Back to Journals » Journal of Hepatocellular Carcinoma » Volume 8
Concurrent Sorafenib and Radiotherapy versus Radiotherapy Alone for Locally Advanced Hepatocellular Carcinoma: A Propensity-Matched Analysis
Authors Liu CM , Huang BS, Yen YH, Wang YM , Huang EY, Hsu HC, Huang TT, Yang YH , Cheng JY
Received 4 June 2021
Accepted for publication 31 July 2021
Published 18 August 2021 Volume 2021:8 Pages 963—973
DOI https://doi.org/10.2147/JHC.S323302
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Manal Hassan
Chieh-Min Liu,1 Bing-Shen Huang,2,3 Yi-Hao Yen,4 Yu-Ming Wang,1,5,6 Eng-Yen Huang,1,5,6 Hsuan-Chih Hsu,1 Tzu-Ting Huang,1 Yao-Hsu Yang,5,7,8 Jen-Yu Cheng1
1Department of Radiation Oncology, Kaohsiung Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Kaohsiung, Taiwan; 2Department of Radiation Oncology, Chang Gung Memorial Hospital and Chang Gung University, Taoyuan, Taiwan; 3Graduate Institute of Clinical Medicine, Chang Gung University, Taoyuan, Taiwan; 4Division of Hepatogastroenterology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Kaohsiung, Taiwan; 5School of Traditional Chinese Medicine, Chang Gung University, Taoyuan, Taiwan; 6Department of Radiation Oncology, Xiamen Chang Gung Hospital, Fujian, People’s Republic of China; 7Department of Traditional Chinese Medicine, Chang Gung Memorial Hospital, Chiayi, Taiwan; 8Health Information and Epidemiology Laboratory of Chang Gung Memorial Hospital, Chiayi, Taiwan
Correspondence: Jen-Yu Cheng
Department of Radiation Oncology, Kaohsiung Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Kaohsiung, Taiwan
Tel +886-9-10722590
Email [email protected]
Purpose: Evidence is lacking concerning the benefit of the combination of sorafenib and radiotherapy to treat advanced hepatocellular carcinoma (HCC). To date, no publication has reported the outcomes of radiotherapy alone versus concurrent therapy. We aimed to compare the effectiveness of radiotherapy alone versus concurrent radiotherapy and sorafenib for locally advanced hepatocellular carcinoma.
Materials and Methods: We conducted a propensity score matching (PSM) cohort study comparing the effectiveness of the concurrent use of sorafenib and external beam radiotherapy versus radiotherapy alone in Barcelona Clinic Liver Cancer (BCLC) stage B or C, nonsurgically managed, nonmetastatic patients with HCC. Two subpopulations were matched based on baseline characteristics. Stratified analysis was also performed to assess the heterogeneous effects of the two arms. Overall survival (OS) was compared. Radiation-induced liver disease (RILD) and overt gastrointestinal (GI) bleeding events were also recorded.
Results: Seven hundred thirty-one BCLC stage B or C nonmetastatic HCC patients were identified from 2007 to 2017. Of these, 347 patients met the inclusion criteria (Radiotherapy alone: 269 patients; concurrent therapy: 78 patients). Propensity score matching yielded 73 patients each in the radiotherapy and concurrent groups. The median OS was 9.6 months in the radiotherapy-alone group and 9.9 months in the concurrent group (hazard ratio (HR): 1.12; 95% CI=0.78– 1.62; p=0.544). Posttreatment toxicities, including radiation-induced liver disease and overt gastrointestinal bleeding, showed no significant differences between the groups.
Conclusion: In our study, the concurrent use of sorafenib and conventional external beam radiotherapy shows no survival benefit over radiotherapy alone for locally advanced hepatocellular carcinoma.
