Back to Journals » Clinical Ophthalmology » Volume 14
Conjunctival Melanoma: Update on Genetics, Epigenetics and Targeted Molecular and Immune-Based Therapies
Authors Gkiala A, Palioura S
Received 9 July 2020
Accepted for publication 17 September 2020
Published 9 October 2020 Volume 2020:14 Pages 3137—3152
DOI https://doi.org/10.2147/OPTH.S271569
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Anastasia Gkiala,1 Sotiria Palioura2
1National and Kapodistrian University of Athens School of Medicine, Athens, Greece; 2Athens Vision Eye Institute, Athens, Greece
Correspondence: Sotiria Palioura
Athens Vision Eye Institute, 328-330 Syngrou Ave., Kallithea, Athens 17673, Greece
Tel +30 698 580 2355
Email [email protected]
Purpose: To present the molecular mechanisms involved in the pathogenesis of conjunctival melanoma (CM) and review the existing literature on targeted molecular inhibitors as well as immune checkpoint inhibitors for the management of locally advanced and metastatic disease.
Methods: A comprehensive review of the literature was performed using the keywords “conjunctival melanoma”, “immune checkpoint inhibitors”, “BRAF inhibitors”, “MEK inhibitors”, “CTLA4 inhibitors”, “PD1 inhibitors”, “c-KIT mutations”, “BRAF mutations”, “NRAS mutations”, “dabrafenib”, “trametinib”, “vemurafenib”, “ipilimumab”, “pembrolizumab”, and “nivolumab”. A total of 250 articles were reviewed and 120 were included in this report.
Results: Mutations of mediators in the MAP kinase pathway, such as RAS, BRAF, MEK and ERK, and mutations of the PI3K/AKT/mTOR pathway play a major role in the pathogenesis of conjunctival melanoma. In addition, alterations of c-KIT, NF1, TERT, chemokine receptors as well as chromosomal copy number alterations and micro RNAs are thought to have a causative association with CM development. Targeted molecular inhibitors, such as BRAF and MEK inhibitors, are currently being implemented in the therapy of BRAF-mutated CM. Furthermore, immune checkpoint PD-1 and CTLA4 inhibitors with favorable clinical outcomes in the treatment of cutaneous melanoma have increased recurrence-free survival and reduced metastatic spread in CM cases.
Conclusion: The complex molecular mechanisms that contribute to the development of CM can be targeted both by molecular inhibitors of oncogenic pathways as well as immune checkpoint inhibitors in order to halt progression of the disease and increase survival.
Keywords: immune checkpoint inhibitors, BRAF inhibitors, MEK inhibitors, CTLA4 inhibitors, PD1 inhibitors, c-KIT mutations, BRAF mutations, NRAS mutations, dabrafenib, vemurafenib, trametinib, ipilimumab, pembrolizumab, nivolumab
Introduction
Conjunctival melanoma is a malignant tumor arising from melanocytes of the ocular surface. Although rare, it can be life threatening due to its metastatic potential. It accounts for 2% of all eye tumors, 5% of melanomas in the ocular region1 and 0.25% of melanomas overall.2 The incidence of conjunctival melanoma is rising over the last few decades and ranges between 0.24 and 0.9 cases per one million person-years.3–6 Conjunctival melanocytic lesions, such as primary acquired melanosis (PAM) and nevi are considered precursors of conjunctival melanoma.7–9 From a histopathology perspective, PAM can also be classified as hypermelanosis and conjunctival melanocytic intraepithelial neoplasia (C-MIN) with scores from 0 to 5 depending on the degree of atypia. PAM with mild atypia can also be referred to as C-MIN with a score of 1, PAM with moderate atypia as C-MIN with a score of 2 to 3, while PAM with severe atypia as C-MIN with a score of 4. C-MIN with a score of 0 is PAM without atypia or complexion-associated/benign acquired melanosis and C-MIN with a score of 5 is conjunctival melanoma in situ. These scores are based on evaluating the horizontal epithelial involvement, the vertical depth and the cellular atypia of the tumor cells.10,11 Pre-existing PAM lesions give rise to 57% to 76% of conjunctival melanomas,7,12 while 13% to 50% of them arise from PAM with severe atypia8 (Figure 1). On the contrary, PAM without atypia, complexion associated/benign acquired melanosis have no established link with the development of conjunctival melanoma.8 De novo development accounts for 15% to 25% of conjunctival melanomas, while pre-existent nevi are reported in 1–6% of cases.7,13
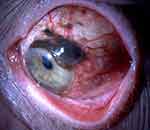 |
Figure 1 Conjunctival melanoma arising from a pre-existing PAM lesion. |
Until recently, the pathogenesis of conjunctival melanoma, as well as its propensity for local invasion and distant metastasis, have remained elusive. A number of molecular studies have now begun to shed light to the genetic and epigenetic alterations that give rise to conjunctival melanoma and may explain its metastatic potential. Moreover, new targeted molecular and immune-based therapies have improved the prognosis and survival of patients with locally invasive and metastatic conjunctival melanoma. This review focuses on the molecular mechanisms behind the pathogenesis of conjunctival melanoma and their increasing role as therapeutic targets in the management of this disease.
Methods
A PubMed search of all articles published in English from January 1995 to June 2020 on the genetics, epigenetics, and targeted therapies for conjunctival melanoma was performed. Searches included a combination of the following terms: “conjunctival melanoma”, “immune checkpoint inhibitors”, “BRAF inhibitors”, “MEK inhibitors”, “CTLA4 inhibitors”, “PD1 inhibitors”, “c-KIT mutations”, “BRAF mutations”, “NRAS mutations”, “dabrafenib”, “vemurafenib”, “trametinib”, “ipilimumab”, “pembrolizumab”, and “nivolumab”. We reviewed a total of 250 pertinent peer-reviewed publications and 120 are included in this review.
