Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Conditioned taste aversion memory extinction temporally induces insular cortical BDNF release and inhibits neuronal apoptosis
Authors Liu DW , Ma L , Zhang XH, Wang YY
Received 10 May 2019
Accepted for publication 5 August 2019
Published 22 August 2019 Volume 2019:15 Pages 2403—2414
DOI https://doi.org/10.2147/NDT.S215289
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jun Chen
Video abstract presented by Ling Ma.
Views: 172
Dian-Wei Liu,1,* Ling Ma,2,* Xu-Hua Zhang,2 Yun-Yan Wang3
1Department of Neurosurgery, Jinan Central Hospital Affiliated to Shandong University, Jinan, Shandong 250013, People’s Republic of China; 2Department of Clinical Laboratory, The Second Hospital of Shandong University, Jinan, Shandong 250033, People’s Republic of China; 3Department of Neurosurgery, QiLu Hospital of Shandong University, Jinan, Shandong 250012, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yun-Yan Wang
Department of Neurosurgery, QiLu Hospital of Shandong University, No. 44 Wen Hua Xi Road, Jinan, Shandong 250012, People’s Republic of China
Tel +86 5 311 370 892 8040
Fax +86 5 318 569 5463
Email [email protected]
Background: Memory extinction has been reported to be related to psychiatric disorders, such as post-traumatic stress disorder (PTSD). Secretion and synthesis of brain-derived neurotrophic factor (BDNF) have been shown to temporally regulate various memory processes via activation of tropomyosin-related kinase B (TrkB) receptors. However, whether memory extinction induces the synthesis and secretion of BDNF on the basis of its localization is not understood. In this study, we aim to investigate activity-dependent BDNF secretion and synthesis in the insular cortex (IC) in the setting of conditioned taste aversion (CTA) memory extinction.
Materials and methods: Rats were subjected to CTA memory extinction and BDNF antibody (or the equal volume of vehicle) was microinjected into the IC immediately after the extinction testing. Real-time polymerase chain reaction and in situ hybridization were used to detect the gene expression of BDNF, NGF and NT4. The protein levels of BDNF were determined through the enzyme-linked immunosorbent assay. In addition, the levels of phosphorylated TrkB normalized to total TrkB were evaluated using immunoprecipitation and immunoblotting. c-Fos, total extracellular signal-regulated kinase (Erk), phosphorylated Erk, and apoptosis-related protein (caspase-3), were detected by Western blotting.
Results: We found that blocking BDNF signaling within the IC disrupts CTA extinction, suggesting that BDNF signaling in the IC is necessary for CTA extinction. Increased expression levels of c-Fos indicate the induced neuronal activity in the IC during CTA extinction. In addition, temporal changes in the gene expression and protein levels of BDNF in the IC were noted during extinction. Moreover, we found that phosphorylation of TrkB increased prior to the enhanced BDNF expression, suggesting that CTA extinction induces rapid activity-dependent BDNF secretion in the IC. Finally, we found decreased expression of caspase-3 in the IC after CTA extinction.
Conclusion: These results demonstrate that CTA memory extinction temporally induces the release and synthesis of BDNF in the IC and inhibits neuronal apoptosis.
Keywords: brain derived neurotrophic factor, insular cortex, conditioned taste aversion, secretion, apoptosis
Introduction
Memory extinction has been reported to be related to psychiatric disorders, such as post-traumatic stress disorder. Brain derived neurotrophic factor (BDNF), a cognate ligand for the tyrosine kinase receptor B (TrkB), plays important regulatory roles in neuronal survival, differentiation, synaptic plasticity, and long-term potentiation, as well as learning and memory.1–3 BDNF regulates memory storage and is linked to fear extinction.4 Increased levels of BDNF mRNA have been observed in the prefrontal cortex (PFC) following successful fear extinction,5 suggesting induced synthesis of BDNF in PFC. The BDNF Val66Met polymorphism was reported to induce a decrease in activity-dependent BDNF secretion and neuronal dendritic complexity within the ventromedial prefrontal cortex (vmPFC).6,7 Further research confirmed the impaired fear extinction both in mice and humans,7–9 confirming the involvement of BDNF secretion in vmPFC in fear extinction. Additionally, previous research reported that the increased levels of phosphorylated TrkB (p-TrkB) prior to and at the peak of the increased levels of BDNF protein have been found in both the basolateral amygdala (BLA) and the infralimbic prefrontal cortex. This suggests that BDNF is secreted in advance of its synthesis during CTA extinction in those two areas.10 However, whether activity-dependent BDNF secretion and synthesis can be induced in the training of memory extinction in other cerebral regions remains unknown.
