Back to Journals » Clinical Interventions in Aging » Volume 18
Construction and Effect of the Three-Level and Two-Stage Screening Mode for Age-Related Hearing Loss: A Study Based on the Community in Shanghai, China
Authors Ge J, Geng S, Gao Y, Ren G, Sun X, Jiang H
Received 21 June 2023
Accepted for publication 7 August 2023
Published 10 August 2023 Volume 2023:18 Pages 1309—1320
DOI https://doi.org/10.2147/CIA.S423822
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Jianli Ge,1,* Shasha Geng,1,* Yang Gao,2 Guangwei Ren,3 Xiaoming Sun,1,4 Hua Jiang1
1Department of General Practice, Shanghai East Hospital, Tongji University School of Medicine, Shanghai, 200120, People’s Republic of China; 2Department of General Practice, Huamu Community Health Service Center, Shanghai, 201204, People’s Republic of China; 3Science and Education Department, Sanlin Community Health Service Center, Shanghai, 200124, People’s Republic of China; 4Department of General Practice, Zhongshan Hospital, Fudan University Medical School, Shanghai, 200032, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaoming Sun, Fudan University Medical School, Zhongshan Hospital, Fudan University, Shanghai, 200032, People’s Republic of China, Email [email protected] Hua Jiang, Department of General Practice, Shanghai East Hospital, Tongji University School of Medicine, Shanghai, 200120, People’s Republic of China, Email [email protected]
Background: There is a high incidence rate of age-related hearing loss. Severe hearing loss may increase the prevalence of mental illness, cognitive impairment, and even the risk of all-cause death.
Purpose: Construction of the three-level and two-stage screening mode for age-related hearing loss of the community and to evaluate its effectiveness.
Materials and Methods: A total of 401 participants (aged 60 years or older) from five typical communities were enrolled in the study. The risk factors assessment of age-related hearing loss was completed by using a cross-sectional survey and receiver operating characteristic (ROC) curve. Multiple screening method was adopted and verified by serial and parallel tests, respectively. Based on research data, incorporate risk factors assessment, the Hearing Handicap Inventory for the Elderly Screening Version (HHIE-s) and pure tone audiometry (PTA) were used to construct the screening mode.
Results: Multiple screening series testing and multiple screening parallel testing, including risk factors assessment, HHIE-s, and PTA, were used for verification: the sensitivity, specificity, and Kappa index were 70.5% and 9.2%, 95.0% and 71.6%, 0.26 and 0.63, respectively. Finally, the three-level and two-stage screening mode for age-related hearing loss was established. “Three-level” was defined as the risk factors assessment/HHIE-s (high-risk population), PTA (suspect population), and comprehensive hearing loss assessment (confirmed population). “Two-stage” was defined as the population screening by general practitioner in the community and target screening by otolaryngologist of the tertiary hospitals.
Conclusion: The three-level and two-stage screening mode for age-related hearing loss consists of the following framework: from population screening to target screening, from suspicious diagnosis to accurate diagnosis, from primary health care to tertiary hospitals. The study objective is to structure a new secondary prevention and treatment mode for age-related hearing loss with primary health care as the core, so as to help the front-end management of healthy aging.
Keywords: age-related hearing loss, three-level and two-stage mode, screening, general practitioners, self-management
Introduction
Definition of Age-Related Hearing Loss
Age-related hearing loss is known as presbycusis, whose definition is hearing loss caused by aging and degeneration of auditory organs in the older adults. The prevalence of age-related hearing loss is high; recent studies estimate that roughly 33% of the persons over the age of 50 years, 45% of the persons over the age of 60 years1 and 63.1% of the persons over the age of 70 years have been affected.2 As the population ages, hearing loss is expected to continue to rise in prevalence.3 It is the third chronic disease in elderly persons behind arthritis and hypertension in the world. The pathogenesis of age-related hearing loss results from the interaction of multiple physiological mechanisms.4–6
In the early stages of onset, the patient exhibits binaural symmetrical high-frequency hearing loss, often accompanied by tinnitus and a decline in auditory recognition ability.
However, the early subjective sensory speech recognition ability is sufficient for daily communication, so the symptoms are hidden and easily ignored. Mild hearing loss reduces the living ability of older adults, while a severe hearing loss could lead to mental illnesses, cognitive impairment, and the risk of all-cause death.4,7–9 Age-related hearing loss is an important public health issue currently incurable; therefore, early detection and diagnosis are essential.
