Back to Journals » Cancer Management and Research » Volume 11
Concomitant dose escalation with image–guided Tomotherapy in locally advanced mid–low rectal cancer: a single-center study
Authors Zhao J, Liu X, Wang W , Hu K, Zhang F, Hou X , Meng Q
Received 7 November 2018
Accepted for publication 22 January 2019
Published 15 February 2019 Volume 2019:11 Pages 1579—1586
DOI https://doi.org/10.2147/CMAR.S193657
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Jing Zhao,1,* Xiaoliang Liu,2,* Weiping Wang,2 Ke Hu,2 Fuquan Zhang,2 Xiaorong Hou,2 Qingyu Meng2
1Department of Oncology, Beijing Shijitan Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Radiation Oncology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, People’s Republic of China
*These authors contributed equally to this work
Purpose: The purpose of this study was to evaluate the efficacy and toxicity of concomitant dose-escalated Tomotherapy in locally advanced mid–low rectal cancer.
Patients and methods: Patients with locally advanced (T3/T4 or N+), low–mid (≤10 cm from anal verge) rectal carcinoma treated with neoadjuvant chemoradiotherapy followed by surgery between May 2012 and October 2017 in Peking Union Medical College Hospital were included in this study. A dose of 45/50 Gy in 25 fractions was delivered to the pelvis with Tomotherapy, and 55 Gy was prescribed for the primary tumor with a simultaneous, integrated boost. Megavolt computed tomography was performed before every delivery. The concurrent chemotherapy regimen included capecitabine alone and XELOX.
Results: A total of 141 patients were enrolled; 129 patients (91.5%) had stage cT3 or cT4, and 121 patients (85.8%) had positive lymph nodes. The location of the tumors was in the lower rectum in 88 patients (62.4%). After neoadjuvant chemoradiotherapy, 113 patients (80.1%) underwent sphincter-preserving resection. Downstaging was observed in 121 patients (85.8%), including 80 patients (56.7%) with T downstaging and 101 patients (83.5%) with N downstaging. Thirty-two patients (22.7%) obtained pathological complete response (pCR). The median follow-up was 38.5 months (range, 9.3–73.6 months). Only 36 patients (25.5%) experienced treatment failure, including distant metastasis in 29 patients (20.6%) and pelvic recurrent in 7 patients (5.0%). The estimated 5-year overall survival (OS), disease-free survival (DFS), and local control (LC) rates of patients were 75.1%, 70.9%, and 95.5%, respectively. pCR was an independent prognostic factor for DFS (HR 0.13, 95% CI: 0.02–0.93, P = 0.043), but it did not improve OS or LC. Grade 3 or greater acute leukopenia and diarrhea rates were 5.7% and 7.8%, respectively, and 15 patients (10.6%) developed postoperative complications.
Conclusion: This study indicates that neoadjuvant, image-guided Tomotherapy with 55 Gy boosted to the primary tumor was well tolerated and resulted in high rates of sphincter-preserving surgery, pCR, LC, and DFS for locally advanced rectal cancer.
Keywords: rectal cancer, neoadjuvant chemoradiotherapy, dose escalation, pathological complete response, Tomotherapy
Introduction
Neoadjuvant chemoradiotherapy, followed by total mesorectal excision (TME), is the current standard treatment for locally advanced rectal adenocarcinoma.1 Compared with postoperative chemoradiotherapy, neoadjuvant chemoradiotherapy reduced the local relapse rate and decreased acute and long-term toxicities.2,3
After neoadjuvant chemoradiotherapy with conventional dosage (45–50 Gy), 13.9%–19.2% of patients with locally advanced rectal cancer had a pathological complete response (pCR),4–8 and pCR was reported to be associated with better local control (LC) and survival.4,9 In the CAO/ARO/AIO-94 trial, the 10-year cumulative incidence of distant metastasis rates were 10.5% and 39.6% for patients with pCR and poor response (P=0.005), respectively, following neoadjuvant chemoradiotherapy. The corresponding disease-free survival (DFS) rates were 89.5% and 63% (P=0.008), respectively.9 Park et al reported that the 5-year overall survival (OS) rates were 93.4% and 77.3% for the patients in pCR and poor response groups (P=0.002), respectively, and the 5-year recurrence-free survival rates were 90.5% and 58.5% (P<0.001), respectively.4 In addition, pCR or good response will also increase the number of patients eligible for organ-preserving treatment and improve the quality of life of patients.