Keywords: radiotherapy, sorafenib, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is a common cancer with evident geographic and sex disparities regarding its incidence and mortality. Globally, HCC is a male-predominant disease that ranks fourth in cancer incidence and sixth in cancer mortality. HCC is highly prevalent in Asia, accounting for more than 70% of new cases, deaths, and the 5-year prevalence worldwide.1 The management of HCC requires an interdisciplinary approach tailored to each patient considering the extent of liver dysfunction, the tumor burden, the patient’s performance status, and the patient’s preferences. While the Barcelona Clinic Liver Cancer (BCLC) classification is widely adopted for therapeutic approaches, heterogeneity exists among different stages, particularly for intermediate (BCLC stage B)- and advanced (BCLC stage C)-stage HCC. Patients with early-stage HCC are treated with curative intent, including tumor resection, transplantation, and ablative strategies for relatively small lesions. Advanced HCC represents a more complex entity that often requires multimodality management combined with transarterial chemoembolization (TACE), radiation therapy (RT) and systemic agents to prolong the limited survival time.2–5
HCC is considered a radiosensitive tumor. Advances in RT techniques in the recent decade have led to the wider application of RT from curative intent to salvage therapy. Patients with large unresectable HCC, portal vein tumor thrombosis (PVTT), repeated radiofrequency ablation (RFA) or TACE, or other conditions rendering them unsuitable for surgery are potential beneficiaries of RT.6 Sorafenib was the only systemic agent for HCC for ten years after 2007. This oral multikinase inhibitor is indicated for advanced HCC because of its survival benefit suggested in two pivotal trials in East Asian and Western populations.7,8 Preclinical in vitro and in vivo data9,10 have focused on the interaction of radiation and sorafenib; however, the results from limited prospective and retrospective studies11–13 regarding the toxicity and response of combinational therapy are discordant. Although the benefit of local therapy in locally advanced HCC has been underinvestigated, our multidisciplinary team considered RT given the radiosensitivity of HCC and potential benefit of preventing liver failure and associated morbidity by achieving local control of the dominant liver disease. The concept has also been studied in a randomized trial conducted by Yoon et al14 demonstrating that, compared with sorafenib alone, local treatment (TACE and RT) provided improved survival for patients with locally advanced HCC. Because no previous study has directly compared the efficacy of RT alone with that of concurrent RT and targeted therapy, we designed this study to investigate the effect of concurrent therapy in locally advanced HCC.
Materials and Methods
Data Source
This retrospective cohort study used our multi-institutional electronic medical research database, the Chang Gung Research Database (CGRD). The CGRD is the largest multi-institutional electronic medical record database in Taiwan and includes the comprehensive clinical information of all patients from four Chang Gung Memorial Hospitals, accounting for 6.1–21.2% of outpatients and 10.2–12.4% of inpatients in Taiwan.15,16 All the database records are deidentified. The study was approved and exempted from written informed consent by the Institutional Review Boards of the Chang Gung Medical Foundation at Taoyuan, Taiwan (permit number: 201901679B0) and was conducted in compliance with the Declaration of Helsinki and other ethical guidelines.
Radiotherapy
Our institutional policy regarding HCC irradiation targeted PVTT as long as the adjacent primary tumors were the main high-dose planning target volume area. PVTT, if present, was our primary concern, and the degree of comprehensive coverage of all primary tumors depended on the clinical status, including the tumor location, preserved liver volume, and possible toxicities. Based on previous publications,17,18 the HCC tumor responses and local control are correlated with the RT dose, but high doses are associated with possible toxicities. Our institutional practice utilized a biologically effective dose (BED) as high as possible at an acceptable toxicity rate. The dose and fractionation regimens were ultimately decided by the radiation oncologists. All the identified patients were treated using intensity-modulated RT or volumetric modulated arc therapy, and a few proton cases were treated after 2016.