Screening of Age-Related Hearing Loss
According to 2013 data from the Centers for Disease Control and Prevention (CDC) of the United States (US), 97% of the newborns were screened for hearing. By 2021, the rate of hearing screening of newborns has reached more than 60% in China and 100% in Shanghai. However, at present, screening for age-related hearing loss is still in its early stages, and there are no unified data on the screening rate.10,11
Current methods for detecting hearing loss include scale method, subjective testing, and objective testing, such as the Hearing Handicap Inventory for the Elderly adults (HHIE), screening for otologic functional impairments (SOFI), pure tone audiometry (PTA), the subjective faces scale, the whispering experiment, the speech audiometry, otoacoustic emission, etc.12–14 But the US Preventive Services Task Force’s updated evidence report and systematic review demonstrated that although some screening tools of hearing loss have been developed, none of them have sufficient sensitivity, specificity, and positive and negative predictive values.13,15 The screening tool of hearing loss should be weighed according to sensitivity, specificity, technology cost, per capita labor cost and ease of operation for effective screening and referral. Currently, there is no convenient screening method or management model that can be widely promoted.
The Role of Primary Health Care in Screening of Age-Related Hearing Loss
In May 2017, the 70th World Health Assembly adopted the resolution on the Prevention of Deafness and Hearing Loss, requiring ear and hearing care to be included in the primary healthcare framework under universal health coverage.16 The core issues are the active implementation and standardization of deaf prevention as part of health education in grassroots communities, the establishment of ear and hearing health records of community residents, and better screening, diagnosis, referral, follow-up and treatment of ear diseases and hearing loss to reduce the incidence of preventable hearing impairment.
In developing countries, less than 1% of hearing loss patients receive hearing aid treatment compared to 10–40% in developed countries. Given the advancement in hearing loss treatment, the rate of acceptance and usage has not increased significantly in the past 50 years.17 More than 95% of individuals who could benefit from hearing aids do not use them.18 Regarding hearing impairment, the older adults have very low cognition and willingness to seek assistance. Consequently, otolaryngologists are unable to contact undiagnosed and untreated patients timely. Hearing loss screening in the older adults is enormously important but receives little attention. General practitioners may be the first to encounter elderly people at risk of hearing loss, and they can play an important role in early identification and management of hearing loss.19,20
Some simple screening testing, such as self-assessment: “Do you believe you have hearing loss?”, “finger-snapping” and “whispering” can initially identify patients with hearing loss.13,17 However, it is rarely used in clinical settings. General practitioners have limited knowledge and comprehension of age-related hearing loss, including the optimal screening, consultation, and referral procedures, and may focus solely on major diseases such as diabetes and coronary heart disease due to time constraints.21 Despite the availability of treatment methods, 50% of the general practitioners have a negative attitude toward diagnosing and treating age-related hearing loss.16,22 Typically, older adults are unaware of hearing loss or reluctant to reveal it, but if inquired by the general practitioner, patients are more likely to follow their treatment recommendations.23,24 Therefore, research is needed to evaluate the effect management mode of hearing loss screening in the older adults.
The prevention and treatment of noninfectious chronic diseases (NCDs), which adopted a mode of early screening, prevention, and intervention, had achieved certain results General practitioners are the leaders in the management of NCDs among the community population, who are suitable for implementing technologies that can reduce medical expenses and health system costs. Age-related hearing loss is related to factors such as personal behavior and illness, and can refer to screening and management methods for NCDs.25
Materials and Methods
Participants
Adults aged 60 years or older from five typical communities with populations of approximately 100,000, three from urban, one from urban-rural fringe, one from suburban, in Pudong New Area, Shanghai were recruited. A total of 401 participants with no history of hearing loss or audiological rehabilitation experience by self-report were finally recruited (Figure 1).
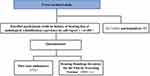 |
Figure 1 Cross-sectional study of age-related hearing loss of the older adults in community. |
Questionnaire
The study selected databases (MEDLINE, PubMed, Embase, and Web of Science) and used searches “presbycusis” (ALL Fields) OR (“age” [ALL Fields] and heating loss [ALL Fields] AND “screening” (ALL Fields)). The study invited ENT experts to supplement and adjust the search content, and jointly developed a questionnaire.
The questionnaire consisted of three parts: 1) general information: age, gender, nationality, work experience, marital status, educational background, whether living alone, exercise habits, eating habits, smoking history, drinking history, body mass index (BMI), family history of hearing loss, and headset wearing habits. 2) History of disease: hypertension, diabetes, hyperlipidemia, hyperuricemia, cardiovascular diseases, hypothyroidism, and chronic otitis media. 3) Hearing Handicap Inventory for the Elderly Screening Version (HHIE-s): 10 scale items involving five emotional and five situational problems. Three options were available for each item, “Yes”, “Sometimes”, “Never” and the corresponding scores were 4, 2, and 0. The total score on the scale was 0–40. According to the American Speech-language-Hearing Association, 0–8 points indicate no obvious hearing loss and 10–40 points denote hearing loss.5
Audiometric Assessment
Pure tone audiometric (PTA) testing was administered in a quiet consulting room with background noise controlled within 40 dB.Prior to the audiometric assessment, the ears were examined for wax or abnormalities. The air conduction thresholds were obtained at 0.5–4 kHz over an intensity range of −10 to 120 dB using a MADSEN audiometer.