Results
Genetic and Epigenetic Changes in Conjunctival Melanoma
Similar to skin melanoma, the majority of conjunctival melanoma tumors harbor mutations in members of the mitogen-activated protein kinase (MAPK) pathway. The major players in the MAPK pathway are the RAS, BRAF, MEK and ERK proteins, which mediate transmission of growth signals from the cellular membrane to the nucleus (Figure 2). Transcriptional factors that control expression of genes with a pivotal role in cellular differentiation, growth and survival are thus activated.14 Although oncogenic activation of the MAPK pathway can be driven by multiple mechanisms, mutations of the BRAF, RAS, cKIT and NF1 genes are the most common culprits both in cutaneous and in conjunctival melanoma cases (Figure 2).15–17
The Cancer Genome Atlas Network (TCGA) study has been pivotal in our understanding of the various molecular signatures of cutaneous melanoma, especially as they relate to the development of a personalized treatment approach.18 DNA, RNA and protein-based analysis of 333 primary and metastatic skin melanomas allowed for their classification into 4 genomic subtypes: mutant BRAF, mutant RAS, mutant NF1 and triple wild-type.18 A total of 166 (52%) tumors harbored BRAF mutations with the V600E substitution being by far the most common one (n = 145), followed by the V600K mutation (n=18). NRAS mutations were found in 28% of the tumors and NF1 ones in 14% of them. Triple wild-type tumors featured KIT mutations, focal amplifications and complex structural rearrangements. Although clinicopathological analysis failed to reveal any correlation of patient outcomes to the assigned genomic classification, immune gene expression and a lymphocytic infiltrate on pathology was linked to better survival of patients with regional metastases.18
The TCGA study also confirmed the mutagenic role of UV light in skin melanoma and its high mutational load, since it showed that 75% of the primary and 84% of the metastatic tumors possessed a UV signature, i.e. C>T mutations accounted for >60% of the total mutation burden or CC>TT transitions accounted for >5% of it.18,19 A similar UV mutation signature and a high mutation burden has recently been identified in conjunctival melanoma samples as well.20,21
The most common driver mutations in conjunctival melanoma are mutations in the BRAF, NRAS, c-KIT and NF1 genes that lead to constitutive activation of the MAPK pathway as well as mutations in NRAS, c-KIT and PTEN that result in activation of the PI3K/AKT/mTOR signaling pathway (Figure 2). Table 1 summarizes the reported number of cases for each mutation, the percentage of mutations detected as well as any associated clinicopathological features.11,15,16,20,22–40 BRAF is a serine-threonine kinase responsible for activating the next kinase in the pathway, MEK. BRAF mutations have been found in 29.7% (n=167) of conjunctival melanomas (n=563) reported to date, both primary and metastatic (Table 1).22,23,25–28,40 In 98.2% (n=165) of cases, BRAF mutations are located in codon 600, where a valine (V) is substituted either by a glutamate (E) in 89.7% (n=148) of them, by a lysine (K) in 9% (n=15) of them or by an arginine (R) in 0.6% (n=1) of them. One undetermined BRAF V600 mutation has also been reported (0.6%).34 Other BRAF mutations, such as G469A and D594G are found in 1.2% (n=2) of the mutant BRAF conjunctival melanomas (Table 1). The V600E, V600K, V600R, D594G and G469A single point mutations lead to constitutive activation of the kinase domain of the BRAF protein, and thus, to downstream activation of the MAPK pathway without the need for phosphorylation by RAS.22,41 These BRAF mutations are also found in up to 52% of cutaneous melanomas,18,26,42,43 represent 50% of the mutations found in benign melanocytic nevi,44,45 but have never been reported in uveal melanoma.46–49 BRAF-mutant conjunctival melanomas seem to be characterized by a more aggressive behavior than BRAF wild-type melanomas (Table 1).11,22,26,27,30,33,36,50 They are more common in younger male patients and are associated with an increased metastatic potential30,33,50 via both the lymphatic and the vascular route.11 Clinically, BRAF-mutant conjunctival melanomas arise more frequently on the bulbar than the palpebral conjunctiva,30,33 which may also indicate the potential pathogenic role of exposure to UV radiation for the development of such tumors. Finally, they are associated with a greater depth of invasion.11,22
 |  |  |
Table 1 Review of Cases Harboring Specific Mutations, Percentage of Mutations Detected and Associated Clinicopathological Factors |
Following BRAF, the most common mutated gene in the MAPK pathway is NRAS. NRAS belongs to the RAS kinase family of small guanine nucleotide-binding proteins (HRAS, KRAS, NRAS) that become activated by receptor tyrosine kinases. NRAS mutations are present in 6.4% (n=36) of conjunctival melanomas and up to 28% of cutaneous melanomas (Table 1)11,16,20,26,27,35,37,39 and are not found in uveal melanomas.49,52 In 88.9% (n = 32) of cases, NRAS mutations are located in codon 61, where a glutamine (Q) is substituted either by an arginine (R) in 40.6% (n=13) of them, by a lysine (K) in 15.6% (n=5) of them, by a histidine (H) in 9.4% (n=3) of them or by a leucine (L) in 9.4% (n=3) of them. In 25% (n=8) of the reported cases, the amino acid substitution at codon 61 has not been characterized. The G13D, G12N and G12C mutations are found in 8.3% (n=3) of the mutant NRAS conjunctival melanomas, while one reported case has not been characterized further.39 The Q61 and G12/13 single point mutations favor the GTP-bound active conformation of the RAS protein, which in turn leads to cellular proliferation via the constitutive activation of the MAPK and PI3K/AKT/mTOR pathways.53 Similar to cutaneous melanomas, NRAS mutations are for the most part mutually exclusive with BRAF mutations26,33,35,54 since only 2 conjunctival melanoma cases with simultaneously mutated BRAF and NRAS genes have been reported to date.11,37
Another commonly mutated gene in conjunctival melanoma is NF1,16,35 which encodes the tumor suppressor protein neurofibromin that negatively regulates the MAPK pathway (Figure 2). NF1 mutations have been identified in 4.4% (n=25) of reported conjunctival melanoma cases (Table 1)16,35 and in 14% of cutaneous melanomas.18 NF1 mutations have been associated with sun-exposed cutaneous melanomas.16,55 More than 25 distinct NF-1 single point mutations have been described (Table 1) that lead to loss of action of this tumor suppressor gene. The result is elevated levels of GTPase activation protein (GAP), which induces RAS signaling and activates the MAPK and PI3K/AKT/mTOR cell proliferation pathways.56 Similar to cutaneous melanoma,18,55 NF1 mutations can coexist with oncogenic BRAF (n=4) or NRAS mutations (n=2) (Table 1).11,16 Co-existence of NF1 and c-KIT gene mutations has been found in about 30% of mucosal melanomas,57 but not in conjunctival melanoma (Table 1).
