Back to Journals » Neuropsychiatric Disease and Treatment » Volume 10
Comprehensive cognitive and cerebral hemodynamic evaluation after cranioplasty
Authors Coelho F, Maynart Oliveira A, Paiva W , Rios Freire F, Tome Calado V, Amorim R , Neville I , Andrade A, Bor-Seng-Shu E, Anghinah R, Teixeira M
Received 12 August 2013
Accepted for publication 4 November 2013
Published 2 May 2014 Volume 2014:10 Pages 695—701
DOI https://doi.org/10.2147/NDT.S52875
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Fernanda Coelho,1 Arthur Maynart Oliveira,2 Wellingson Silva Paiva,2 Fabio Rios Freire,1 Vanessa Tome Calado,1 Robson Luis Amorim,2 Iuri Santana Neville,2 Almir Ferreira de Andrade,2 Edson Bor-Seng-Shu,3 Renato Anghinah,1 Manoel Jacobsen Teixeira2
1Neurorehabilitation Group, Division of Neurology, 2Division of Neurosurgery, 3Neurosonology and Cerebral Hemodynamics Group, University of São Paulo Medical School, São Paulo, Brazil
Abstract: Decompressive craniectomy is an established procedure to lower intracranial pressure and can save patients' lives. However, this procedure is associated with delayed cognitive decline and cerebral hemodynamics complications. Studies show the benefits of cranioplasty beyond cosmetic aspects, including brain protection, and functional and cerebrovascular aspects, but a detailed description of the concrete changes following this procedure are lacking. In this paper, the authors report a patient with trephine syndrome who underwent cranioplasty; comprehensive cognitive and cerebral hemodynamic evaluations were performed prior to and following the cranioplasty. The discussion was based on a critical literature review.
Keywords: cranioplasty, decompressive craniotomy, perfusion CT, traumatic brain injury, cognition, neuropsychological test
Introduction
Decompressive craniectomy (DC) is a treatment option for refractory intracranial hypertension that involves removing a portion of the skull to alleviate swelling.1 Harvey Cushing was the first to describe this technique, and for decades it has been mainly used in the treatment of ischemic stroke and traumatic brain injury.2–5 Despite the reduction of morbidity and mortality associated with this surgical technique, many patients experience early- and late-onset complications such as herniation through the edges of the craniectomy (51%) resulting in brain injury (6% to 58%),6 subdural hygroma (16% to 62%),6–8 hydrocephalus (2% to 29%),9,10 motor impairment,9,11 infection,10 and trephine syndrome (TS) (26%).7
Patients with large skull defects are particularly at risk of developing TS, also known as “sinking skin flap syndrome” or “trephined motor syndrome”. This syndrome is characterized by headache, dizziness, alterations in behavior and mood, seizures, fatigue, motor deficits, and language disturbances.12–14 To date, little is known about the pathophysiological mechanisms underlying this syndrome; however, some authors have hypothesized that abnormal brain pulsatility,15 the effect of atmospheric pressure over the bone defect,13,16–18 changes in cerebrospinal fluid and venous drainage dynamics,19–22 and changes in blood flow and metabolism may all contribute.23–33
Case reports and clinical series published to date suggest improvement in neurological deficits, cognitive function, and brain hemodynamics after bone replacement (cranioplasty),34–40 suggesting potential benefits of this procedure in respect to cognitive aspects. However, little is known about cognitive changes after cranioplasty. The purpose of this paper is to report a comprehensive description of cognitive and hemodynamic changes seen in a patient after cranioplasty.
Materials and methods
Clinical assessment protocol
A patient clinically diagnosed with TS was selected to a prospective protocol. Demographic data, clinical signs, and symptoms were recorded after the completion of a systematic questionnaire regarding the most common symptoms in patients with skull defects. The battery of neuropsychological tests consisted of logical memory and visual reproduction (subtests of the Wechsler Memory Scale – Revised); the Rey Audio-Verbal Learning Test (RAVLT); the Controlled Oral Word Association Test (COWAT-FAS); the Verbal Fluency Test – Animal Category, the Victoria Stroop Test (VST); digits, cubes, arithmetic, sequence of numbers and letters; the Working Memory Index from the Wechsler Adult Intelligence Scale (WMI-WAIS); the Wechsler Memory Scale (WMS) and Wechsler Adult Intelligence Scale; the Trail Making Test A (TMTA) and B (TMTB); the Pfeffer Outpatient Disability Scale (POD); the Rey Complex Figure Test; the Beck Depression Inventory (BDI); the Beck Anxiety Inventory (BAI); the Beck Hopelessness Scale (BHS); the Lipp’s Inventory of Stress Symptoms for adults (ISSL); and the mini-mental state examination (MMSE). The modified Rankin Scale (mRS) and Barthel Index (BI) were recorded to evaluate global functional status. All neuropsychological tests, and the mRS and BI were performed 1 day before cranioplasty. These assessments were repeated 6 months after cranioplasty.