Each individual’s hearing thresholds were annotated as dB/ HL at each of four frequencies (500 Hz, 1 kHz, 2 kHz and 4 kHz). The average of 0.5kHz, 1kHz, 2kHz, and 4kHz pure tone air conduction hearing thresholds of good ears were used as the classification criteria for hearing: ≤25 dB/HL is normal, 26–40dB/HL is mild hearing loss, 41–55dB/HL is moderate hearing loss, 56–70dB/HL is moderately severe hearing loss, 71–90 dB/HL is severe hearing loss and ≥91 dB/HL is profound hearing loss.5,26
Construction of Risk Factors Assessment System
Obtained risk factors for age-related hearing loss based on cross-sectional data and logistic regression analysis. Using conditional logic equations, assign values of 1 or 0 to participants based on whether they have risk factors, and obtain the cumulative scores.
The best cut-off value of the cumulative risk factors scores to predict the risk of age-related hearing loss was obtained by using the subject operating characteristic (ROC) curve. So that the older adults would be divided into low-risk group and high-risk group.
The Referral Management Platform for Age-Related Hearing Loss
The study group had completed the construction of the “1+10+100” mode of local medical consortium in the early stage and achieved a seamless connection between primary healthcare and specialist through hierarchical diagnosis and treatment, two-way referral, and dynamic flow.27 Based on the “1+10+100” mode (Figure 2), the referral management platform for age-related hearing loss could be implemented.
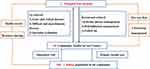 |
Figure 2 “1+10+100” mode of local medical consortium. Notes: 1: One comprehensive hospital. 10: Ten Community Health Service Centers. 100: One million population in the community. |
Screening Mode of Age-Related Hearing Loss
Using two or more screening methods to examine the same subject can improve the sensitivity and specificity, increase screening benefits, including series testing and parallel testing. Series testing consists of a sequence testing that can be determined as positive once a positive result occurs. For parallel testing, if any one result of testing is positive, parallel testing would be considered positive. The study used three methods, risk factors assessment, HHIE-S and PTA, by multiple screening series testing and multiple screening parallel testing to evaluate the screening effects.
Statistical Analysis
EpiData 3.0 was used for data input, and SPSS 25.0 software was used for statistical analysis. For measurement data, the normal distribution was represented by x ± s, and the abnormal distribution was represented by M (P25, P75). The two samples of normal distribution data were compared by t-testing. The Mann–Whitney U-test was carried out to compare the two samples with abnormal distribution data, and the Kruskal–Wallis testing was done for the multiple samples. The classified data were expressed by frequency and rate. The chi-square testing was employed to compare the unordered categorical variables, and the rank sum testing was utilized to compare the ordered categorical variables. Pearson, Spearman, and logistic regression analyses were used to analyze the related factors. P < 0.05 meant the difference was statistically significant.
Results
Risk Factors Assessment for Age-Related Hearing Loss
Logistic Regression Analysis
A total of 401 older adults were enrolled in the cross-sectional study, including 182 (38.4%) males, 219 (54.6%) females, average age (71.0 ± 6.1) years, overweight/obese 181 (45.1%), living alone 30 (7.5%), divorced/widowed 42 (10.5%), education level of primary school and below 66 (17.5%), noise history 45 (11.2%), family history of hearing loss 10 (2.5%), non-light diet 112 (27.9%), non-exercise habits 192 (48.1%), smoking 71 (17.7%), drinking 41 (10.2%), headset wearing habits 29 (7.2%), chronic otitis media 1 (0.2%), hypertension 258 (64.3%), diabetes 123 (30.7%), hyperlipidemia 155 (38.7%), cardiovascular diseases 98 (24.4%), hypothyroidism 37 (9.2%), hyperuricemia 68 (17.0%), and history of ototoxic drugs 5 (1.2%). The prevalence of hearing loss in males was 84.9%, while in females was 78.8%, with no statistically significant difference (χ2=2.691, P=0.101). The constituent ratios of mild, moderate, moderately severe, severe, and profound hearing loss were 48.5%, 23.4%, 7.8%, 1.3% and 0.7%, respectively.
