Back to Journals » Vascular Health and Risk Management » Volume 18
Complex Hybrid Repair of a Secondary Aortoenteric Fistula
Authors Koo MPM, Bookun HR , Robinson D
Received 23 February 2022
Accepted for publication 15 April 2022
Published 27 April 2022 Volume 2022:18 Pages 329—333
DOI https://doi.org/10.2147/VHRM.S363417
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Harry Struijker-Boudier
Supplementary video of "Complex Hybrid Repair of a Secondary Aortoenteric Fistula" [ID 363417].
Views: 317
Mei Ping Melody Koo, Hansraj Riteesh Bookun, Domenic Robinson
Department of Vascular Surgery, St Vincent’s Hospital Melbourne, Melbourne, VIC, Australia
Correspondence: Mei Ping Melody Koo, Email [email protected]
Background: Secondary aortoenteric fistula is a rare, highly morbid and often difficult to diagnose, cause of gastrointestinal bleeding. It is associated with prior aortic surgery or placement of a synthetic aortic graft. Our case features staged hybrid endovascular stent-grafting, graft excision, aortoplasty using a bovine pericardial patch, extra-anatomical bypass and complex bowel repair.
Case Report: An 82-year-old man presented with gastrointestinal bleeding and Streptococcus Anginosus bacteraemia, with previous aorto-bi-iliac bypass surgery for left common iliac occlusive disease 15 years ago. Computed tomography angiography (CTA), gastroscopy, colonoscopy, capsule endoscopy and enteroscopy identified no bleeding source. Repeat CTA showed gas locules and stranding around the graft and the third part of the duodenum, concerning for fistulous communication. On the next day, a Zenith TX2 thoracic 28x80mm stent-graft was deployed into the infrarenal aorta. On laparotomy, a fistula was present between the Dacron graft and fourth part of the duodenum. The Dacron graft was excised, followed by aortic patching with bovine pericardium. A right-to-left femoral-femoral crossover graft was constructed. CT at one-month post-laparotomy showed no signs of perigraft endoleak and interval resolution of gas locules. He was transferred to a rehabilitation facility on the 34th post-operative day with a multidisciplinary follow-up arranged.
Discussion: Aortoduodenal fistula is a challenging entity to diagnose and should be suspected in patients with GI bleeding and prior aortic surgery. Endovascular repair alone is a less invasive option but with higher re-infection and late failure rates. Liberal use of appropriate imaging modalities, a judicious repair strategy, long-term follow-up and multidisciplinary approach are critical for its management.
Keywords: aortoenteric fistula, secondary aortoduodenal fistula, staged hybrid repair
Background
Secondary aortoduodenal fistula is a rare and highly morbid cause of gastrointestinal bleeding, which is often challenging to diagnose. It is associated with prior aortic surgery or placement of a synthetic aortic graft (0.3% to 1.6%) and a mortality rate of close to 100% if left untreated.1–3
The principles of treatment involve total or partial resection of the graft, extra-anatomical or in-situ arterial reconstruction and bowel repair. Endovascular stent grafting has been employed as a primary treatment strategy in instances of high operative risk and limited signs of infection, or as a bridge to definitive repair.2
We present a case of secondary aortoduodenal fistula, which underwent staged hybrid endovascular stent-grafting, graft excision, surgical closure of the fistula using a bovine pericardial patch, extra-anatomical bypass and complex bowel repair (Supplementary Video).
Case Report
An 82-year-old man was transferred to our institution for investigation of upper gastrointestinal bleeding and Streptococcus Anginosus bacteraemia, with a history of aorto-bi-iliac bypass surgery for left common iliac occlusive disease 15 years ago. He was admitted three weeks prior to a peripheral hospital for the same issue and investigated with computed tomography angiography (CTA), gastroscopy, colonoscopy, capsule endoscopy, and push enteroscopy without a clear bleeding source identified.
On arrival, he complained of mild central abdominal discomfort and had melaena. His systolic blood pressure was between 120 and 130 mmHg. Haemoglobin concentration was 86 g/L, leukocyte count 9.8x10^9/L and C-reactive protein 77 mg/L. He received three units of packed red blood cells.