In order to minimize the effects of cumulative learning due to retesting of RAVLT, List A and B, and the recognition test, the words used in the preoperative assessment were replaced by others in the postoperative evaluations. The words used in the tests after surgery were similar with respect to preoperative testing in terms of size (number of letters) and frequency of use by the population. The patient did not undergo a cognitive rehabilitation program in the period between preoperative and postoperative clinical assessments.
Cerebral hemodynamic assessment protocol
The patient was studied with transcranial Doppler ultrasonography (TCD) coupled to a 2-MHz transducer (DWL Doppler Box™, Compumedics Germany GmbH, Singen, Germany) to assess cerebral blood flow velocity from both middle cerebral arteries before and 15 days after cranioplasty. The measurements were conducted while the patient was at rest, in the supine and sitting positions.
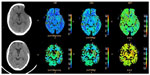
Computed tomography (CT) brain perfusion was also performed before and 30 days after cranioplasty. The parameters of cerebral blood flow (CBF), cerebral blood volume, and mean transit time in four symmetrical brain regions of interest in each hemisphere were evaluated (Figure 1). An Ingenuity Flex32 CT scanner (Philips Healthcare, DA Best, the Netherlands) with 32 channels was used. The scan was based on continuous acquisition at 80 kVp 200 mA for a duration of 40 seconds, allowing a total of 240 images to be obtained; contrast enhancement medium was administered at a rate of 5 mL/second. The CT data were processed using Philips iSite PACS software (Philips Healthcare).
Ethical considerations
This case study was approved by the ethics committee of our institution (CAPPESQ – University of São Paulo Medical School) and informed consent was obtained from the patient or his caregiver.
Literature review
We systematically searched the PubMed database up to September 2013 using the following search terms and combinations: “cranioplasty”, “trephined syndrome”, “sinking skin flap syndrome”, “neurological deficits”, “cerebral decompression”, “brain decompression”, and “decompressive craniotomy”. Reference lists of recovered articles were examined for additional suitable papers. The “Related Articles” feature in PubMed was also used for all selected studies to maximize the probability of locating additional relevant studies. The inclusion criteria for the review was any study including case reports and case series describing clinical improvements after cranioplasty such as improvement of symptoms related to TS or even reversal of neurological deficits and cognitive disturbances. We excluded studies describing only cerebral hemodynamic changes and papers in which interventions other than cranioplasty had been performed (Table 2). Two observers (RLA and WSP) independently reviewed the results of the PubMed search and selected the articles to be included in the analysis; discrepancies were resolved by consensus. We found 1,996 articles, and after matching the inclusion criteria, 29 articles were selected. All studies were case series or case reports.
Case report
A 44-year-old woman with a history of antiphospholipid antibody syndrome (coumarin user) presented with traumatic brain injury after falling to the ground. Upon admission, she scored 12 points on the Glasgow Coma Scale, and a CT scan depicted a massive left frontotemporal subdural hematoma and brain midline shift. The patient underwent a craniotomy to remove the clot and was discharged 2 weeks later. She was conscious and oriented, and exhibited no motor deficit except for a slight difficulty in speaking. Four weeks later, she developed a wound infection. She was treated with antibiotics and the affected bone had to be removed.
The patient was reassessed 8 months later. The bone defect was 8.2 cm × 5.3 cm in size, and the scalp had sunk 2.2 cm from the surface of the skull. A CT scan showed no shift of brain structures, but sulci were enfaced next to the bone defect (Figure 2). The patient presented with headache, tinnitus, dizziness with head movements, incision discomfort, and difficulty in speech. There were no motor deficits, and she received 29 points on the MMSE. The patient scored 2 points on the mRS (ranging 0–6) and 100 points on the BI (ranging 0–100). The patient underwent cranioplasty with methyl methacrylate and was discharged uneventfully on the second postoperative day.
