Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Comprehensive Treatment and Gene Analysis of a Male Patient with Follicular Occlusion Tetrad with Fordyce Granules
Authors Liu B, Wang W , Bi J , Huo R
Received 20 October 2023
Accepted for publication 25 January 2024
Published 1 February 2024 Volume 2024:17 Pages 279—285
DOI https://doi.org/10.2147/CCID.S445823
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Boce Liu,1 Wenjing Wang,1 Jianhai Bi,2,* Ran Huo1,2,*
1Department of Plastic Surgery, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jinan, People’s Republic of China; 2Department of Plastic Surgery, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianhai Bi, Department of Plastic Surgery, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, People’s Republic of China, Tel +86-15110080702, Fax +86 531-68778160, Email [email protected] Ran Huo, Department of Plastic Surgery, Shandong Provincial Hospital of Shandong University, Jinan, Shandong, People’s Republic of China, Tel +86-15168889001, Fax +86 531-68778153, Email [email protected]
Abstract: Follicular occlusion tetrad (FOT) is a chronic inflammatory skin disease that seriously affects patients’ quality of life. At present, there is no standard treatment plan for FOT. We report the case of a 50-year-old male patient diagnosed as having FOT with Fordyce granules and type 2 diabetes mellitus. During hospitalization, the patient received comprehensive and systematic treatment. The patient healed well after surgery and the 10-month follow-up revealed no recurrence. We found eight gene mutations by whole-exome sequencing (WES) of the patient’s peripheral blood.
Keywords: follicular occlusion tetrad, Fordyce granules, gene analysis, comprehensive treatment
Introduction
Follicular occlusion tetrad (FOT) is a complex chronic inflammatory skin disease that refers to the co-occurrence in a person with acne conglobata (AC), hidradenitis suppurativa (HS), perifolliculitis capitis abscedens et suffodiens (PCAS), and pilonidal sinus disease (PSD), which can be accompanied by other diseases and seriously affect patients’ quality of life.1 Fordyce granules (FGs) are ectopic glands in the oral cavity and buccal mucosa.2,3 Many studies have reported FOT; however, few cases are complicated by other diseases. Here, we report a case of FOT with FGs and type 2 diabetes mellitus. The patient healed well after the comprehensive treatment and the 10-month follow-up revealed no recurrence.
Case Report
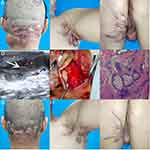
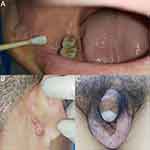
A 50-year-old male patient was hospitalized with multiple systemic abscesses, multiple ulcers, purulent sinus tracts, and sebaceous cysts (Figure 1). He was a Chinese worker with a body mass index of 33.95 kg/m2. The patient was diagnosed with papules, nodules, and pustules in the occipital region, buttocks, and armpits from adolescence, which gradually formed subcutaneous ulcers and sinuses. The sinuses were connected subcutaneously and fused. Some old lesions formed scar hyperplasia and contracture that were accompanied by excruciating pain, which seriously affected the patient’s life. In the past 5 years, the patient had undergone four buttock surgeries and one left axillary surgery at an external hospital; however, the surgical results were unsatisfactory. FGs were observed in the buccal mucosa on the right side of the mouth with no dental abnormalities, a sebaceous cyst had grown behind the right ear for 1 year and there were vitiligo skin lesions on scrotum and glans of the patient (Figure 2). The patient had type 2 diabetes mellitus for >20 years and poor blood glucose control. He also had hearing loss for >5 years and a stutter since childhood. Family history was positive for FOT, the family genetic genealogy is shown in Figure 3.
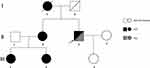 |
Figure 3 This is the family genetic genealogy. |
Owing to the complexity of the patient’s condition, we adopted a comprehensive treatment method involving medication, surgery, and negative-pressure drainage. Before surgery, the patient was injected with insulin to control blood glucose levels. Bacterial culture of secretions from the wounds showed mixed growth of Zurich actinomycetes and Escherichia coli. The patient received intravenous injections of ampicillin and metronidazole before and after surgery. Ultrasonographic examination of the occipital region, buttocks, and armpits revealed thickening of the subcutaneous superficial fascia and mixed echogenic masses with irregular shapes and unclear boundaries. Multiple sinuses were visible between the masses and skin, and strong echoes of hair were observed locally. Gene detection used ethylenediaminetetraacetic acid-anticoagulated peripheral blood of the patient to complete WES. We identified eight gene mutations in CARD14, PARN, GRHL2, FLG, PLEC, RTEL1, BLK, PDX1 and no previously reported mutations in FOT-related (NCSTN, PSENEN, and PSEN1) genes were identified (Table 1). Hair was found in the abscess cavity during surgery. Pathological examination of one lesion revealed suppurative inflammation. The patient underwent debridement and negative-pressure drainage seven times at first. When the drainage volume decreased and fresh granulation tissue grew, the patient underwent a second-stage treatment, including debridement and dressing changes. The FGs did not cause the patient any discomfort, and the patient did not require targeted treatment. After discharge, he was counselled on wound and skin care, oral medication, blood glucose control, weight management and healthy living habits. He was satisfied with the treatment.