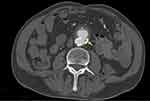
CT angiography, on day 4, showed inflammatory stranding and wall thickening involving the proximal anastomosis of the aorto-bi-iliac graft, and the posterior aspect of the third part of the duodenum. The original aorto-bi-iliac Dacron graft had an end-to-side proximal anastomosis, where small gas locules were present within the wall of the aortic sac where it was crossed by the duodenum, raising the concern for fistulous communication (Figure 1). The native left common iliac artery was occluded, with a patent right common iliac artery and graft limbs. A multi-disciplinary team discussion involving vascular surgery, general surgery, infectious diseases, radiology and gastroenterology units ensued. The decision was made to proceed to a hybrid repair the next day.
A Zenith TX2 thoracic 28×80 mm stent-graft (Cook Medical) was deployed into the proximal infrarenal aorta to cover the origin of the aorto-bi-iliac graft. A fistula was apparent on laparotomy, with the Dacron graft being densely adherent to the fourth part of the duodenum and duodenojejunal flexure. Duodenal resection and primary duodenojejunal anastomosis were carried out. A clamp was applied infrarenally across the aorta with the endograft in-situ, thus preserving visceral circulation. The aorto-bi-iliac graft was excised, and aortic wall and adjacent tissues were debrided. The aortic defect was closed with bovine pericardium patch and the iliac stumps were oversewn. The aortoplasty was performed to ensure that the endograft was not exposed in direct contact with overlying viscera. A right-to-left femoral-femoral crossover bypass graft was constructed with an 8 mm ring-reinforced PTFE.
Intraoperatively, colonic ischaemia from the caecum to the upper rectum was noted, and he underwent subtotal colectomy and ileostomy formation.
Bacterial culture of specimens grew Streptococcus Anginosus, Staphylococcus Haemolyticus and Candida Glabrata. Histopathology revealed an area of perforation in the duodenum with a clean base showing chronic inflammation, and ischaemic colitis from the splenic flexure to the sigmoid colon. Perioperatively, piperacillin-tazobactam, vancomycin and fluconazole were administered intravenously.
The postoperative course was prolonged and characterised by gastrointestinal complications. A CT scan was made one month postoperatively and showed no signs of perigraft endoleak, a patent femoral-femoral crossover graft, a small fluid collection adjacent to the left inferior aspect of the abdominal aorta with interval resolution of internal gas locules (Figure 2). The patient was transferred to a peripheral hospital for rehabilitation on the 34th postoperative day and prescribed amoxicillin and clavulanate, rifampicin, fusidic acid and fluconazole, with a plan of lifelong antibiotics. The patient unfortunately deconditioned further and passed away on the 62th postoperative day from hospital acquired pneumonia.
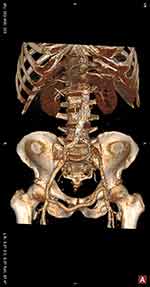 |
Figure 2 (A) CT 3D reconstruction image showing endovascular aortic stent-graft and right-to-left femoral-femoral crossover PTFE graft. |
Discussion
Endovascular repair has been introduced in recent years as a less invasive management option for aortoenteric fistula, with lower early morbidity and mortality. However, endovascular repair alone has been complicated by postoperative recurrence of sepsis and fistula.4–6 Burks et al reported a survival rate of 43% at 36 months (range, 23–67 months) from a five-year experience in endovascular repair of bleeding aortoenteric fistula; a Greek multicentre study also showed similar mortality rate between endovascular and open repair.2,7
This report demonstrates the hybrid management of an aortoduodenal fistula in the context of bacteraemia, incorporating endovascular techniques, definitive excision of polyester vascular prosthesis, and open arterial and bowel reconstruction. Previous reports have demonstrated that duodenorrhaphy with omentum interposition, and omental wrapping for infected aortic graft preservation are associated with long-term sepsis-free survival.8–10 The choice of the bovine pericardial patch for arterioplasty was made in this case to minimise the total operating time, which had been significant at just over 12 hours. Given that there have been cases of re-fistulisation and reinfection following prosthetic aortic repair, long-term close follow-up is warranted.11,12
Aortoduodenal fistula is a challenging entity to diagnose and manage and should be suspected in patients with gastrointestinal bleeding who have had prior aortic surgery. Liberal use of appropriate imaging modalities, a multidisciplinary approach and a high index of suspicion are critical in this process.13 The hybrid approach described provides a staged endovascular bridging option prior to complex open arterial and bowel reconstructions.