Formation of conditioned taste aversion (CTA) is viewed as the pairing of a novel taste (conditioned stimulus, CS) with the subsequent transient malaise (unconditioned stimulus, US) of the food. CTA extinction is a relearning process, which is commonly determined by subjecting the animals to the unreinforced CS 24 hr apart.11,12 During the extinction training, the repeated and non-reinforced CS results in a resumption of drinking the once-avoided taste.13 The long lasting period between two extinction training tests allows us to detect the molecular changes during the extinction. Moreover, it is an excellent method for studying the involvement of different brain regions and the molecular mechanisms of non-declarative memory. According to the accepted CTA paradigm, the amygdala, vmPFC, the parabrachial nucleus, and cerebral regions associated with the gustatory sense are involved in CTA memory extinction.8,14,15
Studies have shown that CTA alters neuronal taste responses within the gustatory cortex.16,17 The insular cortex (IC), which contains the gustatory cortex, plays an important role in CTA extinction.12,18 Acute infusion of BDNF into the IC has been reported to promote CTA extinction,19 while transient inhibition of protein synthesis with cyclosporine A in this region was found to delay CTA extinction.20 However, the roles played by endogenous BDNF were not addressed in these studies. In the present study, we investigated the secretion and synthesis of endogenous BDNF in the IC and its role in CTA extinction.
Materials and methods
Animals
Male Wistar rats (age: 60-days, weight: 250–300 g each, n=149) were individually housed in plastic cages under 12-hr light/dark cycles at a temperature of 22±2°C. Water and food were provided ad libitum except when experimental requirements indicated otherwise. All experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the protocols were approved by the Committee on Institutional Animal Care and Use of Shandong University (Jinan, China). The schedule of the experimental design is shown in Figure 1.
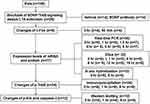 |
Figure 1 Flow diagram of the study.Abbreviations: PCR, polymerase chain reaction; Elisa, Enzyme-linked immunosorbent assay; p-Erk, phosphorylated extracellular signal-regulated kinase. |
Surgical procedures
Before operation, rats were anaesthetized with 5% chloral hydrate (0.6 ml/100 g, intraperitoneal injection) and restrained in a stereotaxic apparatus (RWD Life Science Co., Ltd, 8001, Shenzhen, China). Guide cannulae (23-gauge) were inserted bilaterally aiming for an area 1.0 mm above the IC according to the coordinates below (ie, considering the bregma as a reference point) [anteroposterior: +1.2 mm; lateral: ±5.3 mm; dorsoventral: −5.5 mm]. A stylus was placed into the guide cannulae to prevent clogging. Animals were allowed to recover for at least 1 week after surgery.
Behavioral procedures and drug microinjection
CTA was performed as previously described.21 Rats were deprived of water for 24 hr, and trained for 3–4 days to obtain their baseline water intake within 10 min from two pipettes, each containing 10 mL. On the conditioning day, these rats were granted access for 10 min to saccharin (0.1% w/v, sodium salt), which we considered to be the CS. After 40 min, rats received an intraperitoneal injection of lithium chloride solution (0.15 M, 2% body weight), which we considered to be the US. For CTA extinction training test, three days after CTA conditioning, rats were presented with an array of six pipettes: three contained 5 mL of saccharin and the other three contained 5 mL of water. The amount of liquid consumed was recorded. Results were quantified by an aversion index (AI), defined as [(mL of water)/(mL of water + mL of saccharin)] ×100% consumed in the test. In the first test day, conditioned rats preferred water to saccharin at a ratio of 9:1. For the purpose of memory extinction, conditioned rats were tested for 4 consecutive days.
At the time of infusion, the stylus was removed and a 28-gauge injection needle (extending 1.0 mm from the tip of the guide cannula) was connected to a micro-infusion pump and inserted into the guide cannula. BDNF antibodies (dissolved in 0.1 M phosphate-buffered saline, 1 µg/µl, 0.5 µl/side; Chemicon) or vehicles were microinfused into the IC at the rate of 0.25 μl/min. The injection needle was left in the guide cannula for an additional 2 min to allow drug diffusion.
Quantitative real time-polymerase chain reaction (PCR)
Rats were sacrificed at different time points after the second extinction test. Samples of the bilateral IC of each rat were dissected for RNA extraction using stainless steel brain matrices (RWD Life Science Co., Ltd, 68709, Shenzhen, China) from slices obtained at anteroposterior 1.2 from bregma. Total RNA was extracted using the Ultrapure RNA kit (CW Biotech, CW0597S, Beijing, China) according to the instructions provided by the manufacturer. A total of 0.5 μg RNA from each sample was reversely transcribed into cDNA using the HiFiScript cDNA Synthesis Kit (CW Biotech, CW2569, Beijing, China). Quantitative PCR amplification was performed in a Cycler (MyiQTM2, BIO-RAD) with the use of SYBR green (DRR041A, Takara). Primer sequences were as follows: BDNF forward primer: 5ʹ TAA ATG AAG TTT ATA CAG TAC AGT GGT TCT ACA 3ʹ; BDNF reverse primer: 5ʹ AGT TGT GCG CAA ATG ACT GTT T 3ʹ; nerve growth factor (NGF) forward primer: 5ʹ GCT CAT CCA CCC ACC CAG TCT TCC 3ʹ; NGF reverse primer: 5ʹ CTC GCC CAG CAC TGT CAC CTC CTT 3ʹ; neurotrophin-4 (NT4) forward primer: 5ʹ GAG GCA CTG GCT CTC AGA ATG 3ʹ; NT4 reverse primer: 5ʹ CGA ATC CAG CGC CAG C 3ʹ. β-actin was selected as a control (forward and reverse primers: 5ʹ GGA GAT TAC TGC CCT GGC TCC TA 3ʹ and 5ʹ GAC TCA TCG TAC TCC TGC TTG CTG 3ʹ, respectively). The cycle threshold (Ct) for each sample was selected from the linear range and converted to a starting quantity by interpolation from a standard curve constructed for the same plate and for each set of primers. Each sample was assayed in duplicate and the mRNA levels were normalized to those of β-actin in each well using the 2−ΔΔCt method.