Study Population
We conducted a propensity score-matched cohort study using data from the CGRD, comparing the effectiveness of the concurrent use of sorafenib with external beam RT versus RT alone. The study cohort flowchart is shown in Figure 1. Patients diagnosed with BCLC stage B or C HCC and treated with RT between 2007 and 2017 were identified. To exclude patients treated with palliative intent, we excluded patients who had received external beam radiation with a BED10 below 50 grays (Gy) or equivalent dose in 2-Gy fractions (EQD2) below 41.7 Gy. BED10 was calculated using an α/β value of 10 Gy. Patients with a metastatic status and those who had received RT in fewer than 10 fractions were also excluded.
 |
Figure 1 Study cohort flowchart. Abbreviations: BCLC, Barcelona Clinic Liver Cancer; HCC, hepatocellular carcinoma; RT, radiotherapy; BED10, biologically effective dose; Gy, gray. |
To identify patients primarily treated with RT after diagnosis, we excluded patients who had undergone RT more than 100 days after diagnosis. Medication data were then collected for every prescription of sorafenib in out- and inpatient settings. We defined targeted therapy that started no later than ten days after the commencement of RT as concurrent therapy. Sorafenib started 90 days after RT was not considered a “preplanned” treatment regimen. Institutional follow-up care after RT comprised routine clinical evaluation, serum laboratory examinations and abdominal computed tomography (CT) or magnetic resonance imaging at each visit. Chest CT or other imaging studies were indicated when disease progression was suspected and at the physician’s discretion. Considering potential treatment sequelae and comorbidities in patients with HCC, patients had undergone upper GI endoscopy and colonoscopy when abdominal complaints and signs of anemia were encountered. The intrahepatic disease status was obtained from the detailed image reports at each follow-up visit. Laboratory data and upper endoscopy and colonoscopy reports after RT were collected to compare the occurrences of radiation-induced liver disease (RILD) and overt gastrointestinal (GI) bleeding. Classic and nonclassic RILD was determined based on laboratory data in the subsequent 3 months after RT. Classic RILD was recorded as an increased alkaline phosphatase level by more than twofold that of normal or baseline levels. Nonclassic RILD involved elevated liver transaminases more than five times the normal or baseline levels or a reduction of at least two points in the Child-Pugh score.19 GI bleeding was recorded from the upper endoscopy and colonoscopy reports in the year following RT. Because PVTT is an important adverse prognostic factor and promotes distant metastasis of HCC,20–22 we also performed subgroup analysis according to HCC combined with PVTT.
Statistical Analysis
Statistical analysis was performed using SPSS for Windows (Version 25.0; IBM Corp., Armonk, NY) and SAS 9.4 (SAS Institute, Cary, NC, USA). For all analyses, statistical significance was set at p<0.05. To compare differences between groups, independent t-test was used for numerical variables, and chi-squared test or Fisher’s exact test was used for categorical variables. The results of numerical variables were presented as means (± standard deviation) and those of categorical data were presented as numbers (%).
For propensity score matching (PSM), the propensity score was calculated using logistic regression to model the probability of the receipt of concurrent therapy based on baseline characteristics, including age, sex, Child-Pugh score, TNM stage, tumor size, tumor number, tumor differentiation, severity of liver fibrosis, alpha-fetoprotein (AFP) level, albumin-bilirubin (ALBI) grade, previous TACE, presence of portal vein thrombosis, previously diagnosed hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, and BED10. TNM staging was categorized according to the stage grouping recorded in the registry database, comprising the 6th and 7th editions of the American Joint Committee on Cancer (AJCC) staging system. The IIIA and IIIB subcategories in the 6th edition were manually transformed into IIIAB and IIIC categories according to the 7th edition. Tumor size denoted the largest tumor dimension. Liver fibrosis was defined as an Ishak score of 1 to 4, and cirrhosis was defined as an Ishak score of 5 or 6 or image studies reporting liver cirrhosis. The AFP level was divided into groups by a cutoff value of 200 ng/mL. The greedy method was used for matching at a 1:1 ratio between the study groups with a caliper width 0.25-fold the standard deviation of the propensity score between the study groups. The standardized mean difference (SMD) was used to measure covariate balance.23
The survival rates were estimated using the Kaplan-Meier method, and the Log rank test was used to test the survival difference between the groups. Stratified analysis was also performed to assess the heterogeneous effects of the two arms. We defined the RT-alone group as the reference group, and the Cox regression model was used to estimate the hazard ratio (HR) of the outcomes.