Conjunctival melanomas with mutations in the receptor tyrosine kinase c-KIT represent a relatively rare subset. c-KIT mutations are only present in 0.2% (n=1) of conjunctival melanomas (Table 1)24 and in about 15% of acral, mucosal and chronic sun damaged skin melanomas.55,59 The KIT single point mutations lead to ligand-independent phosphorylation and activation of KIT, which then leads to constitutive activation of the MAPK and the PI3K/AKT/mTOR pathways.60 Similar to cutaneous melanoma,24 they demonstrate mutual exclusivity with NRAS and BRAF mutations (Table 1)17 and overall appear more frequently in older patients.61
Other than the MAPK pathway, an oncogenic NRAS or a mutated c-KIT can also activate the PI3K/AKT/mTOR cell proliferation pathway. This pathway can also be activated by loss of function of the PTEN tumor suppressor (Figure 2).62 An activated PI3K/AKT/mTOR pathway plays a major role in invasion and metastasis of cutaneous melanoma.63 In conjunctival melanoma, an activated PI3K/AKT/mTOR pathway has been linked to higher mitotic index and increased tumor thickness, both of which are poor prognostic features.62 Moreover, the tumor suppressive nuclear fraction of PTEN is low in conjunctival melanomas62 as well as in 65% of cutaneous melanomas.64,65
Epigenetic changes that have been linked to the development of conjunctival melanoma include mutations in the promoter of the TERT gene, increased expression of certain chemokine receptors and microRNAs in tumor cells, and chromosomal copy number alterations. The TERT gene encodes the telomerase reverse transcriptase, a subunit of the telomerase complex that is responsible for adding repetitive sequences at the end of chromosomes, thus making them resistant to degradation. TERT promoter mutations increase the expression of the TERT subunit, are present in 64–68% of primary and metastatic cutaneous melanomas and have been associated with shorter survival rates.66 About 5% (n=28) of conjunctival melanomas possess a single TERT promoter mutation,26,27,29 while 1.4% (n=8) of them also possess either a concomitant BRAF (n=4)20,25 or a concomitant NF1 (n=4)20,26,27 mutation (Table 1). Most TERT mutations consist of the UV signature C>T or CC>TT nucleotide changes.20,21
Chemokines are small secreted proteins that belong to the subfamily of cytokines and are thought to play a role in tumor proliferation, invasion and metastasis of various tumors, including cutaneous and uveal melanoma.67–70 Chemokines are secreted not only by tumor cells but also by stromal and immune cells, and are, thus, involved in regulating the immune response against the tumor.71 Binding of the chemokine CXCL12 to the chemokine receptor CXCR4 activates both the MAPK and the PI3K/AKT/mTOR pathways and prolongs the survival and spread of tumor cells (Figure 2).68 Van Ipenburg et al studied the expression pattern of chemokine receptors CCR10, CCR7, and CXCR4 as it relates to the progression of nevi and PAM to conjunctival melanoma as well as to the metastatic potential of the latter.72 Indeed, chemokine receptor expression was significantly lower in nevi than in melanomas and atypical PAM lesions. Moreover, CXCR4 receptor expression correlated very well with the propensity of conjunctival melanoma for metastasis in a mouse experimental model.72 Thus, chemokine receptors may serve as additional therapeutic targets while changes in their expression profiles may provide valuable information on the prognosis and metastatic potential of conjunctival melanoma tumors.
Chromosomal copy number alterations (CNAs) have also been studied in conjunctival melanomas and have been mostly associated with BRAF/NRAS wild-type tumors.11,26,73 The most frequent CNAs in conjunctival melanoma are losses of 1p, 3q, 6q, 8p, 9p, 9q, 10, 11q, 12q, 13, 15p, 16p, 17p and 19 and gains of 1q, 3p, 4q, 6p, 7, 8q, 11q, 12p, 13q, 14p, 17q and 22q.11,20,26,74,75 A 6p regional gain, which is also present both in cutaneous and in uveal melanomas, seems to be the most common CNA in conjunctival melanoma and has been detected by several groups using a variety of molecular methods ranging from multiplex ligation‐dependent probe amplification to whole-exome sequencing.11,20,25,26 Loss of the 10q region, which includes the PTEN locus, has been linked to lymphatic and metastatic spread as well as to greater tumor thickness and a mutated BRAF gene.11 It is estimated that approximately 30% of BRAF and 43% of NRAS mutations in conjunctival melanoma are due to losses or gains of oncogenic loci.26,73 In particular, NRAS mutations are commonly linked to 1q, 3p or 17q gains and BRAF mutations to loss of chromosome 10.11,26
Finally, micro RNAs have also been implicated in the pathogenesis of conjunctival melanoma. Micro RNAs are small non-coding RNA molecules that act as epigenetic regulators by mediating post-transcriptional silencing of certain genes. These molecules are associated with many cancers, including cutaneous and mucosal melanoma, as they can serve both as oncogenes and tumor suppressors.76–78 High expression of miR-30d, miR-506, miR-509, miR-146 and miR-20b, has been detected both in cutaneous16,30 and in conjunctival melanoma.79,80 Upregulation of miR-20b has been linked to PTEN suppression, while overexpression of miR-3916 may predispose to local recurrences.50,79 Therefore, targeting miRNA expression holds promise for the management of conjunctival melanoma.81