Enzyme-linked immunosorbent assay (ELISA)
The rats were sacrificed at various time points after the second extinction test. Bilateral IC was dissected as described in the real-time PCR section. This was followed by homogenization in radioimmunoprecipitation assay buffer with protease inhibitors (Sigma-Aldrich, St. Louis, MO, USA). ELISA was performed according to the instructions accompanying rat BDNF ELISA Kit (ab213899, abcam). In brief, plates were incubated with BDNF antibodies overnight at 4°C. After blocking non-specific binding with blocking buffer, the test samples were added. Subsequently, a second specific antibody was incubated to bind the captured BDNF. Tested samples were incubated for 1 hr at room temperature with a species-specific antibody conjugated to horseradish peroxidase as a tertiary reactant. Unbound conjugates were removed by subsequent washing. Plates were incubated with chromagenic substrate and substrate absorbance fluorescence was recorded at 450 nm on an ELISA plate reader. All samples were assayed in duplicate.
In situ hybridization
The rats were deeply anesthetized at both 0 hr and 6 hr after the second extinction test. Subsequently, they were transcardially perfused with 4% paraformaldehyde. The brains were removed and post-fixed overnight with 4% paraformaldehyde. After equilibration, the brains were sectioned (40 μm/section) and sections were stored at −20°C.
Riboprobes were prepared as previously described.22 In situ hybridization was performed overnight at 55°C after pre-hybridization. Slices were hybridized with digoxigenin-labeled riboprobes followed by washing according to a stringent protocol. The slices were then covered with anti-digoxigenin antibodies conjugated with alkaline phosphatase (Roche diagnostics, Germany) for 2 hr at room temperature. Finally, sections were incubated in the buffer containing nitro blue-terazolium and 5-bromo-4-chloro-3- indolyl-phosphat (Roche Diagnostics, Germany) for 5 hr at room temperature.
Densitometric analysis was performed on the entire frame observed using a Nikon 80i light microscope equipped with a charge-coupled device camera interfaced to a personal computer under 20×magnification. The threshold value was defined as adjacent background area lacking hybridization. For each section, normalized density = (region of interest density − background density). For statistical analysis, the density of hybridization was reported as the average density of all individual animals ± standard error of the mean in each group (n=5). The mean density of all microphotographs was analyzed with the aid of Image J software.
Immunoprecipitation
The rats were sacrificed at 0, 1 and 8 hr after the second extinction test. Bilateral IC was dissected and homogenized on ice in radioimmunoprecipitation assay buffer, which consisted of 10 mM Tris pH 8.0, 150 mM sodium chloride, 1% NP-40, 1 mM ethylenediaminetetraacetic acid pH 8.0 and 10% glycerol with protease inhibitors (Sigma–Aldrich). After centrifugation, the supernatant was collected. The concentration of total protein was determined using the BCA reagent (Thermo Fisher Scientific, Waltham, MA, USA). Subsequently, 5 mg of homogenized lysate was immunoprecipitated overnight at 4°C using rabbit anti-TrkB antibodies (1:100; Millipore, Temecula, CA, USA). Immunoprecipitated samples coupled with sample buffer were boiled for 5 min to detect the levels of p-TrkB. They were loaded in a 10% sodium dodecyl sulfate-polyacrylamide gel, separated through electrophoresis, and transferred to a polyvinylidene difluoride (PVDF) membrane. The transferred PVDF membrane was blocked with 5% non-fat dry milk in phosphate-buffered saline for 1 hr at room temperature. Subsequently, the blots were separately incubated overnight at 4°C with the following antibodies: mouse anti-phospho-tyrosine antibodies pY99 (1:4000; Santa Cruz, CA, USA) or mouse anti-TrkB antibodies (1:1000; BD Transduction Laboratory, USA). After the blots were washed thrice with tris-buffered saline and Tween 20, they were incubated with horseradish peroxidase-conjugated secondary antibodies (1:5000; Cell Signaling Technology) for 1 hr at room temperature. Western blotting signals were detected using the ECL chemiluminescence system (Millipore). The scanned images were visualized with the Quantity One Manual software. Ratios of immunoprecipitated p-TrkB over total TrkB derived from 0 hr were normalized to 1.0. The values of other groups were normalized according to the 0 hr group.