Results
Seven hundred thirty-one BCLC B or C nonmetastatic HCC patients were identified from 2007 to 2017. Of these, 347 patients met the inclusion criteria (RT alone: 269 patients; concurrent therapy: 78 patients). Thirty-one patients were BCLC stage B, and 316 patients were BCLC stage C. The mean age of all patients was 60.6 years, and 83.3% of them were male. The average BED10 was 72.3 Gy (average EQD2=60.3 Gy; SD=12.8 Gy). The median survival did not differ significantly between the RT alone group (8.9 months) and concurrent group (9.6 months; HR=1.09; 95% CI=0.82–1.44; p=0.572; Figure 2A). In the well-balanced 1:1 PSM cohort, 73 patients adhered to RT alone, and 73 patients adhered to concurrent therapy; 70 and 71 BCLC stage C patients were in the two groups, respectively. The size of the largest tumor exceeded 10 cm in 38.4% of the patients, and approximately half of the patients had tumor numbers exceeding three. Furthermore, 80% of the patients had PVTT, with the main PVTT accounting for 69.9% and 74.0% of the cases in the two groups (N=51, 54). The characteristics of the patients identified before and after PSM are listed in Table 1. In the RT plus sorafenib group, 61.6% (N=45) of patients received standard-dose sorafenib (800 mg per day), and 31.5% (N=23) received half of the dose (400 mg per day). Two patients received 1/4 and 3/4 of the standard dose, and 1 received 1000 mg per day. The median overall survival was 9.6 months (95% CI, 4.4–14.82) in the RT-alone group and 9.9 months (95% CI, 7.3–12.5) in the RT plus sorafenib group (HR=1.12; 95% CI=0.78–1.62; p=0.544; Figure 2B). Stratified analysis revealed that homogeneity existed across all covariates; no significant heterogeneity was observed in the HR after matching (Figure 3).
 |
Table 1 Clinical Characteristics Before and After Matching |
 |
Figure 2 (A) Overall survival before PSM. (B) Overall survival after PSM. Abbreviations: PSM, propensity score matching; RT, radiotherapy. |
The addition of sorafenib to RT did not affect intrahepatic progression-free survival (median time: RT alone=8.2 months, 95% CI=4.6–11.8; concurrent therapy=10.6 months, 95% CI=6.8–14.3; p=0.567; Figure 4). In our HCC combined with PVTT cohort, liver function was inferior in the RT-alone group, while the AFP level was higher in the concurrent group. No survival benefit was observed in the subgroups favoring concurrent treatment (Figure 5).
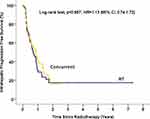 |
Figure 4 Intrahepatic progression-free survival (after matching). |
 |
Figure 5 Subgroup analysis according to HCC combined with PVTT. |
Regarding post-RT toxicities (Table 2), 3 (4.1%) patients in the RT-alone group and 1 (1.4%) patient in the concurrent group developed classic RILD. Twenty-nine (39.7%) patients in the RT-alone group and 26 (35.6%) patients in the concurrent group developed nonclassic RILD (p=0.537). The occurrences of GI bleeding 1 year following RT and concurrent treatment were 1.4% and 8.2% (p=0.116), respectively. Subgroup analysis of patients receiving standard-dose sorafenib showed no significant survival (HR=0.77; p=0.362) or toxicity (RILD events: 37% each; p=0.995) differences compared with patients treated with a reduced dose.
 |
Table 2 Toxicities After Radiotherapy |
Discussion
Our study showed that the concurrent use of RT and sorafenib in patients with locally advanced HCC did not confer a significant survival benefit compared with the RT-alone group in either the entire cohort or PSM cohort. Concurrent therapy did not affect intrahepatic progression-free survival. Subgroup analysis of the entire cohort with PVTT showed no survival benefit when they were initially treated with concurrent therapy. RILD and GI bleeding events after RT showed no significant difference between the groups.