Targeted Molecular Inhibitors for the Management of Conjunctival Melanoma
Both the MAPK and the PI3K/AKT/mTOR pathways have provided valuable molecular targets for the management of cutaneous and conjunctival melanoma. Several inhibitors have been developed and are currently in clinical practice, while various others are under investigation in clinical trials.
In cutaneous melanoma, vemurafenib and dabrafenib were the first inhibitors developed to bind to the active conformation of BRAF and, thus, place a halt to the constitutive activation of the MAPK pathway.82,83 These inhibitors have proven particularly potent for melanomas harboring the BRAF V600E mutation.84 The remarkable and unprecedented tumor responses that were originally observed in metastatic BRAF V600E cutaneous melanomas have not proven sustainable in the majority of patients as melanoma cells develop ways to bypass BRAF inhibition and activate the downstream effector protein, MEK.85–88 Thus, MEK inhibitors, such as trametinib, have also been developed and used successfully in resistant BRAF skin melanomas or in combination with BRAF inhibitors in BRAF mutant melanomas.89,90 In a laboratory study of NRAS mutated skin melanomas, a mutation mutually exclusive with BRAF, MEK inhibitors in combination with PI3K/mTOR inhibitors led to tumor shrinkage.91 This combination treatment scheme is now under investigation in clinical trials.92
The use of targeted molecular inhibitors in the management of conjunctival melanoma has been supported by experimental in vitro data. Use of the BRAF inhibitors vemurafenib and dabrafenib halted growth and proliferation of two BRAF V600E mutant conjunctival melanoma cell lines.15 A MEK and an AKT inhibitor have also demonstrated a dose-dependent action in suppressing proliferation, while combination of these two inhibitors acted synergistically and resulted in cell death in the two BRAF V600E mutant cell lines as well as in one NRAS Q61L mutant one.15 The vemurafenib isoform PLX 4720 has also been tested on two BRAF V600E mutant conjunctival melanoma cell lines and one BRAF wild-type cell line.93 The inhibitor acted dose-dependently and had a cytotoxic effect in one of the two BRAF V600E mutant cell lines. The BRAF wild-type cells were only affected in high concentrations of the inhibitor.93 Finally, in another study, vemurafenib was only marginally effective in halting growth of a BRAF V600E mutant melanoma cell line. In contrast, this cell line was sensitive to the MEK inhibitor trametinib and the PI3K inhibitor pictilisib.36 The same authors also showed that the NRAS Q61L mutant melanoma cell line was moderately sensitive to pictilisib.36
To date, a total of nine conjunctival melanoma cases have been managed with BRAF and BRAF/MEK inhibitors (Table 2)28,31,32,34,35,38,40,94 Four cases were treated solely with BRAF inhibitor monotherapy,28,32,34,94 either with vemurafenib32,34,94 or dabrafenib.94 The remaining five cases received BRAF/MEK inhibitor combination therapy31,35,38,40 with dabrafenib/trametinib31,35,38,40 and vemurafenib/cobimetinib.31 In one case, the anti-PD1 agent pembrolizumab was also used81 and in another case, the anti-PD1 agent nivolumab was given in combination with dabrafenib and trametinib.38
 |
Table 2 Review of Cases, Interventions, and Outcomes of Locally Invasive and Metastatic Conjunctival Melanoma Treated with Targeted Molecular Inhibitors |
Indications for treatment included locally advanced disease in two cases31,35 and metastatic disease in the remaining seven cases.28,32,34,38,40,94 Out of the 4 cases that received BRAF inhibitor monotherapy, one patient demonstrated mixed results, with initial improvement followed by disease progression,28 two patients responded partially to the treatment,32,94 and one patient with metastatic disease was on complete remission at 52 weeks of follow-up.34 Out of the 3 cases that received BRAF/MEK inhibitor combination therapy alone,35,38,40 one patient with metastatic disease demonstrated complete disease remission,38 while another one showed only partial improvement.40 The third patient, who had presented with locally advanced conjunctival melanoma, responded substantially to BRAF/MEK inhibition as neoadjuvant therapy, which allowed for the subsequent resection of the shrunk lesion.35 Finally, two cases describe treatment strategies which include BRAF/MEK inhibitor combination therapy followed by or preceded by BRAF inhibitor monotherapy and anti-PD1 agents. In one case, sequential monotherapy with vemurafenib and pembrolizumab substituted BRAF/MEK inhibition therapy with vemurafenib/trametinib due to intolerable side effects.31 Upon re-initiation of the BRAF/MEK inhibitor scheme, the MEK inhibitor cobimetinib substituted trametinib.38 In another case reported by Kiyohara et al, vemurafenib monotherapy led to serious side effects (erythema multiforme-type skin eruption) and disease progression.38 Thus, the patient was switched to nivolumab and combined BRAF/MEK inhibitor therapy with vemurafenib and trametinib.38 The response was disappointing to both single and combined treatment in contrast to the first case, which demonstrated nearly complete regression after re-initiation of BRAF/MEK inhibitors.