Taking the hearing loss of the older adults as the dependent variable, 22 variables (gender, age, BMI, overweight/obesity, living alone, divorce/widowhood, education level of primary school and below, noise history, family history of deafness, non-light diet, non-exercise habits, smoking, drinking, history of wearing headphones, chronic otitis media, hypertension, diabetes, hyperlipidemia, cardiovascular diseases, hyperuricemia, hypothyroidism, history of ototoxic drugs) were used as independent variables for binary logistic regression analysis. The gender variable was assigned as female = 1 and male = 2; age and BMI were continuous variables; other independent and dependent variables were allocated as none = 0 and yes = 1. The 15 variables with P < 0.2 in the single-factor logistic regression analysis were included in the multi-factor logistic regression analysis. The results found that the age [OR = 1.100, 95% CI (1.037, 1.166)], noise history [OR = 3.886, 95% CI (1.077, 14.022)], non-light diet [OR = 2.445, 95% CI (1.127, 5.305)], hypertension [OR = 1.839, 95% CI (1.015, 3.330)], diabetes [OR = 4.310, 95% CI (1.817, 10.225)] and hyperuricemia [OR = 3.174, 95% CI (1.030, 9.779)] were independent risk factors for age-related hearing loss (P < 0.05) (Tables 1–2).
 |
Table 1 Univariate Logistic Regression Mode of Influencing Factors on Age-Related Hearing Loss |
 |
Table 2 Multivariate Logistic Regression Mode of Influencing Factors on Age-Related Hearing Loss |
Variables as dietary habits, hypertension, diabetes, hyperlipidemia, and noise history were included in the risk factor system. Variable age interacted with hypertension, diabetes, hyperlipidemia, cardiovascular disease, hypothyroidism, hyperuricemia, cardiovascular disease, so hypothyroidism and hyperuricemia were included in the system. The univariate analysis tipped the overweight/obesity, living alone, divorce/widowhood, non-exercise habit, family history of deafness, smoking history, drinking history, history of wearing headphones, history of ototoxic drug was with statistical significance. In combination with the mention rate from literature, the above variables were included in the system. Multiple population surveys suggested that the incidence of hearing loss in males was higher than in females. In this study, there was sex difference in the incidence rate, with no statistical significance. Considering possible inclusion bias, adopted literature14,28–30 and expert opinions, sex was included in the system. Participants of the study were older adults, so age was not included in the system. Finally, 18 risk factors were included.
ROC Curve Analysis
The study used the ROC curve to obtain the threshold for diagnosing age-related hearing loss based on the cumulative scores of risk factors. The area under the ROC curve (AUC) was 0.777 (95% CI 0.721–0.833), the maximum Youden index was 0.534, and the cut-off value was 3.5. The sensitivity and specificity of risk factor assessment for hearing loss were 70.9% and 75.3%, respectively. The threshold value of risk stratification was rounded to 4, and the older adults were divided into two groups based on hearing loss: low-risk (<4) and high-risk (≥4).
Construction of Screening Mode of Age-Relate Hearing Loss
Construction of Screening Mode
Among 401 older adults, the results of a single screening method exhibited that 320 were positive by PTA, 247 by risk factors assessment, and 232 by HHIE-s. Moreover, 161 (39.9%) with positive results were by multiple screening in series testing, and 318 (73.6%) with positive results were by multiple screening in parallel testing. Multiple screening in parallel testing yielded similar results from PTA, but the composition ratio of mild to moderate hearing loss was relatively higher (Table 3).
 |
Table 3 Result of Screening for Age-Related Hearing Loss |
With PTA as the golden indicator, the single and the multiple screening methods were evaluated by three indicators of authenticity, reliability, and positive/negative predictive value. The sensitivity of the multiple screening parallel testing was 21.3%, 19.6% higher than those of the single screening method (risk factor assessment) and single screening method (HHIE-s); meanwhile, the positive predictive rate increased by 0.9% and 18.3%. The multiple screening parallel method had the same specificity as single screening (risk factors assessment); the Kappa index was 0.63, reaching a medium–high consistency (Tables 4–5).
 |
Table 4 Valuation of Single Screening Method |
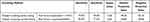 |
Table 5 Valuation of Multiple Screening Method |
Based on the above data, the framework of the screening mode, the content of the screening project, and the implementation strategy were summarized and analyzed. Then, the prototype of three-level and two-stage screening mode for age-related hearing loss was constructed. The mode framework was as follows: 1) Three-level was the hierarchical assessment of risk factors assessment/HHIE-s (high-risk population), PTA (suspect population), and comprehensive assessment (confirmed population). 2) Two-stage was population screening (initial diagnosis and hierarchy of hearing loss) and target screening (qualitative and comprehensive assessment of hearing loss) (Figure 3).