Patients with advanced HCC are commonly prescribed sorafenib, while some are referred for RT. Given the limited number of publications and lack of randomized trial evidence, we reviewed the clinical results from our multi-institutional database. In our study, no significant survival difference was found between the RT-alone group and concurrent group. The present study included patients with advanced HCC without distant metastasis. They were initially managed with RT and had a relatively high tumor burden (38%>10 cm; 50%>3 tumors; 80% PVTT). The results suggest the importance of local treatment. There was evidence supporting locoregional therapy over systemic therapy for selected advanced HCC patients. Nakazawa et al24 conducted a PSM study and reported a survival benefit of RT over sorafenib in patients with unresectable HCC and portal vein tumor thrombosis. For patients with advanced but liver-confined HCC, a randomized control trial14 demonstrated that TACE plus RT provides better outcomes than sorafenib concerning progression-free survival, the response rate, the time to progression, and overall survival. While sorafenib offers relatively suboptimal local effects (tumor response in SHARP trial: 2%; tumor response in Asia-Pacific trial: 3.3%; overall complete response rate: 0%), the advancement of RT provides effective treatment, conferring better local control and acceptable side effects.6,25,26 The previously proposed hypothesis of synergy between RT and sorafenib was mainly based on preclinical results. Some case reports have addressed this possibility, but few clinical studies have directly investigated the clinical benefits and possible side effects resulting from concurrent use. Whether the benefit observed in preclinical studies can be translated into a real-world setting remains unknown.
Similar to our study finding, no previous publication has reported an excessive risk of RILD when patients receive concurrent treatment with RT and sorafenib. However, GI toxicity is another concern. When administering sorafenib with RT, the irradiation field adjacent to the GI tract and dose of sorafenib may be compromised, further affecting tumor control. Radiation fraction size may also play an essential role in this type of toxicity. The Phase I trial conducted by Brade et al12 focused on the combinatory therapeutic regimen of sorafenib with 6 fractions of SBRT. Despite the low number of study participants, they reported significant GI toxicity (2 of 3 evaluable patients: one grade 3 large-bowel bleeding event and one grade 4 bowel obstruction event after SBRT) in patients who required a high volume of liver irradiation. However, the Phase 2 study conducted by Chen et al11 evaluated conventionally fractionated RT combined with sorafenib. Although 45% of patients received sorafenib dose reduction and 10% discontinuation, they reported low rates of grade 2 (5.6%) and 3 (2.8%) gastric or duodenal ulcers. The incidence of GI bleeding events detected in the present study was 8.2% in the concurrent group and 1.4% in the RT-alone group. Despite the lack of statistical significance, GI side effects should not be undervalued.
In addition to sorafenib, four additional targeted agents have been approved for HCC based on positive results in Phase III studies: lenvatinib27 as a first-line therapeutic (noninferior to sorafenib) and regorafenib,28 cabozatinib29 and ramucirumab30 as second-line treatments (sorafenib pretreatment). Aside from the modest results of targeted therapy, immunotherapy is currently drawing substantial attention in the field of HCC. Nivolumab, ipilimumab, and pembrolizumab have been integrated into subsequent-line therapeutic regimens after the occurrence of disease progression,31 and encouraging results from phase Ib studies32,33 have fueled the continued investigation of the combined immunotherapy study design. Additionally, the recent Imbrave150 trial34 confirmed the survival benefit and safety of atezolizumab plus bevacizumab, which outweighed sorafenib for previously untreated, unresectable HCC. Preclinical studies have demonstrated the synergy between RT and immune checkpoint blockers,35–39 but no solid evidence of significant clinical benefits was found in the HCC cohort. Further data are needed to optimize treatment sequences, radiation doses and fractionation and interaction using other systemic agents.