 |
Table 1 Gene Mutations Found by Gene Sequencing |
Discussion
In 1956, Pillsbury et al defined the coexistence of HS, AC, and PCAS as follicular occlusion triad.4 In 1975, Plewig and Kilgman proposed the inclusion of PSD and renamed it follicular occlusion tetrad.5 In 1989, Plewig and Steger found that abnormalities in the epithelium of hair follicles were a common pathogenesis of these diseases. Because the clinical characteristics of the pathogenic site were different from those of acne vulgaris, they proposed acne inversa to replace the previous name FOT.6 The clinical feature of FOT is the repeated occurrence of painful papules, herpes, and nodules in areas with abundant sweat glands or folds, which ultimately form abscesses, sinuses, and scars. Inflammation in patients with FOT is not limited to the skin but is systemic and affects other organs. Patients often experience metabolic syndrome, type 2 diabetes mellitus, atherosclerosis, spondyloarthritis or spondyloarthropathy, inflammatory bowel disease, and depression.1 This disease can be companied by Fordyce disease and other obstructive diseases of the hair follicle.7 The etiology and pathogenesis of FOT are not yet clear and genetic factors play a significant role in the occurrence and development of the disease.1,8 The onset of this disease is also related to other factors, including immune factors, endocrine factors, bacterial infection, smoking, and so on. Recently, Wang et al discovered that γ-secretase gene mutations are associated with the pathogenesis of familial FOT. The three mutated genes are NCSTN, PSENEN, and PSEN1. The γ-secretase function is disrupted after these gene mutations, which can cause the γ-secretase–Notch pathway to not transmit normally, finally causing an imbalance in the body’s immune response and resulting in disease.9 The patient was diagnosed with FOT and FGs, but no FOT related gene mutation was found by WES, suggesting that the onset of FOT may be related to other influence factors. Currently, for FOT patients, a multi-step and comprehensive treatment is recommended. The comprehensive and systematic treatment includes surgical treatments, as well as continuous negative pressure suction and oral medication after surgery. Comprehensive treatment not only treats the patient’s condition, but also ensures that the patient’s condition can not recur during long-term follow-up. There are reports that a comprehensive therapy scheme of 5-aminolevulinic acid-mediated interstitial photodynamic therapy (ALA-iPDT) combined with surgery offered excellent response for FOT with complex diseases.10
FGs are ectopic glands in the oral cavity and buccal mucosa. They may be located on the mandibular retromolar pad or the vermilion border of the lips. They appear as yellow-white, asymptomatic 1–3-mm lesions that remain invariable throughout life.2,3
Mutations in PARN and RTEL1 are associated with dyskeratosis congenita (DC).11,12 DC increase the risk of solid tumors in patients, typically head and neck squamous cell carcinoma.11 The risk of secondary cutaneous squamous cell carcinoma increases in patients with FOT owing to the continuous destruction and repair of the lesions and the continuous stimulation of scar growth.8,13 This indicates that the patient had a significantly increased risk of developing squamous cell carcinoma of the skin. This case should be a warning for both patients and doctors.
The present patient had type 2 diabetes mellitus for >20 years. Considering that diabetes occurred in early adulthood and the patient had obesity, insufficient insulin secretion, we believe that the onset of diabetes is not just a coincidence, but may be caused by common mutations in the BLK and PDX1 genes.14,15 The patient’s hearing also has declined without obvious inducement in the past 5 years. The GRHL2 gene has been confirmed to be related to autosomal dominant hereditary hearing loss,16,17 We suspect that his hearing loss is related to a GRHL2 gene mutation.
Conclusion
At present, there are rare reports of FOT complicated with FGs. There is currently no consensus on the treatment of FOT. During the patient’s hospitalization, he received comprehensive and systematic treatment, including 8 surgical treatments, as well as continuous negative pressure suction and oral medication after surgery. The patient’s postoperative healing was good and there was no recurrence during the 10-month follow-up, confirming the effectiveness of our treatment plan. This comprehensive treatment method is innovative, different from the singularity and one-sidedness of existing treatment methods. Comprehensive treatment not only treats the patient’s condition, but also ensures that the patient’s condition can not recur during long-term follow-up. The results of whole exome gene sequencing also provide reference and inspiration for exploring the pathogenesis of FOT.