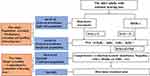 |
Figure 3 Three-level and two-stage screening mode for age-related hearing loss. |
Evaluation of Screening Mode
Among 401 older adults, 29 (11.7%) participants from the high-risk group of stratified risk factors received referrals; 37 (16.2%) participants with positive results of HHIE-s received referrals; 28 (17.5%) participants with positive results of multiple screening series testing received referrals; 38 (13.0%) participants with positive results of multiple screening parallel testing received referrals.
Compared with the referral rate 11.8% of the participants with positive results of PTA, the above methods increased the referral rates by 0.9%, 4.4%, 5.7%, 5.70%, and 1.2%, respectively. Therefore, through multiple screening methods, participants’ understanding of the disease would be improved and their compliance with treatment would be improved (Table 6).
 |
Table 6 The Referral Rate of the Population with Positive Screening Results |
Discussion
Risk Factors Assessment System for Age-Related Hearing Loss Provides Evidence-Based Medicine Basis for Constructing the Screening Mode of Hearing Loss
The pathogenesis of age-related hearing loss is pluralism,6 resulting from multiple physiological mechanisms related to mitochondrial DNA mutation, glutamate over-secretion, changes in immune reactants, free radical damage, and changes in temporal bone tissue.31 A cross-sectional study of 164,770 adults aged 40–69 in the United Kingdom (UK) found that smoking and passive smoking were associated with increased hearing loss.32 A European multicenter study involving 4083 subjects aged 53–67 demonstrated that high BMI was associated with hearing loss.33 A hearing impairment study of 16,000 Korean adults suggested that individuals with cardiovascular and cerebrovascular risk factors (hypertension, diabetes, smoking, and elevated serum cholesterol) were at risk of hearing impairment.33
This study adopted PTA as the gold standard. Univariate logistic regression analysis showed age, BMI, overweight/obesity, divorce/widowhood, noise history, non-light diet, non-exercise habits, hypertension, diabetes, hyperlipidemia, cardiovascular diseases, hyperuricemia, and hypothyroidism were the influencing factors. Multivariate logistic regression analysis disclosed that age, noise history, non-light diet, hypertension, diabetes, and hyperuricemia were independent risk factors.
Regarding education level, male, family history of deafness, smoking, drinking, and history of wearing headphones, chronic otitis media, and ototoxic drug use mentioned in other literature, there was no statistical significance in our study. The reasons were as follows: 1) the study used convenient sampling, and the included population had intention bias. 2) There was no statistically significant effect with gender, smoking and drinking; the reason might be the proportion of included men and women (182/219). 3) The actual number of cases with a history of wearing a headset, chronic otitis media, and ototoxic drugs were too small, leading to deviation in statistical results.
Among the risk factors, there were 13 (72.2%) factors (non-light diet, hypertension, diabetes, hyperlipidemia, cardiovascular diseases, hyperuricemia, hypothyroidism, overweight, non-exercise habits, smoking, drinking, and history of wearing earphones, and ototoxic drugs) that were preventable, controllable, delayed, and improved risk factors. Compared with chronic diseases such as diabetes and hypertension, age-related hearing loss had a high prevalence rate, resulting in adverse events and a large disease burden. Although it cannot be accurately prevented at the primary prevention, it can realize secondary prevention, that is, early detection, early diagnosis, and early intervention.
Predicting Age-Related Hearing Loss Using Cumulative Scores of Risk Factors Based on ROC Curves
The receiver operating characteristic curve (ROC curve) is also known as the sensitivity curve. The ROC curve is a curve drawn based on a series of different binary classification methods (boundary values or determination thresholds), with true positive rate (sensitivity) as the vertical axis and false-positive rate (1-specificity) as the horizontal axis. The area value under the ROC curve is between 1.0 and 0.5. When AUC>0.5, the closer AUC is to 1, the better the diagnostic effect. AUC has low accuracy at 0.5–0.7, certain accuracy at 0.7–0.9, and high accuracy above 0.9. When AUC=0.5, it indicates that the diagnostic method is completely ineffective and has no diagnostic value. The area under the ROC curve (AUC) in the study was 0.777 (95% CI 0.721–0.833), which basically met the diagnostic evaluation requirements. The sensitivity and specificity of the assessment of risk factors for hearing loss were 70.9% and 75.3%, respectively. The threshold for risk stratification was determined to be 4, and older adults would be divided into two groups: low risk (<4), high risk (≥ 4). This method has been proven to be practical in research. It was also the first time to be applied to the screening of hearing loss and fully embodying the perspective of holistic management of disease in general practice medicine.