To our best knowledge, the present study is the largest-sized cohort study comparing RT alone and the concurrent use of RT and sorafenib to treat locally advanced HCC. However, this study has potential limitations. The observed results are based on medical record data retrieved retrospectively. Although baseline characteristics were obtained as much as possible, patients who sought treatment in places other than our medical institutes could not be traced. For multiple nodules, the tumor size was recorded as the dimension of the largest single lesion. Previous studies have addressed the value of prognosticators, including the total tumor diameter, diameter of the largest tumor, tumor volume, and tumor number.40–46 Although the largest tumor size serves as a reference material, it may also cause uncertainty when evaluating HCC with multiple lesions. The definition of concurrent treatment was retrospectively delimited rather than prospectively determined with intention to treat. The RT treatment plan could not be further obtained or analyzed because of the nature of the database. Whether the synergistic effect of sorafenib and RT existed and was further perceptible in clinical use was inconclusive in our study. The results from ongoing trials are pending, including phase I and Phase II studies of concurrent RT with sorafenib (ClinicalTrials.gov numbers NCT00892658 and NCT03535259, respectively), phase III studies of sorafenib with or without SBRT (ClinicalTrials.gov numbers NCT01730937 and NCT04387695, respectively), and a study on proton therapy combined with sorafenib (ClinicalTrials.gov number NCT04387695).
Conclusion
In our study, the concurrent use of sorafenib and conventional external beam RT did not show a survival benefit over RT alone in nonsurgically managed and radiotherapy-treatable locally advanced HCC patients. Further investigation of the synergistic combination of RT and systemic agents is warranted.
Acknowledgments
We thank Chih-Yun Lin, Hsin-Yi Chien and the Biostatistics Center at Kaohsiung Chang Gung Memorial Hospital for valuable biostatistics consultations. The abstract of this manuscript was presented at the American Society for Radiation Oncology 2020 Annual Meeting as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in International Journal of Radiation Oncology Biology Physics: https://www.redjournal.org/article/S0360-3016(20)33202-8/fulltext.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ferlay JEM, Lam F, Colombet M, et al. Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer; 2018.
2. Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462.
3. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380.
4. Liver EAFTSOT. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
5. Lu S-N, Wang J-H, Su C-W, et al. Management consensus guideline for hepatocellular carcinoma: 2016 updated by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J Formosan Med Assoc. 2018;117(5):381–403.
6. Yu Y, Feng M. Radiotherapy for hepatocellular carcinoma. Semin Radiat Oncol. 2018;28(4):277–287.
7. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. New Engl J Med. 2008;359(4):378–390.
8. Cheng A, Kang Y, Chen Z, et al. Randomized phase III trial of sorafenib versus placebo in Asian patients with advanced hepatocellular carcinoma. J Clin Oncol. 2008;26(15_suppl):4509.
9. Li Q, Hu Y, Xi M, He L, Zhao L, Liu M. Sorafenib modulates the radio sensitivity of hepatocellular carcinoma cells in vitro in a schedule-dependent manner. BMC Cancer. 2012;12(1):485.
10. Huang C-Y, Lin C-S, Tai W-T, et al. Sorafenib enhances radiation-induced apoptosis in hepatocellular carcinoma by inhibiting STAT3. Int J Radiat Oncol Biol Phys. 2013;86(3):456–462. doi:10.1016/j.ijrobp.2013.01.025
11. Chen SW, Lin LC, Kuo YC, Liang JA, Kuo CC, Chiou JF. Phase 2 study of combined sorafenib and radiation therapy in patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2014;88(5):1041–1047.
12. Brade AM, Ng S, Brierley J, et al. Phase 1 trial of sorafenib and stereotactic body radiation therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2016;94(3):580–587.
13. Wada Y, Takami Y, Matsushima H, et al. The safety and efficacy of combination therapy of sorafenib and radiotherapy for advanced hepatocellular carcinoma: a retrospective study. Intern Med. 2018;57(10):1345–1353.
14. Yoon SM, Ryoo BY, Lee SJ, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. 2018;4(5):661–669.
15. Shao SC, Chan YY, Kao Yang YH, et al. The Chang Gung research database—a multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol Drug Saf. 2019;28(5):593–600.
16. Tsai M-S, Lin M-H, Lee C-P, et al. Chang Gung research database: a multi-institutional database consisting of original medical records. Biomed J. 2017;40(5):263–269.
17. Park HC, Seong J, Han KH, Chon CY, Moon YM, Suh CO. Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2002;54(1):150–155.
18. Holliday EB, Tao R, Brownlee Z, et al. Definitive radiation therapy for hepatocellular carcinoma with portal vein tumor thrombus. Clin Transl Radiat Oncol. 2017;4:39–45.