Western blotting
The rats were sacrificed at 0 hr and 90 min after the second extinction test to assess the expression levels of c-Fos. The other rats were sacrificed at 0, 1 and 8 hr after the second extinction test to detect the levels of Erk, p-Erk and caspase-3. Bilateral IC was dissected and homogenized with radioimmunoprecipitation assay buffer. The protein lysates were loaded into a gel, separated through electrophoresis, and transferred to a PVDF membrane as described in the Immunoprecipitation section. After blocking, the blots were separately incubated overnight at 4°C with the following antibodies: rabbit anti-c-Fos (1:1000; Cell Signaling Technology), mouse anti-TrkB (1:1000; BD Transduction Laboratory), mouse anti-α-tubulin (1:10000; Sigma-Aldrich), rabbit anti-caspase-3 (1:1000; Cell Signaling Technology), rabbit anti-p-Erk (1:1000; Cell Signaling Technology), rabbit anti-Erk (1:1000; Cell Signaling Technology), or rabbit anti-β-actin (1:1000; Cell Signaling Technology, as control). After incubation with second antibodies, Western blotting signals were detected using the ECL chemiluminescence system (Millipore). The scanned images were visualized with Quantity One Manual software. The ratios of c-Fos over β-actin, TrkB over α-tubulin, p-Erk over Erk and caspase-3 over β-actin at 0 hr after the second extinction test were normalized to 1.0. Values of other groups were normalized according to the 0 hr group.
Statistical analyses
Data were analyzed with Student’s t-test, one- or two-way analysis of variance (ANOVA), followed by Least Significant Difference (LSD) post-hoc comparisons. The significance level was set to 0.05 for all statistical analyses, and all values in the text and figures represent means ± standard error of the mean. Data analyses were performed using the SPSS statistical program version 19.0 (IBM Corp., Armonk, NY, USA).
Results
Blockade of BDNF/TrkB signaling in the IC delays CTA extinction
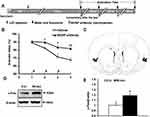
To examine the biological effects exerted by of endogenous BDNF in the IC in CTA extinction, we micro-infused an anti-BDNF antibody into the IC immediately after extinction test. This was repeated for 3 consecutive days (Figure 2A). As shown in Figure 2B, the high aversion index in the first-day test indicated successful CTA conditioning. Rats that underwent treatment with BDNF antibody infusion maintained a high aversion index, whereas the control animals exhibited markedly reduced aversion index, indicating the extinction of CTA by the consumption of saccharin without the association with LiCl injection in the control group. Quantitative analysis revealed a significant delay in the aversion index throughout the 4 extinction days (treatment: F(1,63)=20.123, p=0.000; test day: F(1,63)=13.486, p=0.000; interaction: F(1,63)=2.118, p=0.108), and the t test showed significantly higher AI levels compared with the control group on the second (t test, p=0.027), third (t test, p=0.037), and fourth extinction day (t test, p=0.007). These findings suggest that BDNF/TrkB signaling in the IC is functionally involved in CTA extinction. Figure 2C shows the placement of the cannula in the IC. Anatomical schematic maps were adapted from Paxinos and Watson (2007).23
As a neuronal activity-dependent marker, the expression of c-Fos protein has been shown to be upregulated in the vmPFC 90 min post-CS exposure during CTA extinction tests.24 To investigate whether CTA extinction induced neuronal activity after CTA extinction in the IC, expression levels of c-Fos were measured 0 hr and 90 min after the second extinction test using Western blotting. The levels of c-Fos in the IC were significantly increased 90 min after extinction as compared with those observed at 0 hr (Figure 2D and E; t test, p=0.021), suggesting induced neuronal activity after the CTA extinction test.
Temporal expression of BDNF in the IC during CTA extinction
We subsequently examined the gene expression of BDNF during CTA extinction using real-time PCR to investigate the mechanism through which BDNF in the IC is involved in CTA extinction. As Figure 2B shows, the AI in the control group declined markedly on the third extinction day, which was caused by the extinction training test on the second test day. Therefore, the second test day was chosen to detect the expression levels of BDNF during CTA extinction. The rats were sacrificed at 0, 1, 1.5, 4, 6, or 8 hr after the second extinction test, and the mRNA levels of BDNF in the IC were detected. As shown in Figure 3A, the mRNA levels of BDNF elevated in the IC during CTA extinction (ANOVA: F(5,33)=7.622, p=0.000). The post-hoc analysis showed that the expression of BDNF started to increase from 4 hr (p=0.041, vs 0 hr) and peaked at 6 hr (p=0.002, vs 0 hr). In addition, the mRNA levels of NGF and NT4 were detected at different time points after the second extinction test to assess the possibility of these neurotrophin members also being altered in the IC during CTA extinction. As shown in Figure 3B and C, the mRNA levels of NGF (Figure 3B; ANOVA: F(5,23)=3.824, p=0.015) and NT4 (Figure 3C; ANOVA: F(5,23)=1.174, p=0.36) did not change after the second extinction test as compared with 0 hr, suggesting that CTA extinction selectively induced the gene expression of BDNF in the IC.
The rats were sacrificed at different time points after the second extinction test and ELISA was performed to investigate whether the increases in BDNF mRNA would lead to enhanced BDNF protein levels in the IC. As expected, elevated levels of BDNF protein were observed (Figure 3D; ANOVA: F(5,31)=2.721, p=0.042). This increase was initiated at 8 hr (post-hoc comparisons, p=0.012, vs 0 hr) and peaked at 12 hr (post-hoc comparisons, p=0.005, vs 0 hr). Compared with the time curve of the change in the mRNA levels of BDNF, the delayed increase of BDNF protein levels indicated that the altered protein synthesis occurred corresponding to the increases in gene transcription. These results suggest that CTA extinction may induce the synthesis of BDNF in the IC.
