Back to Journals » OncoTargets and Therapy » Volume 12
Complete response with fluorouracil and irinotecan with a BRAFV600E and EGFR inhibitor in BRAF-mutated metastatic colorectal cancer: a case report
Authors Wang Z, Dai WP, Zang YS
Received 19 July 2018
Accepted for publication 5 December 2018
Published 8 January 2019 Volume 2019:12 Pages 443—447
DOI https://doi.org/10.2147/OTT.S180845
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Zhan Wang*, Wei-Ping Dai*, Yuan-Sheng Zang
Department of Medical Oncology, Changzheng Hospital, Second Military Medical University, Shanghai 200003, China
*These authors contributed equally to this work
Background: Patients with BRAF (v-Raf murine sarcoma viral oncogene homolog B) V600E-mutated metastatic colorectal cancer (mCRC) have a poor prognosis. The Southwest Oncology Group (SWOG) 1406 study evaluated the efficacy of vemurafenib in combination with irinotecan and cetuximab for simultaneous inhibition of epidermal growth factor receptor (EGFR) and BRAF in patients with BRAFV600E-mutated mCRC. Although the combination achieved higher progression-free survival (PFS) and disease control rates (DCRs), there was no complete response (CR) for the drug combination. In this case report, we report the complete recession of metastasis in a patient treated with irinotecan, cetuximab, vemurafenib, and 5-fluorouracil.
Case presentation: A 44-year-old male patient with hepatitis B was diagnosed with right-sided colon adenocarcinoma. He was treated with capecitabine plus oxaliplatin as postoperative adjuvant chemotherapy for eight cycles with a disease-free survival (DFS) of 1 year before the emergence of peritoneal and pelvic metastases. BRAFV600E mutation was positive and chemotherapy included 12 courses of 5-fluorouracil, vemurafenib, irinotecan, and cetuximab. Complete response with recession of metastases was observed.
Conclusion: The combination of fluorouracil and irinotecan with a BRAFV600E and EGFR inhibitor may have synergistic action, leading to recession of secondary metastases in patients with BRAFV600E-mutated colorectal cancer.
Keywords: mCRC, BRAFV600E mutation, fluorouracil, vemurafenib, irinotecan, cetuximab
Background
Colorectal cancer (CRC) is the third and second most frequent cancer in men and women, respectively.1 It is one of the leading causes of cancer-associated mortalities in Chinese population.2 Although histologically similar, CRCs are diverse with respect to the underlying molecular mechanism which could be explored for planning treatment strategies. Chromosomal instability, microsatellite instability, and errors in DNA repair machinery are the most frequent molecular mechanisms involved in various subgroups of CRCs.3
BRAF (v-raf murine sarcoma viral oncogene homolog B1) serine/threonine protein kinase is a downstream signaling protein in the epidermal growth factor receptor (EGFR)-activated mitogen-activated protein kinase (MAPK) pathway.4 The V600E mutation in BRAF leads to constitutive activation of MAPK pathway, and it is mostly associated with epigenetic activation of MLH1, leading to a microsatellite instability phenotype in patients with CRC.5 The RAS/RAF/MAPK pathway is downstream of EGFR and mutation in any gene involved in this pathway also contributes to progression of CRC.6 BRAFV600E mutation defines a specific CRC subgroup with poor prognosis.7,8 BRAF and extended RAS mutations are mutually exclusive with mutation in one of the genes, signifying wild-type phenotype in the other, which might be due to the redundancy of the mutations in both the genes for CRC development.9
A previous randomized crossover clinical trial evaluated the efficacy of irinotecan and cetuximab with and without vemurafenib in patients with BRAFV600E-mutated CRC. The rationale behind the strategy is the simultaneous inhibition of EGFR and BRAFV600E-mutant along with a cytotoxic agent to control metastatic CRC (mCRC). The addition of vemurafenib led to an increase in median progression-free survival (PFS) (4.4 vs 2 months) and disease control rate (DCR) (67% vs 22%). However, there was no complete response indicated by the lack of metastatic tumor mass recession.10
In this case report, we report the successful treatment to a 44-year-old hepatitis B-positive male patient diagnosed with right-sided colon adenocarcinoma with peritoneal and pelvic metastases, with vemurafenib, irinotecan, and cetuximab along with 5-fluorouracil.
Case presentation
A 44-year-old male patient was diagnosed with right-sided colonic carcinoma (hepatic flexure) by electronic colonoscope, which was confirmed by biopsy (December 22, 2016). A family enquiry revealed no incidence of CRC in first- or second-degree relatives ruling out hereditary nonpolyposis colorectal cancer (HNPCC). Furthermore, serological analysis revealed that the patient was positive for hepatitis B (HBsAg+ and HBeAg+), which led to the immediate initiation of telbivudine therapy (600 mg qd). Serum carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9 were 1.09 μg/L and 272.3 U/L, respectively. A full-body computed tomography (CT) found no metastases prompting open surgery for CRC (May 1, 2017). Surgical pathology reported poorly differentiated adenocarcinoma with sub-serosa pericolic fat invasion. One of the 16 regional lymph nodes was positive, and the resection margin was negative for cancerous tissue. The overall pathology report indicated a T3 N1 M0 stage of adenocarcinoma with probability of metastases.
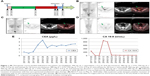
The surgical adjuvant therapy included oxaliplatin, 200 mg, d1 + capecitabine, 1.5 g, d1 to d14 for eight cycles (February 9, 2017 to August 10, 2017). Evidence suggestive of metastases was not observed for almost 1 year with normal CEA and CA 19-9 levels. A positron emission tomography (PET) scan on January 3, 2018 indicated peritoneal and pelvic metastases (Figure 1B), with concomitant rise in CEA (3.15 μg/L) and CA 19-9 (886 U/L) levels. On January 12, 2018, he was switched to FOLFIRI (irinotecan, 380 mg, d1 + 5-fluorouracil, 750 mg, d1 + maintenance dose of 4.75 gm, 5-fluorouracil for 46 hours) treatment.