Common toxicities of the BRAF inhibitor vemurafenib have been described in cases of skin melanoma and include cutaneous manifestations, such as rash, keratoacanthoma and cutaneous squamous cell carcinoma, arthralgia, fatigue, nausea, diarrhea, photosensitivity, alopecia and liver function abnormalities.86,95 Photosensitivity and rash typically manifest first, within the first days of drug administration, while arthralgia, diarrhea, fatigue, alopecia and skin lesions appear weeks to months later.96 Photophobia may persist with drug use, while other side effects usually regress after the first few months.95,96 Dabrafenib is associated with pyrexia, fewer skin manifestations overall and rarely with photosensitivity.96,97 BRAF/MEK inhibitor therapy has been linked to the BRAF inhibitor-related aforementioned adverse effects as well as to QT prolongation and uveitis.95–97 Such side effects lead to dose modification or treatment interruption in 38% of patients receiving vemurafenib for skin melanoma.86,96 The MEK inhibitor trametinib has been associated with decreased left ventricular ejection fraction, peripheral edema, interstitial lung disease, pneumonitis and ocular side effects, such as central serous retinopathy, retinal vein occlusion and retinal pigment epithelial detachments.89,98
Among the reported cases of patients who received single BRAF inhibitor for conjunctival melanoma, cutaneous side effects were the most common. A low-grade skin rash developed in two patients,32,34 while one patient developed an erythema-multiforme-like eruption and keratinous nodules on the chest and scalp.38 Arthralgia and diarrhea were also reported in one patient.34 BRAF/MEK inhibitor combination therapy was associated with severe nausea and vomiting, which in one case led to discontinuation of the treatment,31 as well as with elevation of liver enzymes and pyrexia, which was successfully treated with paracetamol.40 In total, four of the nine reported cases tolerated BRAF inhibitor monotherapy or BRAF/MEK inhibitor combination therapy well with no reported side effects.28,35,38,94
Immune Checkpoint Inhibitors for the Management of Conjunctival Melanoma
Immune checkpoint inhibitors are monoclonal antibodies that target molecules, such as the T cell receptors CTLA4 and PD1, both referred to as immune checkpoints. The ligands of these receptors are expressed on tumor cells and inhibit the activation of the T cells, thus acting as “brakes” of the immune response and allowing tumor cells to escape immune destruction. Immune checkpoint inhibitors, also known under the wider term immunotherapy, stop the inhibitory action of the checkpoints, thus enabling T cells to fight their target.99
Over the last 10 years, immune checkpoint inhibitors have significantly increased the survival of patients with metastatic cutaneous melanoma. The first checkpoint inhibitor that revolutionized the treatment of metastatic cutaneous melanoma was ipilimumab, a monoclonal antibody against CTLA4.100 Since then, two additional monoclonal antibodies against the PD-1 receptor have been approved by the Food and Drug Administration in the United States for the management of metastatic skin melanoma, nivolumab and pembrolizumab. Combination therapy with ipilimumab and nivolumab or pembrolizumab and nivolumab has further improved the overall survival of metastatic skin melanoma patients compared to monotherapy with either of the three agents, albeit at the cost of higher toxicities.101,102 Specifically, in the randomized controlled trial conducted by Larkin et al, patients with untreated, unresectable or metastatic stage III or stage IV BRAF V600E mutant skin melanoma demonstrated a 52% overall 5-year survival when on combined nivolumab-plus-ipilimumab therapy.101 In contrast, 5-year survival was 44% when nivolumab was given alone and 26% when ipilimumab was used as monotherapy.101 With respect to uveal melanoma, use of checkpoint inhibitors has not led to favorable outcomes due to the low mutational burden and the low PD-L1 expression in uveal melanoma cells.103,104
Clinical application of immune checkpoint inhibitor therapy in the treatment of conjunctival melanoma has been reported in thirteen cases so far (Table 3).34,37,39,105–107 The clinical indication for treatment in the majority of cases was metastatic disease.34,37,105,106 In four cases, checkpoint inhibitors were given for the management of locally advanced disease37,107 and in one case both for locally advanced and metastatic disease.39 Two patients with locally advanced disease were also given topical or intralesional interferon α2b along with the systemic immunotherapy agents.37
 |
Table 3 Review of Cases, Interventions, and Outcomes of Locally Invasive and Metastatic Conjunctival Melanoma Treated with Immunotherapy |
Immunotherapy was administered as a single agent in six cases,34,105–107 as sequential therapy in three cases37,106 and as combination immunotherapy in four cases.37,39 In the three patients who received sequential immunotherapy, a treatment switch was done either due to adverse effects37 or to disease progression.37,106 Among the four patients who received combination therapy, pembrolizumab and low dose ipilimumab were used in two cases,37 while ipilimumab and nivolumab were used in the other two.37,39 Among the six patients who were given single-agent immunotherapy, four received nivolumab105,106 and two pembrolizumab.34,107 Overall, the treatment response rate was favorable, with eight of the thirteen patients showing complete regression.34,37,105–107 Four patients responded partially,37,39 while one patient showed an initial partial response followed by disease progression.106
Immune checkpoint inhibitors have serious side effects, as they activate the immune system not only against tumor cells but also against normal tissue. The most common side effects are immune-related adverse events as well as the reignition of pre-existing autoimmune diseases.108,109 The most common toxicities involve the gastrointestinal tract such as colitis, cholangitis, pancreatitis and hepatitis,110–112 followed by endocrine dysfunction such as adrenal insufficiency, hypothyroidism and type I diabetes and cardiovascular complications such as myocarditis.110,113–115 Pneumonitis and acute kidney injury can also occur,110,116 while ocular side effects have rarely been described as well and include dry eyes, conjunctivitis, episcleritis, keratitis, anterior and posterior uveitis, and orbital inflammation.117,118 In the thirteen reported cases of locally advanced and metastatic conjunctival melanoma treated with immune checkpoint inhibitors, six patients did not experience any adverse events34,37,105,107 while hepatitis and colitis were seen in four37,39,106 and three patients37,106 respectively. Other side effects included adrenal insufficiency and skin rash in one patient,37 pneumonitis in one patient37 and infusion reaction in another one.39
Conclusion
Conjunctival melanoma is a rare, but deadly malignancy given its metastatic potential. The gold standard for the treatment of localized disease is wide surgical excision with adjunctive cryotherapy. Until recently, the prognosis of patients with metastatic disease was grim with a median survival of only 8 months after systemic metastasis.119 However, better understanding of the molecular pathways responsible for the pathogenesis of conjunctival melanoma has paved new ways towards novel promising treatment agents. The molecular nature of conjunctival melanoma is partially similar to cutaneous melanoma, thus, many of the targeted management options used in skin melanoma are also benefitting locally advanced and metastatic conjunctival melanoma patients. Targeted molecular inhibitors against mutated intracellular mediators, such as BRAF and MEK, have been used with favorable results. Moreover, immune checkpoint inhibitors, such as anti-PD1 and anti-CTLA4 agents, have also produced complete or partial regression in patients with a wild-type BRAF gene. These targeted molecular and immunotherapy agents have given rise to a new era of substantially improved survival for advanced conjunctival melanoma patients. At this time, testing for underlying mutations is not standard practice in patients with localized disease who undergo complete excision with cryotherapy. As genetic testing becomes more readily available, a concerted effort to characterize these tumors at a molecular level, even when they are localized, will increase our understanding of their biology and evolution as they recur or metastasize. This will allow for further personalized treatment with increased surveillance of patients with a high-risk genetic background in their primary tumor.
Acknowledgments
We hereby confirm that all figures are original. Figure 2 is created with BioRender.com.
Funding
There is no funding to report.
Disclosure
Dr. Palioura is a consultant for Alcon and reports personal fees from Alcon Laboratories, outside the submitted work. Dr. Gkiala has no financial disclosures. The authors report no conflicts of interest for this work.
References
1. Isager P, Engholm G, Overgaard J, Storm H. Uveal and conjunctival malignant melanoma in Denmark 1943–97: observed and relative survival of patients followed through 2002. Ophthalmic Epidemiol. 2006;13(2):85–96. doi:10.1080/09286580600553330
2. Chang AE, Karnell LH, Menck HR. The national cancer data base report on cutaneous and non-cutaneous melanoma: a summary of 84,836 cases from the past decade. The American college of surgeons commission on cancer and the American cancer society. Cancer. 1998;83(8):1664–1678. doi:10.1002/(SICI)1097-0142(19981015)83:8<1664::AID-CNCR23>3.0.CO;2-G
3. Triay E, Bergman L, Nilsson B, All-Ericsson C, Seregard S. Time trends in the incidence of conjunctival melanoma in Sweden. Br J Ophthalmol. 2009;93(11):1524–1528. doi:10.1136/bjo.2009.157933
4. Yu GP, Hu DN, McCormick S, Finger PT. Conjunctival melanoma: is it increasing in the United States? Am J Ophthalmol. 2003;135(6):800–806. doi:10.1016/S0002-9394(02)02288-2
5. Kaštelan S, Gverović Antunica A, Beketić Orešković L, Salopek Rabatić J, Kasun B, Bakija I. Conjunctival melanoma - epidemiological trends and features. Pathol Oncol Res. 2018;24(4):787–796.
6. Virgili G, Parravano M, Gatta G, et al. Incidence and survival of patients with conjunctival melanoma in Europe. JAMA Ophthalmol. 2020;138(6):601–608. doi:10.1001/jamaophthalmol.2020.0531
7. Shields CL, Markowitz JS, Belinsky I, et al. Conjunctival melanoma: outcomes based on tumor origin in 382 consecutive cases. Ophthalmology. 2011;118(2):389–95.e952. doi:10.1016/j.ophtha.2010.06.021
8. Shields JA, Shields CL, Mashayekhi A, et al. Primary acquired melanosis of the conjunctiva: experience with 311 eyes. Trans Am Ophthalmol Soc. 2007;105:61–72.