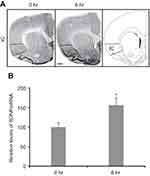
Subsequently, in situ hybridization further confirmed the changes in the mRNA levels of BDNF during extinction (Figure 4A). Similar to the results obtained from the real-time PCR analysis, the mRNA levels of BDNF increased in the IC (Figure 4B, t test, p=0.032) 6 hr after the second extinction test compared with those measured in the 0 hr group.
CTA extinction induces activity-dependent BDNF secretion
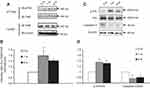
The release and binding of BDNF to its specific receptor (TrkB) triggers its dimerization and autophosphorylation, resulting in activation of downstream signaling pathways that ultimately exert its biological effects. We next investigated whether CTA extinction induced activity-dependent BDNF secretion. The levels of p-TrkB in the IC were examined by immunoprecipitation. We selected the 1 hr and 8 hr timepoints after the second extinction test to detect the levels of total TrkB and p-TrkB (Figure 5A and B; ANOVA: F(2,9)=6.237, p=0.02). The levels of p-TrkB/TrkB, as detected by immunoprecipitation, were found to be significantly higher at both the 1 hr (post-hoc comparisons, p=0.007) and 8 hr (post-hoc comparisons, p=0.04) timepoints after CTA extinction test than those reported in the 0 hr group in the IC. However, the Western blotting analysis did not show any changes in the levels of TrkB in total brain lysate at both timepoints after CTA extinction test compared with 0 hr (Figure 5A). The levels of BDNF protein and p-TrkB increased significantly at 8 hr. This effect was probably due to the release of newly synthesized BDNF. In addition, the elevated levels of p-TrkB at 1 hr after extinction, when both gene expression and protein levels of BDNF were unaltered, suggest that secretion of BDNF is responsible for this effect. Although NT4 also induces phosphorylation of TrkB, there were no changes noted in the expression levels of NT4 after CTA extinction (Figure 3C). These results indicate that CTA extinction training induces rapid activity-dependent BDNF secretion in advance of the increased synthesis of BDNF in the IC.
To further investigate the BDNF release after CTA extinction, expression levels of phosphorylated Erk and total Erk, molecules of the downstream signaling pathway, were detected (Figure 5C and D; ANOVA: F(2,9)=12.816, p=0.002). Similar to the changes observed in p-TrkB, the expression levels of p-Erk were increased at both the 1 hr (post-hoc comparisons, p=0.003) and 8 hr (post-hoc comparisons, p=0.007) timepoints after CTA extinction. In contrast, there were no changes found in the levels of total Erk in the IC after CTA extinction. These results further confirmed the release of BDNF after CTA extinction.
CTA extinction attenuates neuronal apoptosis
The apoptosis of neurons is related to memory deficits. To investigate the neuron apoptosis after CTA extinction, the levels of caspase-3 in the IC was detected (Figure 5C and D; ANOVA: F(2,9)=6.866, p=0.015). Contrary to the changes observed in the levels of p-TrkB, the expression levels of caspase-3 decreased at both the 1 hr (post-hoc comparisons, p=0.007) and 8 hr (post-hoc comparisons, p=0.018) timepoints in the IC after CTA extinction. These results further confirmed that CTA extinction inhibits neuronal apoptosis.
Discussion
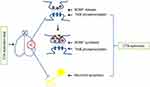
In the present study, we demonstrated that CTA extinction induced the secretion and synthesis of BDNF in the IC. We also found that inhibition of BDNF/TrkB signaling in the IC blocked CTA extinction. Moreover, we found that CTA extinction activated neuronal activity and elevated the mRNA and protein levels of BDNF in the IC. We noted temporally increased levels of p-TrkB and p-Erk after CTA extinction, highlighting the mechanism of BDNF release. Finally, we found that CTA extinction inhibited neuronal apoptosis. As shown in Figure 6, our findings suggest that increased BDNF release and suppressed neuronal apoptosis were temporally induced in the IC throughout CTA extinction.
Previous studies have confirmed that BDNF plays important roles in a variety of memory processes, including hippocampal and amygdala-dependent learning and memory functions.25,26 Hippocampus-specific deletion of BDNF in adult mice was reported to impair extinction of fear memory.27 In addition, overexpression of dominant-negative TrkB (TrkB.T1) in the BLA was found to antagonize BDNF signaling and disrupt extinction retention.28 However, there is limited evidence regarding the role of BDNF in hippocampal-independent memory processes, such as CTA memory extinction. In this study, we found that the mRNA and protein levels of BDNF were temporally induced in the IC after CTA extinction, whereas those of other neurotrophins, such as NGF and NT4, remained unchanged. Together with our previous research, this study further detailed that CTA extinction induced a temporal increase in the expression of BDNF in the BLA and infralimbic prefrontal cortex,10 as well as in the IC.
