinase from Bacillus subtilis natto. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010;66(12):1670–1673. | ||
Fujita M, Nomura K, Hong K, Ito Y, Asada A, Nishimuro S. Purification and characterization of a strong fibrinolytic enzyme (nattokinase) in the vegetable cheese natto, a popular soybean fermented food in Japan. Biochem Biophys Res Commun. 1993;197(3):1340–1347. | ||
Sumi H, Hamada H, Tsushima H, Mihara H, Muraki H. A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto; a typical and popular soybean food in the Japanese diet. Experientia. 1987;43(10):1110–1111. | ||
Xu J, Du M, Yang X, Chen Q, Chen H, Lin DH. Thrombolytic effects in vivo of nattokinase in a carrageenan-induced rat model of thrombosis. Acta Haematol. 2014;132(2):247–253. | ||
Kurosawa Y, Nirengi S, Homma T, et al. A single-dose of oral nattokinase potentiates thrombolysis and anti-coagulation profiles. Sci Rep. 2015;5:11601. | ||
Jang JY, Kim TS, Cai J, et al. Nattokinase improves blood flow by inhibiting platelet aggregation and thrombus formation. Lab Anim Res. 2013;29(4):221–225. | ||
Berkner KL. Vitamin K-dependent carboxylation. Vitam Horm. 2008;78:131–156. | ||
Fujita M, Hong K, Ito Y, et al. Transport of nattokinase across the rat intestinal tract. Biol Pharm Bull. 1995;18(9):1194–1196. | ||
Ero MP, Ng CM, Mihailovski T, Harvey NR, Lewis BH. A pilot study on the serum pharmacokinetics of nattokinase in humans following a single, oral, daily dose. Altern Ther Health Med. 2013;19(3):16–19. | ||
Murakami K, Yamanaka N, Ohnishi K, Fukayama M, Yoshino M. Inhibition of angiotensin I converting enzyme by subtilisin NAT (nattokinase) in natto, a Japanese traditional fermented food. Food Funct. 2012;3(6):674–678. | ||
Kim YK, Kim SM, Kim JY, Kwon O. The culture filtrates from Bacillus subtilis natto lowers blood pressure via renin–angiotensin system in spontaneously hypertensive rats fed with a high-cholesterol diet. J Korean Soc Appl Bio Chem. 2011;54(6):959–965. | ||
Sumi H, Hamada H, Nakanishi K, Hiratani H. Enhancement of the fibrinolytic activity in plasma by oral administration of nattokinase. Acta Haematol. 1990;84(3):139–143. | ||
Fujita M, Hong K, Ito Y, Fujii R, Kariya K, Nishimuro S. Thrombolytic effect of nattokinase on a chemically induced thrombosis model in rat. Biol Pharm Bull. 1995;18(10):1387–1391. | ||
Kim JY, Gum SN, Paik JK, et al. Effects of nattokinase on blood pressure: a randomized controlled trial. Hypertens Res. 2008;31:1583–1588. | ||
Ueda T, Kawakami R, Nishida T, et al. Plasma renin activity is a strong and independent prognostic indicator in patients with acute decompensated heart failure treated with renin–angiotensin system inhibitors. Circ J. 2015;79(6):1307–13014. | ||
Haymond S, Clarke NJ, Reitz RE, McPhaul MJ, Wu Z. Plasma renin activity: the importance of correct sample type. Clin Chem. 2016;62(2):408–409. | ||
Baker HN, Murphy R, Lopez E, Garcia C. Conversion of a capture ELISA to a Luminex xMAP assay using a multiplex antibody screening method. J Vis Exp. 2012;65:4084. | ||
Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. | ||
Esler M, Zweifler A, Randall O, Julius S, DeQuattro V. The determinants of plasma-renin activity in essential hypertension. Ann Intern Med. 1978;88:746–752. | ||
Resnick LM, Muller FB, Laragh JH. Calcium-regulating hormones in essential hypertension: relation to plasma renin activity and sodium metabolism. Ann Intern Med. 1986;105(5):649–654. | ||
Kim JM, Kim TH, Lee HH, Lee SH, Wang T. Postmenopausal hypertension and sodium sensitivity. J Menopausal Med. 2014;20(1):1–6. | ||
Felmeden DC, Lip GYH. The renin–angiotensin–aldosterone system and fibrinolysis. J Renin Angiotensin Aldosterone Syst. 2000;1(3):240–244. | ||
Bongers TN, de Maat MP, van Goor ML, et al. High von Willebrand factor levels increase the risk of first ischemic stroke: influence of ADAMTS13, inflammation, and genetic variability. Stroke. 2006;37(11):2672–2677. | ||
Wieberdink RG, van Schie MC, Koudstaal PJ, et al. High von Willebrand factor levels increase the risk of stroke: the Rotterdam study. Stroke. 2010;41(10):2151–2156. | ||
Jenkins PV, O’Donnell JS. ABO blood group determines plasma von Willebrand factor levels: a biologic function after all? Transfusion. 2006;46:1836–1844. | ||
Jennewein C, Tran N, Paulus P, Ellinghaus P, Eble JA, Zacharowski K. Novel aspects of fibrin(ogen) fragments during inflammation. Mol Med. 2011;17(5–6):568–573. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.