9. Shields CL, Shields JA, Gündüz K, et al. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Arch Ophthalmol. 2000;118(11):1497–1507. doi:10.1001/archopht.118.11.1497
10. Damato B, Coupland SE. Conjunctival melanoma and melanosis: a reappraisal of terminology, classification and staging. Clin Exp Ophthalmol. 2008;36(8):786–795. doi:10.1111/j.1442-9071.2008.01888.x
11. Kenawy N, Kalirai H, Sacco JJ, et al. Conjunctival melanoma copy number alterations and correlation with mutation status, tumor features, and clinical outcome. Pigment Cell Melanoma Res. 2019;32(4):564–575. doi:10.1111/pcmr.12767
12. Shields CL, Kaliki S, Al-Dahmash SA, Lally SE, Shields JA. American joint committee on cancer (AJCC) clinical classification predicts conjunctival melanoma outcomes. Ophthalmic Plast Reconstr Surg. 2012;28(5):313–323. doi:10.1097/IOP.0b013e3182611670
13. Shields CL, Fasiuddin AF, Mashayekhi A, Shields JA. Conjunctival nevi: clinical features and natural course in 410 consecutive patients. Arch Ophthalmol. 2004;122(2):167–175. doi:10.1001/archopht.122.2.167
14. McCubrey JA, Steelman LS, Chappell WH, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773(8):1263–1284. doi:10.1016/j.bbamcr.2006.10.001
15. Cao J, Heijkants RC, Jochemsen AG, et al. Targeting of the MAPK and AKT pathways in conjunctival melanoma shows potential synergy. Oncotarget. 2016;8(35):58021–58036. doi:10.18632/oncotarget.10770
16. Scholz SL, Cosgarea I, Süßkind D, et al. NF1 mutations in conjunctival melanoma. Br J Cancer. 2018;118(9):1243–1247. doi:10.1038/s41416-018-0046-5
17. Wallander ML, Layfield LJ, Emerson LL, et al. KIT mutations in ocular melanoma: frequency and anatomic distribution. Mod Pathol. 2011;24(8):1031–1035. doi:10.1038/modpathol.2011.57
18. Akbani R, Akdemir K, Aksoy B, Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell. 2015;161(7):1681–1696. doi:10.1016/j.cell.2015.05.044
19. Brash DE. UV signature mutations. Photochem Photobiol. 2015;91(1):15–26. doi:10.1111/php.12377
20. Swaminathan SS, Field MG, Sant D, et al. Molecular characteristics of conjunctival melanoma using whole-exome sequencing. JAMA Ophthalmol. 2017;135(12):1434–1437. doi:10.1001/jamaophthalmol.2017.4837
21. Rivolta C, Royer-Bertrand B, Rimoldi D, et al. UV light signature in conjunctival melanoma; not only skin should be protected from solar radiation. J Hum Genet. 2016;61(4):361–362. doi:10.1038/jhg.2015.152
22. Gear H, Williams H, Kemp EG, Roberts F. BRAF mutations in conjunctival melanoma. Invest Ophthalmol Vis Sci. 2004;45(8):2484–2488. doi:10.1167/iovs.04-0093
23. Goldenberg-Cohen N, Cohen Y, Rosenbaum E, et al. T1799A BRAF mutations in conjunctival melanocytic lesions. Invest Ophthalmol Vis Sci. 2005;46(9):3027–3030. doi:10.1167/iovs.04-1449
24. Beadling C, Jacobson-Dunlop E, Hodi FS, et al. KIT gene mutations and copy number in melanoma subtypes. Clin Cancer Res. 2008;14(21):6821–6828. doi:10.1158/1078-0432.CCR-08-0575
25. Lake SL, Jmor F, Dopierala J, Taktak AF, Coupland SE, Damato BE. Multiplex ligation-dependent probe amplification of conjunctival melanoma reveals common BRAF V600E gene mutation and gene copy number changes. Invest Ophthalmol Vis Sci. 2011;52(8):5598–5604. doi:10.1167/iovs.10-6934
26. Griewank KG, Westekemper H, Murali R, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin Cancer Res. 2013;19(12):3143–3152. doi:10.1158/1078-0432.CCR-13-0163
27. Griewank KG, Murali R, Schilling B, et al. TERT promoter mutations in ocular melanoma distinguish between conjunctival and uveal tumors. Br J Cancer. 2013;109(2):497–501. doi:10.1038/bjc.2013.312
28. Weber JL, Smalley KS, Sondak VK, Gibney GT. Conjunctival melanomas harbor BRAF and NRAS mutations–letter. Clin Cancer Res. 2013;19(22):6329–6330. doi:10.1158/1078-0432.CCR-13-2007
29. Koopmans AE, Ober K, Dubbink HJ, et al. Prevalence and implications of TERT promoter mutation in uveal and conjunctival melanoma and in benign and premalignant conjunctival melanocytic lesions. Invest Ophthalmol Vis Sci. 2014;55(9):6024–6030. doi:10.1167/iovs.14-14901
30. Larsen AC, Dahmcke CM, Dahl C, et al. A retrospective review of conjunctival melanoma presentation, treatment, and outcome and an investigation of features associated with BRAF mutations. JAMA Ophthalmol. 2015;133(11):1295–1303. doi:10.1001/jamaophthalmol.2015.3200
31. Dagi Glass LR, Lawrence DP, Jakobiec FA, Freitag SK. Conjunctival melanoma responsive to combined systemic BRAF/MEK inhibitors. Ophthalmic Plast Reconstr Surg. 2017;33(5):e114–e116. doi:10.1097/IOP.0000000000000833
32. Maleka A, Åström G, Byström P, Ullenhag GJ. A case report of a patient with metastatic ocular melanoma who experienced a response to treatment with the BRAF inhibitor vemurafenib. BMC Cancer. 2016;16(1):634. doi:10.1186/s12885-016-2657-7
33. Larsen AC, Dahl C, Dahmcke CM, et al. BRAF mutations in conjunctival melanoma: investigation of incidence, clinicopathological features, prognosis and paired premalignant lesions. Acta Ophthalmol. 2016;94(5):463–470. doi:10.1111/aos.13007
34. Pinto Torres S, André T, Gouveia E, Costa L, Passos MJ. Systemic treatment of metastatic conjunctival melanoma. Case Rep Oncol Med. 2017;2017:4623964.
35. Demirci H, Demirci FY, Ciftci S, et al. Integrative exome and transcriptome analysis of conjunctival melanoma and its potential application for personalized therapy. JAMA Ophthalmol. 2019;137(12):1444–1448. doi:10.1001/jamaophthalmol.2019.4237
36. El Zaoui I, Bucher M, Rimoldi D, et al. Conjunctival melanoma targeted therapy: MAPK and PI3K/mTOR pathways inhibition. Invest Ophthalmol Vis Sci. 2019;60(7):2764–2772. doi:10.1167/iovs.18-26508
37. Finger PT, Pavlick AC. Checkpoint inhibition immunotherapy for advanced local and systemic conjunctival melanoma: a clinical case series. J Immunother Cancer. 2019;7(1):83. doi:10.1186/s40425-019-0555-7
38. Kiyohara T, Tanimura H, Miyamoto M, et al. Two cases of BRAF-mutated, bulbar conjunctival melanoma, and review of the published literature. Clin Exp Dermatol. 2020;45(2):207–211. doi:10.1111/ced.14060
39. Chang M, Lally SE, Dalvin LA, Orloff MM, Shields CL. Conjunctival melanoma with orbital invasion and liver metastasis managed with systemic immune checkpoint inhibitor therapy. Indian J Ophthalmol. 2019;67(12):2071–2073. doi:10.4103/ijo.IJO_663_19
40. Rossi E, Maiorano BA, Pagliara MM, et al. Dabrafenib and trametinib in BRAF mutant metastatic conjunctival melanoma. Front Oncol. 2019;9:232. doi:10.3389/fonc.2019.00232
41. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi:10.1038/nature00766
42. Glitza IC, Davies MA. Genotyping of cutaneous melanoma. Chin Clin Oncol. 2014;3(3):27.
43. Platz A, Egyhazi S, Ringborg U, Hansson J. Human cutaneous melanoma; a review of NRAS and BRAF mutation frequencies in relation to histogenetic subclass and body site. Mol Oncol. 2008;1(4):395–405. doi:10.1016/j.molonc.2007.12.003
44. Pollock PM, Harper UL, Hansen KS, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33(1):19–20. doi:10.1038/ng1054
45. Uribe P, Wistuba II, González S. BRAF mutation: a frequent event in benign, atypical, and malignant melanocytic lesions of the skin. Am J Dermatopathol. 2003;25(5):365–370. doi:10.1097/00000372-200310000-00001
46. Cohen Y, Goldenberg-Cohen N, Parrella P, et al. Lack of BRAF mutation in primary uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44(7):2876–2878. doi:10.1167/iovs.02-1329