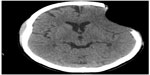  | Figure 2 Cranial computed tomography before performing cranioplasty. |
Six months after surgery, the patient reported complete resolution of symptoms, without changes in motor examination, and a subjective report of improvement in verbal fluency. Neuropsychological assessment before and after cranioplasty showed improvement in memory capacity, language, executive functions, and activities of daily living (Table 1). Postoperative MMSE showed a slight variation (down 1 point). No changes were observed in BI and mRS.
Regarding the cerebral hemodynamic changes, postoperative TCD showed a 20% and 16% increase in the cerebral blood flow velocity in the middle cerebral artery in the supine and the sitting positions, respectively. There was no significant increment of CBF velocity on the contralateral middle cerebral artery.
Perfusion CT study revealed an increase in CBF: 18 to 58 mL/100 g/minute ipsilateral to the cranioplasty and 19 to 70 mL/100 g/minute on the contralateral side. Interestingly, there was a reduction in the mean transit time on both sides: 14.7 to 5.2 seconds (ipsilateral to the cranioplasty) and 13.6 to 4.8 seconds (contralateral side). The increment in cerebral blood volume was less evident: 4.03 to 5.11 mL/100 g (ipsilateral to the cranioplasty) and from 4.34 to 5.46 mL/100 g (contralateral side). The values of blood pressure and heart rate were similar at the time of the tests (Figure 3).
Discussion
The findings of this case report indicate that a skull defect can lead to an objective neuropsychological impairment, not exclusively due to the primary injury. Generally, patients submitted to DC are at risk of death and, consequently, can present with a constellation of neurological symptoms. An implication of this study is that the replacement of the bone (which had been considered merely a matter of cosmesis for decades) may actually confer functional benefits to the patient. Although the first report describing the symptoms caused by the skull defect comes from 1939 (Grant and Norcross),12 researchers have not investigated the late effects of DC and cranioplasty in much detail. Grant and Norcross12 described the first clinical series of patients who underwent cranioplasty and presented with the syndrome of the trephined. Of the 83 operated patients, 43 had symptoms compatible with TS, and improvement occurred in approximately 25% of cases. The improvement in motor signs and symptoms was noted in 14% of patients. Despite the observation that there was cognitive improvement in three cases, there was no information about the variables used in cognitive assessment. Grantham and Landis15 presented similar results in 1948, showing advance in 29% of TS patients and improvement of verbal fluency in some cases.
TS has been described in patients who develop progressive loss of hand strength contralateral to the skull defect in the late phase post-DC.12–14 In 1977, Yamaura and Makino showed that patients with sinking skin at the site of bone defects can present with progressive motor deficit, reversible in 30% of cases after cranioplasty.13 They found that regardless of the underlying disease that led to DC, patients who had more prominent concavity tended to develop motor deficits that could be reversed with cranioplasty. In addition, they observed improvement in neurological deficits in 30% of patients and correlated these findings with modification of standard electroencephalogram. These authors believed that the compressive effects of atmospheric pressure on the brain parenchyma led to neurological impairment, and that the risk of TS occurrence was higher in patients with large bone defects.16,20,34,41 Our patient presented with a significant “brain compression” that was totally reversed after the cranioplasty.
With the advent of techniques for assessing cerebral circulation, studies have shown reduced local cerebral blood flow associated with the compromised cerebral hemisphere. Richaud et al,21 through examinations with inhaled xenon 133, showed a 15%–30% increase in cerebral perfusion and improvement in neurological deficit in approximately eight of 15 patients evaluated. Stiver et al in 200831 showed, through CT perfusion, a possible relationship between the development of neurological deficits and delayed worsening of cerebrospinal fluid circulation by facilitating the passage of liquor into the brain parenchyma adjacent to the previous contused areas. Winkler et al24 used TCD to suggest improvement of cerebrovascular reactivity after cranioplasty, and that restoration of cerebral blood flow was related to improvement in glucose metabolism in the cerebral hemispheres, including areas contralateral to the bone defect. In this study, the mean increase in the rate of glucose metabolism (12.1% on the cranioplasty side and 4.5% on the contralateral side) was associated with a positive clinical prognosis and elevated satisfaction. Of the 13 patients enrolled, seven (53%) had reversal of hemiparesis, two (15%) of hemineglect, two (15%) of aphasia, and seven (53%) showed improvement in cognitive functions (not quantified) after cranioplasty. In our case, there was an increase in cerebral blood flow demonstrated by CT perfusion study and by TCD. However, despite reports of cognitive improvement, the data provided by publications continue to be more qualitative than quantitative.