Consent Statements
Consent has been obtained from the patient’s next of kin for publication purposes. Institutional approval was not required for case publication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Khan A, Ahmad E, Javaid S, Sankari M. An insidious gastrointestinal bleeding from secondary aortoduodenal fistula leading to septic shock. Case Rep Gastrointest Med. 2019;2019:1–4. doi:10.1155/2019/6261526
2. Burks J, Faries P, Gravereaux E, Hollier L, Marin M. Endovascular repair of bleeding aortoenteric fistulas: a 5-year experience. J Vasc Surg. 2001;34(6):1055–1059. doi:10.1067/mva.2001.119752
3. Lemos DW, Raffetto JD, Moore TC, et al. Primary aortoduodenal fistula: a case report and review of the literature. J Vasc Surg. 2003;37:686–689. doi:10.1067/mva.2003.101
4. Biancari F, Romsi P, Perälä J, Koivukangas V, Cresti R, Juvonen T. Staged endovascular stent-grafting and surgical treatment of a secondary aortoduodenal fistula. Eur J Vasc Endovasc Surg. 2006;31(1):42–43. doi:10.1016/j.ejvs.2005.09.007
5. González-Fajardo J, Gutiérrez V, Martín-Pedrosa M, Del Rio L, Carrera S, Vaquero C. Endovascular repair in the presence of aortic infection. Ann Vasc Surg. 2005;19(1):94–98. doi:10.1007/s10016-004-0144-0
6. Chuter T, Lukaszewicz G, Reilly L, et al. Endovascular repair of a presumed aortoenteric fistula: late failure due to recurrent infection. J Endovasc Ther. 2000;7(3):240–244. doi:10.1177/152660280000700312
7. Kakkos S, Antoniadis P, Klonaris C, et al. Open or endovascular repair of aortoenteric fistulas? A multicentre comparative study. Eur J Vasc Endovasc Surg. 2011;41(5):625–634. doi:10.1016/j.ejvs.2010.12.026
8. Rodrigues Dos Santos C, Casaca R, Mendes de Almeida J, Mendes-Pedro L. Enteric repair in aortoduodenal fistulas: a forgotten but often lethal player. Ann Vasc Surg. 2014;28(3):756–762. doi:10.1016/j.avsg.2013.09.004
9. Jamieson R, Burns P, Dawson A, Fraser S. Aortic graft preservation by debridement and omental wrapping. Ann Vasc Surg. 2012;26(3):
10. Andrade D, Vinck E, Niño Torres L. Two-stage omental flap approach for ascending aortic graft infection. Braz J Cardiovasc Surg. 2020;35(3). doi:10.21470/1678-9741-2020-0040
11. Lutz B, Reeps C, Biro G, Knappich C, Zimmermann A, Eckstein H. Bovine pericardium as new technical option for in situ reconstruction of aortic graft infection. Ann Vasc Surg. 2017;41:118–126. doi:10.1016/j.avsg.2016.07.098
12. Arima D, Suematsu Y, Kurahashi K, Shimizu T, Nishi S, Yoshimoto A. Recurrence of aortoenteric fistula after endovascular aortic repair. Ann Vasc Dis. 2020;13(1):90–92. doi:10.3400/avd.cr.19-00106
13. Yoshimoto K, Shiiya N, Onodera Y, Yasuda K. Secondary aortoenteric fistula. J Vasc Surg. 2005;42(4):805. doi:10.1016/j.jvs.2004.06.029
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.