Community-Based Three-Level and Two-Stage Screening Mode for Age-Related Hearing Loss is Appropriate and Effective
Screening refers to the application of rapid and simple methods to screen out high-risk patients from healthy people for further diagnosis and treatment. The three-level and two-stage screening mode for age-related hearing loss is a selective, active and therapeutic screening method for the older adults; the construction connotation is as follows: the risk factors assessment/HHIE-s (screening out high-risk population), the PTA (screening out suspect population); the population screening (performed by a general practitioner in the community) and target screening (performed by otorhinolaryngology specialty of the tertiary hospitals), the results of the preliminary screening showed that the proportion of mild and moderate hearing loss was relatively higher, indicating the possibility of early warning hearing loss. All these conformed to the fundamental principles of early screening and early diagnosis.
The method to improve screening efficiency are as follows: 1) screening in high-risk groups, namely selective screening, improving the positive prediction rate and screening benefit. 2) Selecting a testing with high sensitivity and utilizing two or more screening methods to examine the same subject can improve sensitivity, specificity, and benefit, including using series and parallel testing. The series testing is conducted in multiple testing sequences. Once a negative result occurs, it can be determined as negative. It has the benefit of increased specificity and decreased rate of misdiagnosis but decreased sensitivity and increased rate of missed diagnosis. If any one result is positive among all testing sequences, it is regarded as positive in parallel testing. It has the advantage of increased sensitivity and decreased missed diagnoses. The disadvantages are reduced specificity and increased misdiagnosis. The three-level and two-stage screening mode for hearing loss constructed in this study was validated by serial testing and parallel testing, respectively. The sensitivity, specificity and Kappa index of the former were 70.5%, 95.0% and 0.26, and the sensitivity, specificity and Kappa index of the latter were 92.2%, 71.6% and 0.63, respectively. The results indicated that multiple screening parallel testing could obtain excellent screening sensitivity, consistency, and specificity. The evaluation of screening mode tips that the mode has enforceability, accessibility, and convenience.
Through multiple screening, it is expected to improve the awareness of hearing loss and medical compliance in the older adults, to realize the management of hearing loss starts with active self-health intervention.
The Management Mode of Age-Related Hearing Loss Based on General Practitioners is Relatively Underdeveloped
Age-related hearing loss is called an “imperceptible disability”. The implementation of screening for age-related hearing loss has been proven to improve the detection rate7 and the acceptance rate of hearing loss compensation treatment (including wearing hearing aids).34 A Dutch hearing screening program found that 57% of general practice outpatient patients were screened for hearing loss.35 The Australian government encourages the older adults (≥75 years) to receive routine hearing assessments through medical insurance funding and added relevant content to their health examination programs.36 Therefore, early detection and referral of high-risk groups/patients by general practitioners is one of the key steps of hearing loss screening, but there is no active and effective management mode.
As mentioned by experts, general practitioners are responsible for the prevention of common and chronic diseases, medical treatment, health care, rehabilitation, health education and other comprehensive services of primary health care. At the same time, screening, and management of hearing loss for the older adults are also their responsibilities. Based on the management mode of chronic diseases, a screening mode of hearing loss for the older adults led by general practitioners was constructed. Through stratified screening, we can find high-risk groups from large populations and suspicious groups from high-risk groups to achieve secondary prevention of age-related hearing loss.
Conclusions
Age-related hearing loss is related to many behavioral factors and chronic diseases. As the disease progresses, it will cause great harm to health and is relatively irreversible and unable to resolve itself. The three-level and two-stage screening mode for hearing loss has high efficiency and enforceability, relying on a “1+10+100” medical consortium: from population screening to target screening, suspect to diagnosis, and primary healthcare to the tertiary hospitals. The proportion of mild and moderate hearing loss found in screening was relatively higher, and the proportion of patients receiving referrals was also higher, indicating an age-related hearing loss can be screened, intervened, and delayed. The establishment of a new mode of prevention and control of age-related hearing loss, with the general practitioners as the main body, had realized the conceptual change from “specialist passive waiting for patients” to “general practitioners actively screening suspected patients”, which is conducive to the front-end management of healthy aging. Through empirical study, from large population to high-risk population to suspected population, from simple preliminary screening to accurate diagnosis, the study provides an accessible, appropriate, and popularized mode. Through the screening mode, it is expected to increase the awareness rate, treatment rate and control rate of hearing loss in the older adult, which is conducive to the front-end management of healthy aging.
Strengths and Limitations
The study realized the conceptual transformation from “specialist waiting for patients” to “general practitioner actively screening suspected patients”. From large populations to high-risk populations to suspect populations, from simple preliminary screening to accurate diagnosis, a new mode of secondary prevention, management, and control of age-related hearing loss has been established.