Some authors claim that cranioplasty may result in improved cognition and neurological signs and symptoms of TS.12,24,39,42–47 Maeshima et al26 used several scales (Word Fluency Test, Frontal Lobe Assessment Battery, Auditory Verbal Learning Test, Raven’s Colored Progressive Matrices and Revised WAIS, MMSE, and Behavioral Inattention Test) to quantify cognitive improvement, correlating these findings with cerebral perfusion data. Likewise, Agner et al48 showed improvement in neurocognitive analysis of 48.3% in the Cognistat scores (Neurobehavioral Cognitive Status Examination, which independently assess different aspects of language, the ability to perform complex constructions, memory, calculations, and reasoning) and of 32.95% in the EXIT interview (Executive Interview, assesses executive functions in the bedside) after cranioplasty.
In our case, the comparative results between the two neuropsychological assessments showed significant improvement in the task of verbal fluency by phonemic category of the COWAT-FAS, having initially received a lower score than expected for the level of schooling and, at a later point in time, scoring above average. It also highlighted better scores for the task of verbal fluency (semantic category of animals), episodic memory (logical memory), audio-verbal learning (RAVLT), information processing speed (TMTA, TMTB, and VST), and visual-constructive functions (copy of Rey Complex Figure Test and cubes).
Our patient attained similar performance with respect to visual memory, logical reasoning ability, and inhibitory control, while demonstrating worsening performance on the attentional tasks (digits, TMTA, TMTB), and occupational memory index (arithmetic, digits, and sequence of numbers and letters). The capacity to perform daily activities and emotional processing showed the most promising results. Taken together, these findings show us that improvement of symptoms and signs of TS, as well as cognitive impairment, could be related to an improvement in the hemodynamic brain patterns in both hemispheres. This report served as a pilot study of a prospective cohort study that includes the entire assessments described here, aiming to evaluate patients with large and small skull defects, due to traumatic or non-traumatic causes.
Conclusion
Although it is difficult to draw conclusions from a single case report, our study provides additional evidence with respect to the possible pathophysiological mechanisms involved in TS, which can be related to a global hemodynamic dysfunction. Moreover, the benefits provided by the cranioplasty suggest that skull defects can also lead, in the long term, to neuropsychological impairments. Further work needs to be done to test these hypotheses with a larger sample size. Therefore, a prospective cohort study is being prepared to address the correlation between brain hemodynamics and clinical findings in such patients.
Disclosure
The authors report no conflicts of interest in this work.
References
Bohman LE, Schuster JM. Decompressive craniectomy for management of traumatic brain injury: an update. Curr Neurol Neurosci Rep. 2013;13(11):392. | |
Cushing H. The establishment of cerebral hernia as a decompressive measure for inaccessible brain tumors; with the description of intramuscular methods of making the bone defect in temporal and occipital regions. Surg Gynecol Obstet 1905;1:297–314. | |
Amorim RL, Bor-Seng-Shu E, Gattás GS, Paiva W, de Andrade AF, Teixeira MJ. Decompressive craniectomy and cerebral blood flow regulation in head injured patients: a case studied by perfusion CT. J Neuroradiol. 2012;39(5):346–349. | |
Kjellberg RN, Prieto A Jr. Bifrontal decompressive craniotomy for massive cerebral edema. J Neurosurg. 1971;34(4):488–493. | |