In attempts to improve the pCR and survival, several large, randomized, Phase III trials addressed the addition of oxaliplatin to 5-Fu or capecitabine.5–8,10–12 However, most studies (ACCORD 12, STAR-01, R-04) demonstrated that the combined chemotherapy regimen could not improve the pCR and survival rates and were associated with higher toxicities.6,7,10,11 Based on these results, the addition of oxaliplatin to neoadjuvant chemotherapy is not recommended at present.
The tumor response was associated with the dose delivered.13 An alternative strategy to improve the pCR and survival was dose-escalated radiotherapy. Combined with an accelerator with a high modulation capability and a megavolt computed tomography (MVCT), Tomotherapy fully integrates intensity-modulated radiation therapy (IMRT) and image-guided radiation therapy. For rectal cancer patients, Tomotherapy allows more accurate and precise boost irradiation delivered to the primary tumor than IMRT and three-dimensional conformal radiation therapy (3D-CRT) did, which were used in most previous studies.14 In this study, we reported the efficacy and toxicity of dose-escalated Tomotherapy combined with chemotherapy in mid–low, locally advanced rectal carcinoma patients.
Patients and methods
Patients
We conducted this study from May 2012 to October 2017. The eligibility criteria were as follows: histologically confirmed rectal carcinoma; local advanced stage (cT3-4N0-2M0 or cT1-4N1-2M0); mid–low rectal cancer (≤10 cm from anal verge). Patients received surgery after concurrent chemoradiotherapy. Pretreatment evaluation included complete history and physical examination, digital rectal examination (DRE), blood count, renal and liver function, colonoscopy, biopsy, chest and abdomen computed tomography (CT), pelvic magnetic resonance imaging (MRI), and transrectal ultrasound. Some patients received positron emission tomography/computed tomography (PET/CT).
Radiotherapy
All patients received a CT simulation (16-slice Philips Brilliance CT BigBore, Deventer, Netherlands) in the supine position with oral and intravenous contrast agents. Bladder and rectum preparation were conducted before the CT scan. The gross tumor volume (GTV) and clinical target volume (CTV) were contoured on the axial CT slices. The GTV included the primary tumor (GTV-T) and involved lymph nodes (GTV-N). The GTV-T scans were delineated based on the pelvic MRI and taking the DRE, endoscopy, and pelvic ultrasound into consideration. Involved lymph nodes were defined as short diameter >1 cm or confirmed by diffusion-weighted imaging or PET/CT. CTV covered GTV-T, GTV-N (if any), the complete mesorectum, and pelvic lymph node region (including presacral, internal iliac, obturator), with an upper margin at the L5–S1 interspace. The planning clinical target volume (PCTV) was the CTV plus 8 mm margin craniocaudal direction, and 6 mm in anteroposterior and left–right directions. The planning gross tumor volume (PGTV) was created by adding a 5 mm margin to the GTV-T and GTV-N.
A dose of 45/50 Gy in 25 fractions was prescribed to the PCTV. PGTV (including PGTV-T and PGTV-N) was boosted to 55 Gy with a simultaneously integrated boost. Radiotherapy plans were generated on the Tomotherapy treatment planning system (Tomotherapy Inc., Madison, WI, USA). The Tomotherapy planning parameters were a 2.5-cm field width, a pitch of 0.25, and a maximum modulation factor of 2.5. The planning goals were delivering at least 95% of the prescribed dose to 95% of the PCTV or PGTV. MVCT was used for image guidance before each treatment delivery. Patients were repositioned after co-registration of MVCT images with the planning kilovolt CT images.