Anatomical studies have highlighted the involvement of several brain structures in CTA memory processes8,15 and IC plays an important role in CTA memory process. Firstly, IC is involved in CTA memory acquisition. It was reported that microinfusion of BDNF into IC preceding CTA training enhanced the CTA retention29 and reversed CTA memory deficits induced by inhibition of protein synthesis.30 Consistent with these findings, our previous study confirmed that endogenous BDNF in the IC was required for CTA memory formation.22 Secondly, IC is necessary for CTA extinction. Studies have shown that inhibition of acid-sensing ion channel 1a, a molecule playing important roles in synaptic plasticity, learning and memory, immediately after extinction test in the IC attenuates CTA memory extinction.31 In addition, inhibition of protein synthesis or β-adrenergic receptors in the IC immediately after the second CTA extinction test blocks further CTA extinction.12,32 Here, we found that blocking BDNF/TrkB signaling in the IC impaired CTA extinction, directly indicating that endogenous BDNF in the IC is required for CTA extinction. Collectively, these results suggest that BDNF in the IC is required for both CTA acquisition and extinction. Moreover, previous study has shown that CTA extinction is related to c-Fos expression in the insular cortex.33 Consistant with this study, we found that CTA extinction induced neuronal activity in the IC after the second extinction test, which indicates that successful CTA extinction is related to the activated neuronal activity in the IC.
We found that CTA extinction induced activity-dependent BDNF secretion in the IC. Activity-dependent BDNF secretion plays an important role in neuronal activity and memory process. Studies have shown that the human BDNF Val66Met polymorphism leads to decreased activity-dependent BDNF secretion.6 Decreased BDNF secretion was also associated with hippocampal-dependent episodic short-term memory deficit.7 In addition, the BDNF Val66Met was associated with smaller grey matter volume and decreased neuronal dendritic complexity in the vmPFC, significantly affecting CTA extinction.8 These data further suggest that activity-dependent BDNF secretion may be involved in CTA extinction. In this study, we noted increased levels of p-TrkB prior to increases in the mRNA and protein levels of BDNF in the IC, suggesting that TrkB can be activated subsequent to the stimulated release of BDNF. Our data are consistent with previous findings reporting activity-induced BDNF secretion from cultured neurons to be dependent on N-methyl-D-aspartate receptor activities.34 The use of a BDNF antibody to neutralize the released BDNF in the IC blocked CTA extinction, indicating that activity-dependent BDNF secretion in the IC is involved in CTA extinction.
We found that CTA extinction attenuates the neuronal apoptosis. BDNF can protect central and peripheral nerves from damage by inhibiting neuronal apoptosis and promoting the growth of neuron axons.35–38 A previous study has shown that the binding of BDNF to TrkB regulates neuronal activity mainly through activating the mitogen-activated protein kinase (MAPK)/PI3K/Erk pathway. The activation of Erk1/2 and p38 MAPK participates in numerous cellular activities, including cell proliferation, differentiation, death, and survival. Moreover, it improves impaired learning and memory in patients with Alzheimer’s disease by inducing the survival mechanisms.39 In addition, p-Erk can inhibit neuronal apoptosis by activating the anti-apoptotic pathway.40,41 Recently, the up-regulation of BDNF-TrkB and the activation of Erk exert protective effects on the retina against acute ocular hypertension by reducing retinal cell apoptosis. Consistent with this study, the increased levels of p-Erk and decreased levels of caspase-3 in our study further demonstrate the participation of BDNF-TrkB/Erk signaling in the attenuation of neuronal apoptosis in the IC after CTA extinction.
Evidence suggests that BDNF–TrkB signaling plays a key role in the pathophysiology of stress-related psychiatric disorders.42,43 Infusion of BDNF into the hippocampus caused an anti-depressant effect in a learned helplessness rat model.44 Zhan et al reported that decreased BDNF-TrkB signaling in mPFC may play a role in susceptibility to chronic social defeat stress.45,46 Chronic restraint stress can induce abnormal pain sensitivity through the BDNF-mammalian target of rapamycin signaling pathway in the spinal cord.47 In addition, a number of psychiatric disorders such as panic disorder, phobias, social anxiety disorder (SAD), and posttraumatic stress disorder, share a failure of memory extinction.48–50 In this study, the impaired CTA extinction after inhibition of BDNF-TrkB further confirmed the involvement of BDNF in the psychiatric disorders.