Kondziolka D, Fazl M. Functional recovery after decompressive craniectomy for cerebral infarction. Neurosurgery. 1988;23(2):143–147. | |
Honeybul S. Complications of decompressive craniectomy for head injury. J Clin Neurosci. 2010;17(4):430–435. | |
Stiver SI. Complications of decompressive craniectomy for traumatic brain injury. Neurosurg Focus. 2009;26(6):E7. | |
Jiang JY, Xu W, Li WP, et al. Efficacy of standard trauma craniectomy for refractory intracranial hypertension with severe traumatic brain injury: a multicenter, prospective, randomized controlled study. J Neurotrauma. 2005;22(6):623–628. | |
Chibbaro S, Tacconi L. Role of decompressive craniectomy in the management of severe head injury with refractory cerebral edema and intractable intracranial pressure. Our experience with 48 cases. Surg Neurol. 2007;68(6):632–638. | |
Yang XJ, Hong GL, Su SB, Yang SY. Complications induced by decompressive craniectomies after traumatic brain injury. Chin J Traumatol. 2003;6(2):99–103. | |
Flint AC, Manley GT, Gean AD, Hemphill JC 3rd, Rosenthal G. Post-operative expansion of hemorrhagic contusions after unilateral decompressive hemicraniectomy in severe traumatic brain injury. J Neurotrauma. 2008;25(5):503–512. | |
Grant FC, Norcross NC. Repair of cranial defects by cranioplasty. Ann Surg. 1939;110(4):488–512. | |
Yamaura A, Makino H. Neurological deficits in the presence of the sinking flap following decompressive craniectomy. Neurol Med Chir (Tokyo). 1977;17(1 Pt 1):43–53. | |
Stiver SI, Wintermark M, Manley GT. Motor trephine syndrome: a mechanistic hypothesis. Acta Neurochir Suppl. 2008;102:273–277. | |
Grantham EG, Landis HP. Cranioplasty and the post-traumatic syndrome. J Neurosurg. 1948;5(1):19–22. | |
Tabaddor K, LaMorgese J. Complication of a large cranial defect: case report. J Neurosurg. 1976;44(4):506–508. | |
Stula D. [Intracranial pressure measurement in large skull defects]. Neurochirurgia (Stuttg). 1985;28(4):164–169. German. | |
Farrington PR. Closure of a defect of the skull with tantalum. Rocky Mt Med J. 1945;42:842–844. | |
Langfitt TW. Increased intracranial pressure. Clin Neurosurg. 1968;16:436–471. | |
Fodstad H, Love JA, Ekstedt J, Fridén H, Liliequist B. Effect of cranioplasty on cerebrospinal fluid hydrodynamics in patients with the syndrome of the trephined. Acta Neurochir (Wien). 1984;70(1–2):21–30. | |
Royall DR, Mahurin RK, Gray KF. Bedside assessment of executive cognitive impairment: the executive interview. J Am Geriatr Soc. 1992;40(12):1221–1226. | |
Segal DH, Oppenheim JS, Murovic JA. Neurological recovery after cranioplasty. Neurosurgery. 1994;34(4):729–731; discussion 731. | |
Kuo JR, Wang CC, Chio C, Cheng TJ. Neurological improvement after cranioplasty – analysis by transcranial Doppler ultrasonography. J Clin Neurosci. 2004;11(5):486–489. | |
Winkler PA, Stummer W, Linke R, Krishnan KG, Tatsch K. Influence of cranioplasty on postural blood flow regulation, cerebrovascular reserve capacity, and cerebral glucose metabolism. J Neurosurg. 2000;93(1):53–61. | |
Isago T, Nozaki M, Kikuchi Y, Honda T, Nakazawa H. Sinking skin flap syndrome: a case of improved cerebral blood flow after cranioplasty. Ann Plast Surg. 2004;53(3):288–292. | |
Maeshima S, Kagawa M, Kishida Y, et al. Unilateral spatial neglect related to a depressed skin flap following decompressive craniectomy. Eur Neurol. 2005;53(3):164–168. | |
Richaud J, Boetto S, Guell A, Lazorthes Y. [Effects of cranioplasty on neurological function and cerebral blood flow]. Neurochirurgie. 1985;31(3):183–188. French. | |
Suzuki N, Suzuki S, Iwabuchi T. Neurological improvement after cranioplasty. Analysis by dynamic CT scan. Acta Neurochir (Wien). 1993;122(1–2):49–53. | |
Sakamoto S, Eguchi K, Kiura Y, Arita K, Kurisu K. CT perfusion imaging in the syndrome of the sinking skin flap before and after cranioplasty. Clin Neurol Neurosurg. 2006;108(6):583–585. | |