Chemotherapy
All patients received concurrent chemoradiotherapy. The concurrent chemotherapy regimen included oral capecitabine (825 mg/m2 twice per day, 5 days per week) with or without oxaliplatin at a dose of 50 mg/m2 once per week for 6 weeks. Different chemotherapy regimens were given according to the patient’s condition. Postoperative chemotherapy was individualized.
Surgery
Before surgery, patients received a pelvic MRI/transrectal ultrasound for reassessment of staging and resectability by surgeons. TME surgery was performed for rectal cancer patients at least 6 weeks after neoadjuvant radiotherapy. Whether to perform sphincter-preserving surgery or not was decided by the attending surgeon, based on the distance of the tumor to the anal sphincter, the clinical response to neoadjuvant treatment, and the patient’s preference. Prophylactic ileostomy was performed for patients with low rectal cancer receiving sphincter-preserving TME. Transanal endoscopic microsurgery (TEM) was conducted for some patients with very low rectal cancer (<3 cm from the anal verge), acquired clinical complete response (cCR) after neoadjuvant treatment, and a strong desire to preserve the sphincter.
Follow-up and evaluation of toxicities
Patients had follow-up examinations every 3 months during the first 2 years, every 6 months during the next 3–5 years, and then once each year. Carbohydrate antigen 19–9 and carcinoembryonic antigen levels were measured every 3 months together with a rectal examination that included an MRI of the pelvis and CT scan of the thorax and abdomen. Chemoradiotherapy-related toxicities and postoperative complications were recorded. Acute toxicities during chemoradiotherapy were evaluated every week. Toxicities were evaluated with Common Terminology Criteria for Adverse Events, version 3.0.
Statistics
The primary end point of this study was the pCR rate. The secondary end points, including resection rate, sphincter-preserving rate, downstaging rate, acute and postoperative complications, pattern of failure, and survival, were also calculated. OS, DFS, and LC rates were estimated with the Kaplan–Meier methods, and the univariate log rank test was used to evaluate the significance of prognostic factors for survival. Multivariate analysis, using the Cox proportional regression method, was performed for the covariates selected in the univariate analysis. An equivalent dose in 2-Gy fractions (EQD2) was calculated with α/β=10 for the tumor. A significance level of 0.05 was used. All the statistical analyses were performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA).
Results
Patients
A total of 141 patients were enrolled. Patients’ and tumors’ characteristics are detailed in Table 1. All patients were diagnosed with locally advanced rectal cancer, 129 patients (91.5%) with stage cT3 or cT4; 121 patients (85.8%) had positive lymph nodes. The location of tumors was in the lower rectum in 88 patients (62.4%; Table 1).
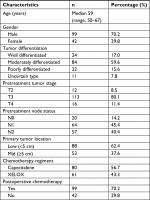  | Table 1 Characteristics of patients and tumors |
Treatment and acute toxicities
A dose of 50 Gy in 25 fractions was delivered to the pelvic of 55 patients (39.0%), and 45 Gy was prescribed for 86 patients (61.0%). All patients completed radiotherapy. The median duration of radiotherapy was 35 days (range, 34–50 days). One patient prolonged the radiotherapy time due to grade III leukopenia, but the total dose remained unchanged. Capecitabine alone was used in 56.7% (80/141) of patients and XELOX in 43.3% (61/141) of patients. Adjuvant chemotherapy was conducted in 70.2% (99/141) patients, including capecitabine in 36 patients and XELOX in 63 patients. Grades 3–4 leukopenia, anemia, and thrombocytopenia were observed in 8 (5.7%) patients, 2 (1.4%) patients, and 4 (2.8%) patients, respectively. Eleven patients (7.8%) developed grade 3 diarrhea.
Surgery and postoperative complications
Surgery was performed on all patients, including TEM in seven patients. Negative margins were observed in 138 patients (97.9%), and 113 patients (80.1%) underwent sphincter-preserving resection. TEM was conducted in seven patients: T3N1bM0 in four patients, T3N0M0 in two patients, and T3bN2M0 in one patient, and obtained cCR after chemoradiotherapy. Five of them had pathologic CR and the other two patients had pathologic T2 disease (with spotted and focal residual tumor in the muscle layer) after surgery.