Ethics Approval and Informed Consent
The patient has signed the informed consent and agreed to publish it. He gave her consent for the publication of identifiable details, which can include photographs and/or case history and/or details within the text to be published in the article. He confirmed that he had seen and been given the opportunity to read the article.
Consent for Publication
Approval for the publication of the patient’s case details was obtained from Shandong Provincial Hospital.
Acknowledgments
We would like to acknowledge the patient who participated in the test and we are grateful for his kind contributions.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sabat R, Jemec GBE, Matusiak Ł, Kimball AB, Prens E, Wolk K. Hidradenitis suppurativa. Nat Rev Dis Primers. 2020;6(1):18. PMID: 32165620. doi:10.1038/s41572-020-0149-1
2. Diajil AR, Goodson ML. Blood group in relation to oral Fordyce’s granules and serum cholesterol level. J Oral Pathol Med. 2023;52(6):521–527. PMID: 37038041. doi:10.1111/jop.13432
3. De Felice C, Parrini S, Chitano G, Gentile M, Dipaola L, Latini G. Fordyce granules and hereditary non-polyposis colorectal cancer syndrome. Gut. 2005;54(9):1279–1282. PMID: 15879014; PMCID: PMC1774669. doi:10.1136/gut.2005.064881
4. Pillsbury DM, Shelley WB, Kligman AM. Dermatology. Vol. 482–484. Philadelphia: Saunders; 1956:489.
5. Plewig G, Kligman AM. Acne. Morphogenesis and Treatment. Berlin: Springer; 1975:192–193.
6. Plewig G, Steger M. Acne inversa (alias acne triad, acne tetrad or hidradenitis suppurativa). In: Marks R, Plewig G, editors. Acne and Related Disorders. London: Martin Dunitz; 1989:345–357.
7. Atzori L, Zanniello R, Pilloni L, Rongioletti F. Steatocystoma multiplex suppurativa associated with hidradenitis suppurativa successfully treated with Adalimumab. J Eur Acad Dermatol Venereol. 2019;33(Suppl 6):42–44. PMID: 31535759. doi:10.1111/jdv.15848
8. Jemec GB. Clinical practice. Hidradenitis suppurativa. N Engl J Med. 2012;366(2):158–164. PMID: 22236226. doi:10.1056/NEJMcp1014163
9. Wang B, Yang W, Wen W, et al. Gamma-secretase gene mutations in familial acne inversa. Science. 2010;330(6007):1065. doi:10.1126/science.1196284
10. Yan J, Zhang G, Liao C, Wang X, Shi L. ALA-iPDT for follicular occlusion tetrad concomitant with pachyonychia congenital type II and ankylosing spondylitis. Photodiagnosis Photodyn Ther. 2022;39:102891. PMID: 35490959. doi:10.1016/j.pdpdt.2022.102891
11. Tummala H, Walne A, Collopy L, et al. Poly(A)-specific ribonuclease deficiency impacts telomere biology and causes dyskeratosis congenita. J Clin Invest. 2015;125(5):2151–2160. PMID: 25893599; PMCID: PMC4463202. doi:10.1172/JCI78963
12. Walne AJ, Vulliamy T, Kirwan M, Plagnol V, Dokal I. Constitutional mutations in RTEL1 cause severe dyskeratosis congenita. Am J Hum Genet. 2013;92(3):448–453. PMID: 23453664; PMCID: PMC3591859. doi:10.1016/j.ajhg.2013.02.001
13. Altunay IK, Gökdemir G, Kurt A, Kayaoglu S. Hidradenitis suppurativa and squamous cell carcinoma. Dermatol Surg. 2002;28(1):88–90. PMID: 11991278. doi:10.1046/j.1524-4725.2002.01090.x
14. Borowiec M, Liew CW, Thompson R, et al. Mutations at the BLK locus linked to maturity onset diabetes of the young and beta-cell dysfunction. Proc Natl Acad Sci U S A. 2009;106(34):14460–14465. PMID: 19667185; PMCID: PMC2732833. doi:10.1073/pnas.0906474106
15. Zhang Y, Fang X, Wei J, et al. PDX-1: a promising therapeutic target to reverse diabetes. Biomolecules. 2022;12(12):1785. PMID: 36551213; PMCID: PMC9775243. doi:10.3390/biom12121785
16. Trebusak Podkrajsek K, Tesovnik T, Bozanic Urbancic N, Battelino S. Novel GRHL2 gene variant associated with hearing loss: a case report and review of the literature. Genes. 2021;12(4):484. PMID: 33810548; PMCID: PMC8066333. doi:10.3390/genes12040484
17. Vona B, Nanda I, Neuner C, Müller T, Haaf T. Confirmation of GRHL2 as the gene for the DFNA28 locus. Am J Med Genet A. 2013;161A(8):2060–2065. PMID: 23813623; PMCID: PMC3884766. doi:10.1002/ajmg.a.36017
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.