The subjects of this study are older adults from the community who participate in annual physical examinations, so they have higher initiative and enthusiasm for self-health management. The participants of the study represented the characteristics of the older adults in the communities of Shanghai, while the characteristics of them in other provinces and cities might be differences. Therefore, there may be partial selection bias in the data. It is necessary to expand geographical selection and increase sample size to further verify the applicability of the screening model. The implementation of the screening mode could be affected by many factors, such as the general practitioner’s quality, performance allocation, and health management ability. The further research objective is to construct a prospective cohort of older adults from different characteristic communities and verify effectiveness and improve mode management.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article. All authors agree to the data sharing.
Ethics Approval and Consent to Participate
Ethics approval and consent to participate Ethics approval by the Academic Ethics Committee of Shanghai East Hospital (wf2021094) is acquired before the current study, which does not involve any ethical issue. All participants provided informed consent before being included in this research. The submission was performed in accordance with the Declaration of Helsinki.
Consent for Publication
Written informed consent for publication was obtained from all participants.
Acknowledgments
Jianli Ge and Shasha Geng are co-first authors for this study. The authors express thanks to Professor Jirong Duan from Hearing Clinic, Punan Hospital, Shanghai. The abstract of this paper was presented at “WONCA 2023, WONCA Sydney World Conference” and will be as an abstract presentation with interim findings in aging forum.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Scientific research project of Shanghai Health and Family Planning Commission (201940235), General Practice Medical Education and Teaching Research Project (B-YXGP20210301-03), and School Level Education Research Project of Nanjing Medical University (2021zc087).
Disclosure
The authors report no competing interests in this work.
References
1. Goman AM, Lin FR. Prevalence of hearing loss by severity in the United States. Am J Public Health. 2016;106(10):e1. doi:10.2105/AJPH.2016.303299
2. Lin FR, Thorpe R, Gordonsalant S, Ferrucci L. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol. 2011;66(5):582. doi:10.1093/gerona/glr002
3. Erwin DZ, Chen P. Hearing loss in the elderly. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. PMID: 35593873.
4. Nieman CL, Oh ES. Hearing Loss. Ann Intern Med. 2020;173(11):ITC81–ITC96. doi:10.7326/AITC202012010
5. Shahidipour Z, Geshani A, Khosravifard E, et al. Auditory memory deficit in elderly people with hearing loss. Iran J Otorhinolaryngol. 2013;25(72):169–176.
6. Tavanai E, Mohammadkhani G. Role of antioxidants in prevention of age-related hearing loss: a review of literature. Eur Arch Otorhinolaryngol. 2017;274(4):1821–1834. doi:10.1007/s00405-016-4378-6
7. Wallhagen MI, Strawbridge WJ. Hearing loss education for older adults in primary care clinics: benefits of a Concise Educational Brochure. Geriatr Nurs. 2017;38(6):527–530. doi:10.1016/j.gerinurse.2017.03.015
8. Louw C, Swanepoel W, Eikelboom RH. Self-reported hearing loss and pure tone audiometry for screening in primary health care clinics. J Prim Care Community Health. 2018;90:1–8.
9. Griffithst D, Lad M, Kumar S, et al. How can hearing loss cause dementia? Neuron. 2020;108(3):401–412. doi:10.1016/j.neuron.2020.08.003
10. Castiglione A, Casa M, Gallo S, et A. Correspondence between cognitive and audiological evaluations among the elderly: a preliminary report of an audiological screening model of subjects at risk of cognitive decline with slight to moderate hearing loss. Front Neurosci. 2019;13:1279. doi:10.3389/fnins.2019.01279
11. Man J, Chen H, Zhang T, et al. Global, regional, and national burden of age-related hearing loss from 1990 to 2019. Aging. 2021;13(24):25944–25959. doi:10.18632/aging.203782
12. Tomioka K, Ikeda H, Hanaie K, et al. The Hearing handicap inventory for elderly-screening (HHIE-s) versus a single question: reliability, validity and relations with quality of life measures in the elderly community. Japan Qual Life Res. 2013;22(5):1151–1159. doi:10.1007/s11136-012-0235-2
13. Feltner C, Wallace IF, Kistler CE, et al. Screening for hearing loss in older adults: updated evidence reports and systematic review for the US preventive Services Task Force. JAMA. 2021;325(12):1202–1215. doi:10.1001/jama.2020.24855
14. Costa-Guarisco LP, Dalpubel D, Labanca L, et al. Perception of hearing loss: use of the subjective faces scale to screen hearing among the elderly. Cien Saude Colet. 2017;22(11):3579–3588. doi:10.1590/1413-812320172211.277872016
15. Labanca L, Guimarães FS, Costa-Guarisco LP, et al. Screening of hearing in elderly people: assessment of accuracy and reproducibility of the whispered voice test. Cien Saude Colet. 2017;22(11):3589–3598. doi:10.1590/1413-812320172211.31222016
16. World Health Organization. World Health Assembly Resolution on Prevention of Deafness and Hearing Loss. Geneva: Seventieth World Health Assembly, WHA; 2017.