Fifteen of 141 patients (10.6%) experienced postoperative complications, including 6 patients with bowel obstruction, 3 patients with rectovaginal fistula, 1 patient with uroschesis, 2 patients with anastomotic fistula, 1 patient with anal fistula, 1 patient with rectovesical fistula, and 1 patient with anastomotic stenosis.
Pathologic response
Downstaging was observed in 121 patients (85.8%), including 80 patients (56.7%) with T downstaging and 101 patients (83.5%) with N downstaging. A pCR was observed in 32 patients (22.7%). Compared with capecitabine alone, XELOX presented no significant difference in pCR (P=0.119). Pathologic T0 was also found in 32 patients (22.7%). Of the 121 patients with positive lymph nodes before treatment, negative lymph node involvement was observed in 90 patients (74.4%).
Pattern of failure and survival
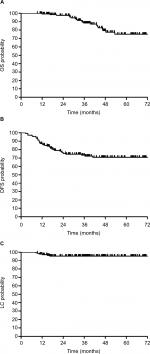
The median follow-up was 38.5 months (range, 9.3–73.6 months). Only 36 patients (25.5%) experienced treatment failure, including distant metastasis in 29 patients (20.6%) and pelvic recurrent in 7 patients (5.0%). The most common metastases were lung, followed by liver and bone. The estimated 5-year OS, DFS, and LC rates of patients were 75.1% (95% CI: 63.1%–83.7%), 70.9% (95% CI: 61.7%–78.3%), and 95.5% (95% CI: 90.2%–98.0%), respectively (Figure 1A–C). Univariate analysis showed that pCR (P=0.019) and sphincter preservation (P=0.027) were prognostic factors of DFS but did not improve OS and LC (Table 2, Figure 2). Multivariate analysis demonstrated that pCR was independently significant for DFS (HR =0.13, 95% CI: 0.02–0.93, P = 0.043; Table 3).
  | Table 2 Univariate analysis of prognostic factors in OS, DFS, and LC Abbreviations: DFS, disease-free survival; LC, local control; OS, overall survival; pCR, pathological complete response. |
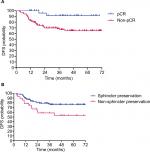  | Figure 2 Patients with pCR (A) and sphincter-preservation (B) had longer DFS (P=0.019, P=0.027, respectively). Abbreviations: DFS, disease-free survival; pCR, pathological complete response. |
  | Table 3 Multivariate analysis of the prognosis of DFS Abbreviation: DFS, disease-free survival; pCR, pathological complete response. |
Discussion
Compared with the addition of oxaliplatin,6,7,10,11 the ability to achieve a pCR appears promising with the use of primary tumor dose escalation for locally advanced rectal cancer patients treated with neoadjuvant chemoradiotherapy followed by surgery. In a trial from China, 63 patients with locally advanced mid–low rectal cancer underwent radiotherapy at two dose levels (41.8 Gy and 50.6 Gy in 22 fractions simultaneously) and concurrent chemotherapy. The pCR rate was 31.0%. Only four (6.9%) patients experienced postoperative complications.15 In another Chinese study, by Zhu et al, 50 Gy was delivered to the pelvic area, and the primary tumor was boosted to 55 Gy. PCR was observed in 23.7% of patients. The postoperative complication incidence rate was 17.1%.16 A Slovenia study enrolled 51 patients with stages II–III rectal cancer. A dose of 41.8 Gy in 22 fractions was delivered to the pelvic area. T2–T3 tumors were concomitantly boosted to 46.2 Gy and a T4 tumor was boosted to 48.4 Gy. PCR was achieved in 12 (25.5%) patients, and only 2 patients developed ≥ grade 3 acute toxicities.17 In the study by Tey et al, with 55 Gy in 25 fractions to the primary tumor, 35% of patients obtained pCR.18 A meta-analysis including 487 patients also showed a high pCR rate (20.4%) with ≥60 Gy dose-escalated radiotherapy.19 pCR rates in these studies were higher than previous reports of 13.9%–19.2% without dose escalation.4–8 In the present study, the pCR rate was 22.7%, which was similar to the meta-analysis with doses ≥60 Gy,19 and the pCR rate was much higher than historical reports on nondose escalated radiotherapy in our institute, in which a dose of 50 Gy in 25 fractions was delivered to the pelvic area with conventional radiotherapy or 3D-CRT, and the pCR rate was 10.1%.20