19. Pan CC, Kavanagh BD, Dawson LA, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76(3):S94–S100.
20. Addario L, Tritto G, Cavaglià E, Amodio F, Giannelli E, Di Costanzo GG. Preserved liver function, portal thrombosis and absence of oesophageal varices are risk factors for metastasis of hepatocellular carcinoma. Digestive Liver Dis. 2011;43(4):319–324.
21. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022.
22. Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–1236.
23. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Statistics Simulation Comput. 2009;38(6):1228–1234.
24. Nakazawa T, Hidaka H, Shibuya A, et al. Overall survival in response to sorafenib versus radiotherapy in unresectable hepatocellular carcinoma with major portal vein tumor thrombosis: propensity score analysis. BMC Gastroenterol. 2014;14(1):84.
25. Rim CH, Kim HJ, Seong J. Clinical feasibility and efficacy of stereotactic body radiotherapy for hepatocellular carcinoma: a systematic review and meta-analysis of observational studies. Radiother Oncol. 2019;131:135–144.
26. Hara K, Takeda A, Tsurugai Y, et al. Radiotherapy for hepatocellular carcinoma results in comparable survival to radiofrequency ablation: a propensity score analysis. Hepatology. 2019;69(6):2533–2545.
27. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. The Lancet. 2018;391(10126):1163–1173.
28. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. 2017;389(10064):56–66.
29. Abou-Alfa GK, Meyer T, Cheng A-L, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. New Engl J Med. 2018;379(1):54–63.
30. Zhu AX, Kang Y-K, Yen C-J, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–296.
31. National Comprehensive Cancer Network Guidelines. Hepatobiliary cancers (Version 5.2020-August 4, 2020). Available from: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf.
32. Llovet J, Shepard KV, Finn RS, et al. A phase Ib trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) in unresectable hepatocellular carcinoma (uHCC): updated results. Ann Oncol. 2019;30:v286–v287.
33. Pishvaian MJ, Lee MS, Ryoo BY, et al. Updated safety and clinical activity results from a phase Ib study of atezolizumab + bevacizumab in hepatocellular carcinoma (HCC). Ann Oncol. 2018;29:viii718–viii719.
34. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905.
35. Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15(17):5379–5388.
36. Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74(19):5458–5468.
37. Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–695.
38. Young KH, Baird JR, Savage T, et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS One. 2016;11(6):e0157164.
39. Kim K-J, Kim J-H, Lee SJ, Lee E-J, Shin E-C, Seong J. Radiation improves antitumor effect of immune checkpoint inhibitor in murine hepatocellular carcinoma model. Oncotarget. 2017;8(25):41242.
40. McPeake J, O’grady J, Zaman S, et al. Liver transplantation for primary hepatocellular carcinoma: tumor size and number determine outcome. J Hepatol. 1993;18(2):226–234.
41. Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transplantation. 2005;11(9):1086–1092.
42. Marelli L, Grasso A, Pleguezuelo M, et al. Tumour size and differentiation in predicting recurrence of hepatocellular carcinoma after liver transplantation: external validation of a new prognostic score. Ann Surg Oncol. 2008;15(12):3503.
43. Germani G, Gurusamy K, Garcovich M, et al. Which matters most: number of tumors, size of the largest tumor, or total tumor volume? Liver Transplantation. 2011;17(S2):S58–S66.
44. Chen YL, Ko CJ, Chien SY, et al. Tumor size as a prognostic factor in resected small hepatocellular carcinoma: a controversy revisited. J Gastroenterol Hepatol. 2011;26(5):851–857.
45. Dai C-Y, Lin C-Y, Tsai P-C, et al. Impact of tumor size on the prognosis of hepatocellular carcinoma in patients who underwent liver resection. J Chinese Med Assoc. 2018;81(2):155–163.
46. Wu G, Wu J, Wang B, Zhu X, Shi X, Ding Y. Importance of tumor size at diagnosis as a prognostic factor for hepatocellular carcinoma survival: a population-based study. Cancer Manag Res. 2018;10:4401.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