Kemmling A, Duning T, Lemcke L, et al. Case report of MR perfusion imaging in sinking skin flap syndrome: growing evidence for hemodynamic impairment. BMC Neurol. 2010;10:80. | |
Stiver SI, Wintermark M, Manley GT. Reversible monoparesis following decompressive hemicraniectomy for traumatic brain injury. J Neurosurg. 2008;109(2):245–254. | |
Yoshida K, Furuse M, Izawa A, Iizima N, Kuchiwaki H, Inao S. Dynamics of cerebral blood flow and metabolism in patients with cranioplasty as evaluated by 133Xe CT and 31P magnetic resonance spectroscopy. J Neurol Neurosurg Psychiatry. 1996;61(2):166–171. | |
Erdogan E, Düz B, Kocaoglu M, Izci Y, Sirin S, Timurkaynak E. The effect of cranioplasty on cerebral hemodynamics: evaluation with transcranial Doppler sonography. Neurol India. 2003;51(4):479–481. | |
Nakamura T, Takashima T, Isobe K, Yamaura A. Rapid neurological alteration associated with concave deformity of the skin flap in a craniectomized patient. Case report. Neurol Med Chir (Tokyo). 1980;20(1):89–93. | |
Ng D, Dan NG. Cranioplasty and the syndrome of the trephined. J Clin Neurosci. 1997;4(3):346–348. | |
Schiffer J, Gur R, Nisim U, Pollak L. Symptomatic patients after craniectomy. Surg Neurol. 1997;47(3):231–237. | |
Kumar GS, Chacko AG, Rajshekhar V. Unusual presentation of the “syndrome of the trephined”. Neurol India. 2004;52(4):504–505. | |
Chieregato A. The syndrome of the sunken skin flap: a neglected potentially reversible phenomenon affecting recovery after decompressive craniotomy. Intensive Care Med. 2006;32(10):1668–1669. | |
Han PY, Kim JH, Kang HI, Kim JS. “Syndrome of the sinking skin-flap” secondary to the ventriculoperitoneal shunt after craniectomy. J Korean Neurosurg Soc. 2008;43(1):51–53. | |
Joseph V, Reilly P. Syndrome of the trephined. J Neurosurg. 2009; 111(4):650–652. | |
Sarov M, Guichard JP, Chibarro S, et al; DECIMAL investigators. Sinking skin flap syndrome and paradoxical herniation after hemicraniectomy for malignant hemispheric infarction. Stroke. 2010;41(3):560–562. | |
Bijlenga P, Zumofen D, Yilmaz H, Creisson E, de Tribolet N. Orthostatic mesodiencephalic dysfunction after decompressive craniectomy. J Neurol Neurosurg Psychiatry. 2007;78(4):430–433. | |
Gottlob I, Simonsz-Tòth B, Heilbronner R. Midbrain syndrome with eye movement disorder: dramatic improvement after cranioplasty. Strabismus. 2002;10(4):271–277. | |
Sugiyama K, Kondo T, Hirayama K, Tobimatsu Y, Urushiyama Y, Izumi S. A case of neurological improvement and facilitation of rehabilitation after cranioplasty. Jpn J Rehabil Med. 2004;41:104–109. | |
Chibbaro S, Vallee F, Beccaria K, et al. [The impact of early cranioplasty on cerebral blood flow and its correlation with neurological and cognitive outcome. Prospective multi-centre study on 24 patients]. Rev Neurol (Paris). 2013;169(3):240–248. French. | |
Di Stefano C, Sturiale C, Trentini P, et al. Unexpected neuropsychological improvement after cranioplasty: a case series study. Br J Neurosurg. 2012;26(6):827–831. | |
Honeybul S, Janzen C, Kruger K, Ho KM. The impact of cranioplasty on neurological function. Br J Neurosurg. 2013;27(5):636–641. | |
Agner C, Dujovny M, Gaviria M. Neurocognitive assessment before and after cranioplasty. Acta Neurochir (Wien). 2002;144(10):1033–1040; discussion 1040. | |
Mokri B. Orthostatic headaches in the syndrome of the trephined: resolution following cranioplasty. Headache. 2010;50(7):1206–1211. | |
de Quintana-Schmidt C, Clavel-Laria P, Asencio-Cortes C, Vendrell-Brucet JM, Molet-Teixido J. [Sinking skin flap syndrome]. Rev Neurol. 2011;52(11):661–664. Spanish. | |
Carota A, Pintucci M, Zanchi F, D’Ambrosio E, Calabrese P. ‘Cognitive’ sinking skin flap syndrome. Eur Neurol. 2011;66(4):227–228. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.