IMRT was used in most previous reports of dose-escalated neoadjuvant chemoradiotherapy.15–18,20 It decreased the dose to the bowel, bladder, and femoral head21 and reduced the toxicities22,23 compared with 3D-CRT. In comparison with IMRT and volumetric modulated arc therapy, patients treated with Tomotherapy had lower doses to the bladder and femoral head and better conformity index and homogeneity index.24Another advantage of Tomotherapy was the use of the MVCT modality, which allows very precise daily patient positioning. Rectal motion is a potential obstacle for dose escalation of the primary tumor. With daily MVCT, the mean shift of mesorectum space was <3.2 mm.25 The clinical target volume–planning target volume (CTV–PTV) margin was safely reduced, and the mean PTV was decreased from 1,857.4 to 1,462.0 cc (P<0.01). The V15 of the small bowel decreased from 110.9 to 81.4 cc (P<0.01).26 In the present study, we used margins of 5 mm for the primary tumor and 6–8 mm for CTV, which were smaller than most studies with IMRT.16,17 The margins proved to be safe – the pelvic failure rate was low (5.0%). Previous studies of primary tumor dose escalation with Tomotherapy were limited but showed good survival or LC and favorable toxicities.27–30
The dose delivered to the primary tumor in dose-escalated neoadjuvant chemotherapy for rectal cancer was inclusive. The dose fractionation used in previous studies included 46.2 Gy in 22 fractions (EQD2 =46.6 Gy), 48.4 Gy in 22 fractions (EQD2 =49.1 Gy),17 50.6 Gy in 22 fractions (EQD2 =51.8 Gy),15 55 Gy in 25 fractions (EQD2 =55.9 Gy),16,18 55.2 Gy in 23 fractions (EQD2 =57.0 Gy),27,28,30 50 Gy in 25 fractions (EQD2 =50 Gy),29,31 and EQD2 ≥60 Gy (pooled analysis, with sequential/simultaneous external beam radiotherapy or brachytherapy).19 In most studies without dose escalation, 45–50 Gy was delivered to the pelvic area.4–8 The dose-escalated study by But-Hadzic et al delivered only 46.2/48.4 Gy to the primary tumor and 41.8 Gy to the pelvic area in 22 fractions.17 This study just reduced the dose delivered to the pelvic area rather than primary dose escalation. In the present study, the dose fractionation was 55 Gy in 25 fractions, with an EQD2 of 55.9 Gy. With this dose fractionation delivered with Tomotherapy, 80.1% of patients received sphincter-preserving surgery. The pCR rate was 22.7%. Only 36 patients (25.5%) experienced treatment failure, and 7 patients (5.0%) developed pelvic failure. The treatment outcome was promising.
In this study, grade 3 or greater acute leukopenia and diarrhea rates were just 5.7% and 7.8%, respectively, and only 10.6% of patients developed postoperative complications. The toxicities were not significantly higher than previous studies without dose escalation4–8 and were similar to the previous, dose-escalated studies.15–18,21,27–30 The toxicity was also lower than our non-dose escalated study with conventional radiotherapy or 3D-CRT, in which 7.9% of patients developed anastomotic fistula, and 6.5% of patients developed bowel obstruction.20
It is worth noting that one patient developed postoperative anastomotic stenosis. This patient was diagnosed with T3bN1aM0 disease located 5 cm from the anal verge, underwent sphincter-preserving TME, and obtained pCR. However, ileostomy closure could not be performed because of postoperative anastomotic stenosis. Considering the potential association with dose escalation, we constrained the dose to the sigmoid with D2cc ≤45 Gy, and no anastomotic stenosis was observed thereafter.