Conclusion
In this study, we reported temporally and specific changes in the gene expression of BDNF in the IC in memory extinction. In addition, we found that BDNF in the IC is necessary for CTA extinction. We also confirmed that CTA extinction induces BDNF secretion and inhibits neuronal apoptosis in the IC. This study further details the role of BDNF in CTA extinction. Memory extinction is closely related to psychiatric disorders; therefore, understanding memory extinction may be helpful for the treatment of psychiatric disorders.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant number 81701169), the China Postdoctoral Science Foundation (2019M652398), the Distinguished Middle-Aged and Young Scientist Encouragement and Reward Foundation of Shandong Province (grant number BS2014SW002), the Medical Health Technology Development Plan of Shandong Province (grant number 2017WS657), and the Science and Technology Program of Health and Family Planning Commission of Jinan (grant number 2017-1-19).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi:10.1038/nrn1078
2. Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi:10.1146/annurev.neuro.24.1.677
3. Ortiz JB, Anglin JM, Daas EJ, Paode PR, Nishimura K, Conrad CD. BDNF and TrkB mediate the improvement from chronic stress-induced spatial memory deficits and CA3 dendritic retraction. Neuroscience. 2018;388:330–346. doi:10.1016/j.neuroscience.2018.07.049
4. Rosas-Vidal LE, Do-Monte FH, Sotres-Bayon F, Quirk GJ. Hippocampal–prefrontal BDNF and memory for fear extinction. Neuropsychopharmacology. 2014;39:2161–2169. doi:10.1038/npp.2014.64
5. Bredy TW, Wu H, Crego C, et al. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learning Memory. 2007;14:268–276. doi:10.1101/lm.500907
6. Chen ZY, Patel PD, Sant G, et al. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24:4401–4411. doi:10.1523/JNEUROSCI.0348-04.2004
7. Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi:10.1016/s0092-8674(03)00035-7
8. Yu H, Wang Y, Pattwell S, et al. Variant BDNF Val66Met polymorphism affects extinction of conditioned aversive memory. J Neurosci. 2009;29:4056–4064. doi:10.1523/JNEUROSCI.5539-08.2009
9. Soliman F, Glatt CE, Bath KG, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi:10.1126/science.1181886
10. Xin J, Ma L, Zhang TY, et al. Involvement of BDNF signaling transmission from basolateral amygdala to infralimbic prefrontal cortex in conditioned taste aversion extinction. J Neurosci. 2014;34:7302–7313. doi:10.1523/JNEUROSCI.5030-13.2014
11. Brooks DC, Vaughn JM, Freeman AJ, Woods AM. An extinction cue reduces spontaneous recovery of ataxic ethanol tolerance in rats. Psychopharmacology. 2004;176:256–265. doi:10.1007/s00213-004-1882-y
12. Berman DE, Dudai Y. Memory extinction, learning anew, and learning the new: dissociations in the molecular machinery of learning in cortex. Science. 2001;291:2417–2419. doi:10.1126/science.1058165
13. Mickley GA, Kenmuir CL, McMullen CA, et al. Dynamic processing of taste aversion extinction in the brain. Brain Res. 2004;1016:79–89. doi:10.1016/j.brainres.2004.04.071
14. Akirav I, Khatsrinov V, Vouimba RM, et al. Extinction of conditioned taste aversion depends on functional protein synthesis but not on NMDA receptor activation in the ventromedial prefrontal cortex. Learning Memory. 2006;13:254–258. doi:10.1101/lm.191706
15. Gallo M, Bielavska E, Roldan G, Bures J. Tetrodotoxin inactivation of the gustatory cortex disrupts the effect of the N-methyl-D-aspartate antagonist ketamine on latent inhibition of conditioned taste aversion in rats. Neurosci Lett. 1998;240:61–64. doi:10.1016/s0304-3940(97)00897-5
16. Yasoshima Y, Yamamoto T. Short-term and long-term excitability changes of the insular cortical neurons after the acquisition of taste aversion learning in behaving rats. Neuroscience. 1998;84:1–5. doi:10.1016/s0306-4522(97)00636-2
17. Accolla R, Carleton A. Internal body state influences topographical plasticity of sensory representations in the rat gustatory cortex. Proc Natl Acad Sci U S A. 2008;105:4010–4015. doi:10.1073/pnas.0708927105
18. Rivera-Olvera A, Nelson-Mora J, Gonsebatt ME, Escobar ML. Extinction of aversive taste memory homeostatically prevents the maintenance of in vivo insular cortex LTP: calcineurin participation. Neurobiol Learn Mem. 2018;154:54–61. doi:10.1016/j.nlm.2018.04.005
19. Rodriguez-Serrano LM, Ramirez-Leon B, Rodriguez-Duran LF, et al. Acute infusion of brain-derived neurotrophic factor in the insular cortex promotes conditioned taste aversion extinction. Neurobiol Learn Mem. 2014;116:139–144. doi:10.1016/j.nlm.2014.10.007
20. Hadamitzky M, Orlowski K, Schwitalla JC, et al. Transient inhibition of protein synthesis in the rat insular cortex delays extinction of conditioned taste aversion with cyclosporine A. Neurobiol Learn Mem. 2016;133:129–135. doi:10.1016/j.nlm.2016.06.008
21. Desmedt A, Hazvi S, Dudai Y. Differential pattern of cAMP response element-binding protein activation in the rat brain after conditioned aversion as a function of the associative process engaged: taste versus context association. J Neurosci. 2003;23:6102–6110.
22. Ma L, Wang DD, Zhang TY, et al. Region-specific involvement of BDNF secretion and synthesis in conditioned taste aversion memory formation. J Neurosci. 2011;31:2079–2090. doi:10.1523/JNEUROSCI.5348-10.2011
23. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Amsterdam; Boston: Academic Press/Elsevier; 2007.
24. Mickley GA, Kenmuir CL, Yocom AM, Wellman JA, Biada JM. A role for prefrontal cortex in the extinction of a conditioned taste aversion. Brain Res. 2005;1051:176–182. doi:10.1016/j.brainres.2005.05.033
25. Kesslak JP, So V, Choi J, Cotman CW, Gomez-Pinilla F. Learning upregulates brain-derived neurotrophic factor messenger ribonucleic acid: a mechanism to facilitate encoding and circuit maintenance? Behav Neurosci. 1998;112:1012–1019.
26. Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–535. doi:10.1038/75698
27. Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–670. doi:10.1038/sj.mp.4001957
28. Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci. 2006;9:870–872. doi:10.1038/nn1718
29. Castillo DV, Figueroa-Guzman Y, Escobar ML. Brain-derived neurotrophic factor enhances conditioned taste aversion retention. Brain Res. 2006;1067:250–255. doi:10.1016/j.brainres.2005.10.085
30. Moguel-Gonzalez M, Gomez-Palacio-Schjetnan A, Escobar ML. BDNF reverses the CTA memory deficits produced by inhibition of protein synthesis. Neurobiol Learn Mem. 2008;90:584–587. doi:10.1016/j.nlm.2008.06.003
31. Li WG, Liu MG, Deng S, et al. ASIC1a regulates insular long-term depression and is required for the extinction of conditioned taste aversion. Nat Commun. 2016;7:13770. doi:10.1038/ncomms13770
32. Berman DE, Hazvi S, Stehberg J, et al. Conflicting processes in the extinction of conditioned taste aversion: behavioral and molecular aspects of latency, apparent stagnation, and spontaneous recovery. Learning Memory. 2003;10:16–25. doi:10.1101/lm.53703
33. Hadamitzky M, Bosche K, Engler A, et al. Extinction of conditioned taste aversion is related to the aversion strength and associated with c-fos expression in the insular cortex. Neuroscience. 2015;303:34–41. doi:10.1016/j.neuroscience.2015.06.040
34. Park H, Popescu A, Poo MM. Essential role of presynaptic NMDA receptors in activity-dependent BDNF secretion and corticostriatal LTP. Neuron. 2014;84:1009–1022. doi:10.1016/j.neuron.2014.10.045
35. Mantilla CB, Gransee HM, Zhan WZ, Sieck GC. Motoneuron BDNF/TrkB signaling enhances functional recovery after cervical spinal cord injury. Exp Neurol. 2013;247:101–109. doi:10.1016/j.expneurol.2013.04.002
36. Weishaupt N, Li S, Di Pardo A, Sipione S, Fouad K. Synergistic effects of BDNF and rehabilitative training on recovery after cervical spinal cord injury. Behav Brain Res. 2013;239:31–42. doi:10.1016/j.bbr.2012.10.047
37. Singer W, Panford-Walsh R, Knipper M. The function of BDNF in the adult auditory system. Neuropharmacology. 2014;76:
38. Weber AJ, Harman CD. BDNF treatment and extended recovery from optic nerve trauma in the cat. Invest Ophthalmol Vis Sci. 2013;54:6594–6604. doi:10.1167/iovs.13-12683
39. Bartolome F, de Las Cuevas N, Munoz U, et al. Impaired apoptosis in lymphoblasts from Alzheimer’s disease patients: cross-talk of Ca2+/calmodulin and ERK1/2 signaling pathways. Cell Mol Life Sci. 2007;64:1437–1448. doi:10.1007/s00018-007-7081-3
40. Ahn JY, Liu X, Liu Z, et al. Nuclear Akt associates with PKC-phosphorylated Ebp1, preventing DNA fragmentation by inhibition of caspase-activated DNase. Embo J. 2006;25:2083–2095. doi:10.1038/sj.emboj.7601111
41. Arthur JS, Fong AL, Dwyer JM, et al. Mitogen- and stress-activated protein kinase 1 mediates cAMP response element-binding protein phosphorylation and activation by neurotrophins. J Neurosci. 2004;24:4324–4332. doi:10.1523/JNEUROSCI.5227-03.2004
42. Bjorkholm C, Monteggia LM. BDNF - a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi:10.1016/j.neuropharm.2015.10.034
43. Lindholm JS, Castren E. Mice with altered BDNF signaling as models for mood disorders and antidepressant effects. Front Behav Neurosci. 2014;8:143. doi:10.3389/fnbeh.2014.00143
44. Shirayama Y, Chen AC, Nakagawa S, et al. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261.
45. Zhan G, Huang N, Li S, et al. PGC-1alpha-FNDC5-BDNF signaling pathway in skeletal muscle confers resilience to stress in mice subjected to chronic social defeat. Psychopharmacology. 2018;235:3351–3358. doi:10.1007/s00213-018-5041-2
46. Yang C, Kobayashi S, Nakao K, et al. AMPA receptor activation-independent antidepressant actions of ketamine metabolite (S)-norketamine. Biol Psychiatry. 2018;84:591–600. doi:10.1016/j.biopsych.2018.05.007
47. Huang N, Yang C, Hua D, et al. Alterations in the BDNF-mTOR signaling pathway in the spinal cord contribute to hyperalgesia in a rodent model of chronic restraint stress. Neuroscience. 2019;409:142–151. doi:10.1016/j.neuroscience.2019.03.052
48. Cannistraro PA, Rauch SL. Neural circuitry of anxiety: evidence from structural and functional neuroimaging studies. Psychopharmacol Bull. 2003;37:8–25.
49. Duits P, Cath DC, Lissek S, et al. Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depress Anxiety. 2015;32:239–253. doi:10.1002/da.22353
50. Lebois LAM, Seligowski AV, Wolff JD, et al. Augmentation of extinction and inhibitory learning in anxiety and trauma-related disorders. Annu Rev Clin Psychol. 2019;15:257–284. doi:10.1146/annurev-clinpsy-050718-095634
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.