17. Weinstein BE. Screening for otologic functional impairments in the elderly: whose job is it anyway? Audiol Res. 2011;1(E12):42–48. doi:10.4081/audiores.2011.e12
18. Dane J, Genther MD, Kevin D, et al. Association of hearing loss with hospitalization and burden of disease in older adults. JAMA. 2013;309(22):1–6.
19. Johnson CE, Danhauer JL, Koch LL, Celani KE, Lopez IP, Williams VA. Hearing and balance screening and referrals for Medicare patients: a national survey of primary care physicians. J Am Acad Audiol. 2008;19(2):171–190. doi:10.3766/jaaa.19.2.7
20. Schneider JM, Gopinath B, McMahon CM, et al. Role of general practitioners in managing age-related hearing loss. Med J Aust. 2010;192(1):20–23. doi:10.5694/j.1326-5377.2010.tb03395.x
21. Krist AH, Davidson KW, Mangione CM, et al. Screening for hearing loss in older adults: US preventive Services Task Force recommendation statement. JAMA. 2021;325(12):1196–1201. doi:10.1001/jama.2021.2566
22. Reed NS, Altan A, Deal JA, et al. Trends in health care costs and utilization associated with untreated hearing loss over 10 years. JAMA Otolaryngol Head Neck Surg. 2019;145(1):27–34. doi:10.1001/jamaoto.2018.2875
23. Wang Y, Mo LY, Li YG, et al. Analyzing use of the Chinese HHIE-s for hearing screening of elderly in a northeastern industrial area of China. Int Audiol. 2016;56(4):242–247. doi:10.1080/14992027.2016.1263399
24. Chang JE, Weinstein B, Chodosh J, et al. Hospital readmission risk for patients with self-reported hearing loss and communication trouble. J Am Geriatr Soc. 2018;66(11):2227–2228. doi:10.1111/jgs.15545
25. Hands S. Hearing loss in over-65s: is routine questionnaire screening worthwhile? J Laryngol Otol. 2000;114(9):661–666. doi:10.1258/0022215001906633
26. Clark JG. Uses and abuses of hearing loss classification. ASHA. 1981;23(7):493–500.
27. Ge J, Geng S, Jing L, Jiang H, Sun X. Exploration and practice of the “1+10+1100000” model. Int J Health Plan Manage. 2019;34(3):1065–1072. doi:10.1002/hpm.2875
28. Aziz A, Md Daud MK, Nik Othman NA, et al. Early detection of high-frequency presbycusis among normal hearing individuals. Otol Neurotol. 2020;41(8):e989–e992. doi:10.1097/MAO.0000000000002725
29. von Gablenz P, Holube I. Hearing loss and speech recognition in the elderly. Laryngorhinootologie. 2017;96(11):759–764. doi:10.1055/s-0043-119388
30. Chien C-Y, Tai S-Y, Wang L-F. Metabolic syndrome increases the risk of sudden sensorineural hearing loss in Taiwan: a case-control study. Otolaryngol Head Neck Surg. 2015;153(1):105–111. doi:10.1177/0194599815575713
31. Lis S, Ye H, Chen AT, et al. Characteristics of hearing loss in elderly outpatients over 60 years of age: an annual cross-sectional study. Acta Otolaryngol. 2021;141(8):762–767. doi:10.1080/00016489.2021.1912386
32. Bowlm R, Dawsions J. Age-related hearing loss. Cold Spring Harb Perspect Med. 2019;9(8):a033217. doi:10.1101/cshperspect.a033217
33. Hong JW, Jeon JH, Ku CR, et al. The prevalence and factors associated with hearing impairment in the Korean adults: the 2010–2012 Korea National Health and Nutrition Examination Survey (observational study). Medicine. 2015;94(10):e611. doi:10.1097/MD.0000000000000611
34. Louw C, Swanpoel DW, Eikelboomr H. Self-reported hearing loss and pure tone audiometry for screening in primary health care clinics. Prim Care Commun Health. 2018;9:2150132718803156.
35. Sprinzlg M, Riechelmann H. Current trends in treating hearing loss in elderly people: a review of the technology and treatment options - a mini-review. Gerontology. 2010;56(3):351–358. doi:10.1159/000275062
36. Ransen E F, Topsakal V, Hendrickxj J, et al. Occupational noise, smoking, and a high body mass index are risk factors for age-related hearing impairment and moderate alcohol consumption is protective: a European population-based multicenter study. J Assoc Res Otolaryngol. 2008;9(3):264–276. doi:10.1007/s10162-008-0123-1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