Considering the comparatively low toxicity of dose-escalated radiotherapy,15–18,21,27–30 a higher escalated dose was tried. The RECTAL BOOST study was a randomized control trial to compare 65 Gy (50 Gy in 25 fractions plus 15 Gy in 5 fractions, EQD2 =66.3 Gy) to 50 Gy for locally advanced rectal–cervical cancer patients.32 Recruitment for this study was started in September 2014.
Tumor regression after chemoradiotherapy, especially pCR, was regarded as an important prognostic factor. A retrospective study by the MD Anderson Cancer Center showed that tumor response was associated with 5-year recurrence-free survival, distant-metastasis rate, and local recurrence.4 In our study, pCR was independently prognostic of DFS but could not improve OS and LC. The results may be related to short follow-up time. Long-term follow-up is needed.
Conclusion
This study indicates that neoadjuvant, image-guided Tomotherapy with 55 Gy boosted to the primary tumor was well tolerated and resulted in high rates of sphincter-preserving surgery, pCR, LC, and DFS for locally advanced rectal cancer. Patients’ survival will be evaluated with longer follow-up.
Ethics statement
The study was approved by Peking Union Medical College Hospital Ethics Committee. The study was carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients prior to enrolment.
Acknowledgment
This work was supported by the 13th Five-Year Research Fund of China (Grant No. 2016YFC0105207).
Disclosure
The authors report no conflicts of interest in this work.
References
Bosset J-F, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med Overseas Ed. 2006;355(11):1114–1123. | ||
Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926–1933. | ||
Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–1740. | ||
Park IJ, You YN, Agarwal A, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30(15):1770–1776. | ||
Deng Y, Chi P, Lan P, et al. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC multicenter, open-label, randomized three-arm phase III trial. J Clin Oncol. 2016;34(27):3300–3307. | ||
Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29(20):2773–2780. | ||
Gérard JP, Azria D, Gourgou-Bourgade S, et al. Clinical outcome of the Accord 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol. 2012;30(36):4558–4565. | ||
Rödel C, Liersch T, Becker H, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13(7):679–687. | ||
Fokas E, Liersch T, Fietkau R, et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol. 2014;32(15):1554–1562. | ||
O’Connell MJ, Colangelo LH, Beart RW, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from national surgical adjuvant breast and bowel project trial R-04. J Clin Oncol. 2014;32(18):1927–1934. | ||
Allegra CJ, Yothers G, O’Connell MJ, et al. Neoadjuvant 5-FU or capecitabine plus radiation with or without oxaliplatin in rectal cancer patients: a phase III randomized clinical trial. J Natl Cancer Inst. 2015;107(11):djv248. | ||
Rödel C, Graeven U, Fietkau R, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16(8):979–989. | ||
Appelt AL, Sebag-Montefiore D. Technological advances in radiotherapy of rectal cancer: opportunities and challenges. Curr Opin Oncol. 2016;28(4):353–358. | ||
Sermeus A, Engels B, Urbain D, De Ridder M. Advances in radiotherapy delivery for rectal cancer: a European perspective. Expert Rev Gastroenterol Hepatol. 2015;9(4):393–397. | ||
Li JL, Ji JF, Cai Y, et al. Preoperative concomitant boost intensity-modulated radiotherapy with oral capecitabine in locally advanced mid-low rectal cancer: a phase II trial. Radiother Oncol. 2012;102(1):4–9. | ||
Zhu J, Liu F, Gu W, et al. Concomitant boost IMRT-based neoadjuvant chemoradiotherapy for clinical stage II/III rectal adenocarcinoma: results of a phase II study. Radiat Oncol. 2014;9(1):70. | ||