47. Jager MJ, Shields CL, Cebulla CM, et al. Uveal melanoma. Nat Rev Dis Primers. 2020;6(1):24.
48. Spendlove HE, Damato BE, Humphreys J, Barker KT, Hiscott PS, Houlston RS. BRAF mutations are detectable in conjunctival but not uveal melanomas. Melanoma Res. 2004;14(6):449–452. doi:10.1097/00008390-200412000-00003
49. Bol KF, Donia M, Heegaard S, Kiilgaard JF, Svane IM. Genetic biomarkers in melanoma of the ocular region: what the medical oncologist should know. Int J Mol Sci. 2020;21(15):5231. doi:10.3390/ijms21155231
50. Larsen AC. Conjunctival malignant melanoma in Denmark. Epidemiology, treatment and prognosis with special emphasis on tumorigenesis and genetic profile. Acta Ophthalmol. 2016;94(8):842. doi:10.1111/aos.13207
51. Dahl C, Guldberg P. The genome and epigenome of malignant melanoma. APMIS. 2007;115(10):1161–1176. doi:10.1111/j.1600-0463.2007.apm_855.xml.x
52. Cruz F
53. Muñoz-Couselo E, Adelantado EZ, Ortiz C, García JS, Perez-Garcia J. NRAS-mutant melanoma: current challenges and future prospect. Onco Targets Ther. 2017;10:3941–3947. doi:10.2147/OTT.S117121
54. Jakob JA, Bassett RL
55. Krauthammer M, Kong Y, Bacchiocchi A, et al. Exome sequencing identifies recurrent mutations in NF1 and RASopathy genes in sun-exposed melanomas. Nat Genet. 2015;47(9):996–1002. doi:10.1038/ng.3361
56. Cichowski K, Jacks T. NF1 tumor suppressor gene function: narrowing the GAP. Cell. 2001;104(4):593–604. doi:10.1016/S0092-8674(01)00245-8
57. Hintzsche JD, Gorden NT, Amato CM, et al. Whole-exome sequencing identifies recurrent SF3B1 R625 mutation and comutation of NF1 and KIT in mucosal melanoma. Melanoma Res. 2017;27(3):189–199. doi:10.1097/CMR.0000000000000345
58. Sheng X, Li S, Chi Z, et al. Prognostic factors for conjunctival melanoma: a study in ethnic Chinese patients. Br J Ophthalmol. 2015;99(7):990–996. doi:10.1136/bjophthalmol-2014-305730
59. Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24(26):4340–4346.
60. Longley BJ, Reguera MJ, Ma Y. Classes of c-KIT activating mutations: proposed mechanisms of action and implications for disease classification and therapy. Leuk Res. 2001;25(7):571–576. doi:10.1016/S0145-2126(01)00028-5
61. Gong HZ, Zheng HY, Li J. The clinical significance of KIT mutations in melanoma: a meta-analysis. Melanoma Res. 2018;28(4):259–270.
62. Pópulo H, Soares P, Rocha AS, Silva P, Lopes JM. Evaluation of the mTOR pathway in ocular (uvea and conjunctiva) melanoma. Melanoma Res. 2010;20(2):107–117. doi:10.1097/CMR.0b013e32832ccd09
63. Fedorenko IV, Gibney GT, Sondak VK, Smalley KS. Beyond BRAF: where next for melanoma therapy? Br J Cancer. 2015;112(2):217–226.
64. Tsao H, Mihm MC
65. Westekemper H, Karimi S, Süsskind D, et al. Expression of HSP 90, PTEN and Bcl-2 in conjunctival melanoma. Br J Ophthalmol. 2011;95(6):853–858. doi:10.1136/bjo.2010.183939
66. Hugdahl E, Kalvenes MB, Mannelqvist M, Ladstein RG, Akslen LA. Prognostic impact and concordance of TERT promoter mutation and protein expression in matched primary and metastatic cutaneous melanoma. Br J Cancer. 2018;118(1):98–105.
67. Müller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–56. doi:10.1038/35065016
68. Mishan MA, Ahmadiankia N, Bahrami AR. CXCR4 and CCR7: two eligible targets in targeted cancer therapy. Cell Biol Int. 2016;40(9):955–967. doi:10.1002/cbin.10631
69. Dobner BC, Riechardt AI, Joussen AM, Englert S, Bechrakis NE. Expression of hematogenous and lymphogenous chemokine receptors and their ligands on uveal malignant melanoma in association with liver metastasis. Acta Ophthalmol. 2012;90(8):e638–e644. doi:10.1111/j.1755-3768.2012.02515.x
70. Mitchell B, Mahalingam M. The CXCR4/CXCL12 axis in cutaneous malignancies with an emphasis on malignant melanoma. Histol Histopathol. 2014;29:1539–1546.
71. Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17(9):559–572.
72. van Ipenburg JA, de Waard NE, Naus NC, Jager MJ, Paridaens D, Verdijk RM. Chemokine receptor expression pattern correlates to progression of conjunctival melanocytic lesions. Invest Ophthalmol Vis Sci. 2019;60(8):2950–2957. doi:10.1167/iovs.19-27162
73. Rossi E, Schinzari G, Maiorano BA, et al. Conjunctival melanoma: genetic and epigenetic insights of a distinct type of melanoma. Int J Mol Sci. 2019;20(21):5447. doi:10.3390/ijms20215447
74. McNamara M, Felix C, Davison EV, Fenton M, Kennedy SM. Assessment of chromosome 3 copy number in ocular melanoma using fluorescence in situ hybridization. Cancer Genet Cytogenet. 1997;98(1):4–8.
75. Vajdic CM, Hutchins AM, Kricker A, et al. Chromosomal gains and losses in ocular melanoma detected by comparative genomic hybridization in an Australian population-based study. Cancer Genet Cytogenet. 2003;144(1):12–17. doi:10.1016/S0165-4608(02)00868-3
76. Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6(4):259–269. doi:10.1038/nrc1840
77. Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302(1):1–12.
78. Kunz M. MicroRNAs in melanoma biology. Adv Exp Med Biol. 2013;774:103–120.
79. Xu Y, Brenn T, Brown ER, Doherty V, Melton DW. Differential expression of microRNAs during melanoma progression: miR-200c, miR-205 and miR-211 are downregulated in melanoma and act as tumor suppressors. Br J Cancer. 2012;106(3):553–561. doi:10.1038/bjc.2011.568
80. Philippidou D, Schmitt M, Moser D, et al. Signatures of microRNAs and selected microRNA target genes in human melanoma. Cancer Res. 2010;70(10):4163–4173. doi:10.1158/0008-5472.CAN-09-4512
81. Larsen AC, Mikkelsen LH, Borup R, et al. MicroRNA expression profile in conjunctival melanoma. Invest Ophthalmol Vis Sci. 2016;57(10):4205–4212. doi:10.1167/iovs.16-19862
82. Waizenegger IC, Baum A, Steurer S, et al. A novel RAF kinase inhibitor with DFG-out-binding mode: high efficacy in BRAF-mutant tumor xenograft models in the absence of normal tissue hyperproliferation. Mol Cancer Ther. 2016;15(3):354–365. doi:10.1158/1535-7163.MCT-15-0617
83. Bollag G, Hirth P, Tsai J, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467(7315):596–599. doi:10.1038/nature09454
84. Wellbrock C, Arozarena I. The complexity of the ERK/MAP-kinase pathway and the treatment of melanoma skin cancer. Front Cell Dev Biol. 2016;4:33.
85. Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366(8):707–714. doi:10.1056/NEJMoa1112302
86. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. doi:10.1056/NEJMoa1103782
87. Shi H, Hugo W, Kong X, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 2014;4(1):80–93.
88. Van Allen EM, Wagle N, Sucker A, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 2014;4(1):94–109.
89. Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367(2):107–114. doi:10.1056/NEJMoa1203421
90. Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877–1888. doi:10.1056/NEJMoa1406037
91. Posch C, Moslehi H, Feeney L, et al. Combined targeting of MEK and PI3K/mTOR effector pathways is necessary to effectively inhibit NRAS mutant melanoma in vitro and in vivo. Proc Natl Acad Sci USA. 2013;110(10):4015–4020. doi:10.1073/pnas.1216013110
92. Chamcheu JC, Roy T, Uddin MB, et al. Role and therapeutic targeting of the PI3K/Akt/mTOR signaling pathway in skin cancer: a review of current status and future trends on natural and synthetic agents therapy. Cells. 2019;8(8):803. doi:10.3390/cells8080803
93. Riechardt AI, Maier AK, Nonnenmacher A, et al. B-Raf inhibition in conjunctival melanoma cell lines with PLX 4720. Br J Ophthalmol. 2015;99(12):1739–1745. doi:10.1136/bjophthalmol-2015-306689
94. Griewank KG, Westekemper H, Schilling B, et al. Conjunctival melanomas harbor BRAF and NRAS mutations–response. Clin Cancer Res. 2013;19(22):6331–6332. doi:10.1158/1078-0432.CCR-13-2368
95. Larkin J, Del Vecchio M, Ascierto PA, et al. Vemurafenib in patients with BRAF (V600) mutated metastatic melanoma: an open-label, multicenter, safety study. Lancet Oncol. 2014;15(4):436–444. doi:10.1016/S1470-2045(14)70051-8
96. Welsh SJ, Corrie PG. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol. 2015;7(2):122–136. doi:10.1177/1758834014566428
97. Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicenter, open-label, Phase 3 randomized controlled trial. Lancet. 2012;380(9839):358–365. doi:10.1016/S0140-6736(12)60868-X
98. Infante JR, Fecher LA, Falchook GS, et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a Phase 1 dose-escalation trial. Lancet Oncol. 2012;13(8):773–781. doi:10.1016/S1470-2045(12)70270-X
99. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–214. doi:10.1016/j.cell.2015.03.030
100. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi:10.1056/NEJMoa1003466
101. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–1546. doi:10.1056/NEJMoa1910836
102. Gellrich FF, Schmitz M, Beissert S, Meier F. Anti-PD-1 and novel combinations in the treatment of melanoma-an update. J Clin Med. 2020;9(1):223.
103. Wierenga APA, Cao J, Luyten GPM, Jager MJ. Immune checkpoint inhibitors in uveal and conjunctival melanoma. Int Ophthalmol Clin. 2019;59(2):53–63. doi:10.1097/IIO.0000000000000263
104. Jindal V. Role of immune checkpoint inhibitors and novel immunotherapies in uveal melanoma. Chin Clin Oncol. 2018;7(1):8. doi:10.21037/cco.2018.01.05
105. Ford J, Thuro BA, Thakar S, Hwu WJ, Richani K, Esmaeli B. Immune checkpoint inhibitors for treatment of metastatic melanoma of the orbit and ocular adnexa. Ophthalmic Plast Reconstr Surg. 2017;33(4):e82–e85. doi:10.1097/IOP.0000000000000790
106. Sagiv O, Thakar SD, Kandl TJ, et al. Immunotherapy with programmed cell death 1 inhibitors for 5 patients with conjunctival melanoma. JAMA Ophthalmol. 2018;136(11):1236–1241. doi:10.1001/jamaophthalmol.2018.3488
107. Kini A, Fu R, Compton C, Miller DM, Ramasubramanian A. Pembrolizumab for recurrent conjunctival melanoma. JAMA Ophthalmol. 2017;135(8):891–892.
108. Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6(1):38.
109. Coureau M, Meert AP, Berghmans T, Grigoriu B. Efficacy and toxicity of immune -checkpoint inhibitors in patients with preexisting autoimmune disorders. Front Med (Lausanne). 2020;7:137. doi:10.3389/fmed.2020.00137
110. Bajwa R, Cheema A, Khan T, et al. Adverse effects of immune checkpoint inhibitors (programmed death-1 inhibitors and cytotoxic T-lymphocyte-associated protein-4 inhibitors): results of a retrospective study. J Clin Med Res. 2019;11(4):225–236. doi:10.14740/jocmr3750
111. Aivazian K, Long GV, Sinclair EC, Kench JG, McKenzie CA. Histopathology of pembrolizumab-induced hepatitis: a case report. Pathology. 2017;49(7):789–792. doi:10.1016/j.pathol.2017.07.010
112. Friedman CF, Clark V, Raikhel AV, et al. Thinking critically about classifying adverse events: incidence of pancreatitis in patients treated with nivolumab + ipilimumab. J Natl Cancer Inst. 2016;109(4):djw260. doi:10.1093/jnci/djw260
113. Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–1755. doi:10.1056/NEJMoa1609214
114. Seki T, Yasuda A, Oki M, et al. Secondary adrenal insufficiency following nivolumab therapy in a patient with metastatic renal cell carcinoma. Tokai J Exp Clin Med. 2017;42(3):115–120.
115. Kastrisiou M, Kostadima FL, Kefas A, et al. Nivolumab-induced hypothyroidism and selective pituitary insufficiency in a patient with lung adenocarcinoma: a case report and review of the literature. ESMO Open. 2017;2(4):e000217. doi:10.1136/esmoopen-2017-000217
116. Lucas JA, Menke J, Rabacal WA, Schoen FJ, Sharpe AH, Kelley VR. Programmed death ligand 1 regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice. J Immunol. 2008;181(4):2513–2521. doi:10.4049/jimmunol.181.4.2513
117. Wang W, Lam WC, Chen L. Recurrent grade 4 panuveitis with serous retinal detachment related to nivolumab treatment in a patient with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2019;68(1):85–95. doi:10.1007/s00262-018-2260-7
118. Papavasileiou E, Prasad S, Freitag SK, Sobrin L, Lobo AM. Ipilimumab-induced ocular and orbital inflammation–a case series and review of the literature. Ocul Immunol Inflamm. 2016;24(2):140–146.
119. Tuomaala S, Kivelä T. Metastatic pattern and survival in disseminated conjunctival melanoma: implications for sentinel lymph node biopsy. Ophthalmology. 2004;111(4):816–821. doi:10.1016/j.ophtha.2003.11.001
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.