But-Hadzic J, Anderluh F, Brecelj E, et al. Acute toxicity and tumor response in locally advanced rectal cancer after preoperative chemoradiation therapy with shortening of the overall treatment time using intensity-modulated radiation therapy with simultaneous integrated boost: a phase 2 trial. Int J Radiat Oncol Biol Phys. 2016;96(5):1003–1010. | ||
Tey J, Leong CN, Cheong WK, et al. A phase II trial of preoperative concurrent chemotherapy and dose escalated intensity modulated radiotherapy (IMRT) for locally advanced rectal cancer. J Cancer. 2017;8(16):3114–3121. | ||
Burbach JP, den Harder AM, Intven M, van Vulpen M, Verkooijen HM, Reerink O. Impact of radiotherapy boost on pathological complete response in patients with locally advanced rectal cancer: a systematic review and meta-analysis. Radiother Oncol. 2014;113(1):1–9. | ||
Hu K, Li W, Hou X, et al. The efficacy of neoadjuvant concurrent chemoradiotherapy or radiotherapy alone in patients with locally advanced rectal cancer. Chin J Radiat Oncol. 2011;20(6):502–505. | ||
Lupattelli M, Matrone F, Gambacorta MA, et al. Preoperative intensity-modulated radiotherapy with a simultaneous integrated boost combined with capecitabine in locally advanced rectal cancer: short-term results of a multicentric study. Radiat Oncol. 2017;12(1):139. | ||
Stuyck C, Wegge M, Bulens P, Joye I, Haustermans K. Moderate dose escalation with volumetric modulated Arc therapy improves outcome in rectal cancer. Acta Oncol. 2017;56(11):1501–1506. | ||
Bae BK, Kang MK, Kim JC, et al. Simultaneous integrated boost intensity-modulated radiotherapy versus 3-dimensional conformal radiotherapy in preoperative concurrent chemoradiotherapy for locally advanced rectal cancer. Radiat Oncol J. 2017;35(3):208–216. | ||
Lin JC, Tsai JT, Chen LJ, Li MH, Liu WH. Compared planning dosimetry of TOMO, VMAT and IMRT in rectal cancer with different simulated positions. Oncotarget. 2017;8(26):42020–42029. | ||
Tournel K, De Ridder M, Engels B, et al. Assessment of intrafractional movement and internal motion in radiotherapy of rectal cancer using megavoltage computed tomography. Int J Radiat Oncol Biol Phys. 2008;71(3):934–939. | ||
Engels B, De Ridder M, Tournel K, et al. Preoperative helical tomotherapy and megavoltage computed tomography for rectal cancer: impact on the irradiated volume of small bowel. Int J Radiat Oncol Biol Phys. 2009;74(5):1476–1480. | ||
De Ridder M, Tournel K, Van Nieuwenhove Y, et al. Phase II study of preoperative helical tomotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2008;70(3):728–734. | ||
Engels B, Tournel K, Everaert H, et al. Phase II study of preoperative helical tomotherapy with a simultaneous integrated boost for rectal cancer. Int J Radiat Oncol Biol Phys. 2012;83(1):142–148. | ||
Huang CM, Huang MY, Tsai HL, et al. A retrospective comparison of outcome and toxicity of preoperative image-guided intensity-modulated radiotherapy versus conventional pelvic radiotherapy for locally advanced rectal carcinoma. J Radiat Res. 2017;58(2):247–259. | ||
Engels B, Platteaux N, Van den Begin R, et al. Preoperative intensity-modulated and image-guided radiotherapy with a simultaneous integrated boost in locally advanced rectal cancer: report on late toxicity and outcome. Radiother Oncol. 2014;110(1):155–159. | ||
Huang MY, Chen CF, Huang CM, et al. Helical tomotherapy combined with capecitabine in the preoperative treatment of locally advanced rectal cancer. Biomed Res Int. 2014;2014(8):1–12. | ||
Burbach JP, Verkooijen HM, Intven M, et al. Randomized controlled trial for pre-operAtive dose-escaLation boost in locally advanced rectal cancer (rectal boost study): study protocol for a randomized controlled trial. Trials. 2015;16(1):58. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.