At the same time, the tissue sample from the initial surgery was subjected to next-generation sequencing (NGS) using Colorectal core™ panel (Burning Rock Dx, Guangzhou, China) to check for mutation in 56 different genes with therapeutic implications in targeted CRC therapy. NGS testing identified BRAFV600E mutation with extended wild-type RAS. Other genetic anomalies identified by NGS included the following mutations and amplifications: BRCA2D306V, ATMR337C, L1814F, RNF43R132, TP53R248W, Myc (copy number increase), and MCL1 (copy number increase). The clinical relevance of the mutations other than BRAFV600E in CRC is not yet established.
The NGS test results were confirmed from plasma samples with circulating tumor DNA (ctDNA) in a China Food and Drug Administration (CFDA)-approved real-time PCR assay (Super-ARMS EGFR Mutation Detection Kit, Amoy Diagnostics, Xiamen, China). The real-time PCR assay targeted 17 KRAS, 13 NRAS, and 6 BRAF mutations encompassing multiple exons of the respective genes using highly sensitive probes. The only mutation observed in real-time PCR assay is the BRAFV600E mutation.
With the genetic testing results, his treatment regimen also included vemurafenib and cetuximab (irinotecan, 380 mg, d1 + 5-FU, 750 mg, d1 + 5-FU, 4.75 g, d1 with maintenance for 46 hours + vemurafenib, 960 mg, qd + cetuximab, 900 mg, d1) along with FOLFIRI (folinic acid, fluorouracil, and irinotecan) which was started from January 26, 2018. This treatment regimen was scheduled for 12 cycles (first cycle: irinotecan + 5-FU; second cycle: irinotecan + 5-fluorouracil + vemurafenib + cetuximab) and a PET/CT scan on February 24, 2018 (after second treatment cycle) (Figure 1C) showed recession of metastases with less sero-peritoneal invasion. A subsequent PET/CT scan on May 31, 2018 (after 10th treatment cycle) (Figure 1D) showed complete recession of metastases with no sero-peritoneal invasion. Concomitant decrease in serum CEA (2.79 μg/L) and CA 19-9 (36.92 U/L) (Figure 1E) levels was also observed. The overall clinical progression along with the treatment course is given in Figure 1A.
During the treatment course, the patient experienced grade 1/2 adverse events such as rash, diarrhea, and neutropenia, requiring no specific treatment.
Discussion and conclusion
BRAFV600E mutation in patients with CRC is a unique molecular subtype occurring in ~10% of patients with mCRC and is associated with poor prognosis in the metastatic stage despite good early stage prognosis.11,12 Hence, aggressive combination chemotherapy is required to improve the survival rate in patients with BRAFV600E mCRC.13
The standard therapy includes surgery followed by adjuvant chemotherapy in combination with targeted therapy, which improves overall survival (OS).14 Sequential combination chemotherapy also plays an important role in CRC management, especially in mCRC.15 Cytotoxic drugs such as 5-fluorouracil combined with oxaliplatin or irinotecan are still the preferred chemotherapeutic regimen for mCRC.16 They have a synergistic effect when combined with biological agents such as EGFR and BRAFV600E-specific inhibitors.17
Our patient presented with metastatic CRC a year after surgical removal of right-sided colonic carcinoma and was found to have BRAFV600E mutation by NGS. His metastases were successfully treated by simultaneous inhibition of BRAFV600E and EGFR receptor by vemurafenib and cetuximab along with the cytotoxic drugs such as irinotecan and 5-fluorouracil.
In Southwest Oncology Group (SWOG) 1406 trial, patients with BRAFV600E-mutant mCRC were treated with cetuximab, irinotecan, and vemurafenib. There was no indication of complete response in their study. However, the increased DFS in the vemurafenib-treated group indicated the synergistic activity of cetuximab in combination with vemurafenib.10 Furthermore, the combination of BRAF (encorafenib) and EGFR (cetuximab) inhibitors along with MEK inhibitor (binimetinib) has been reported to be associated with an objective response rate (ORR) of 41% including a case that showed complete response in the BEACON (NCT02928224) trial.18
BRAFV600E mutation modifies the kinase domain of BRAF, leading to the monomeric BRAF, activating the downstream signaling pathways.19 Vemurafenib is a small-molecule tyrosine kinase inhibitor with BRAFV600E-specific (monomer-specific) inhibitor activity which may lead to decreased activity of the MAPK pathway. In patients with BRAFV600E-mutated melanoma, vemurafenib monotherapy was found to be effective, which prompted its use in CRC. However, in patients with CRC, vemurafenib blocks extracellular-signal-regulated kinase (ERK) signaling, which releases upstream receptors from ERK-dependent negative feedback, resulting in increased ligand-dependent signaling that leads to subsequent activation of RAS. This generates RAF inhibitor-resistant RAF dimers.20 This associated rebound in ERK signaling is modest in BRAF-mutant melanomas which is not the case with CRC.21
Cetuximab, which is a monoclonal antibody targeted against EGFR receptor, when combined with vemurafenib, prevents the feedback activation of RAS.22,23 It is not effective in KRAS-mutated (codon 12 and 13) CRCs,24 and the mutually exclusive nature of KRAS and BRAF mutations makes it a viable treatment option for BRAF-mutated CRCs. Furthermore, cetuximab has shown to revert irinotecan resistance in preclinical studies.25 This along with the positive results in animal experiments may have led to the initiation of the SWOG 1406 clinical trial. The treatment regimen in our patients included 5-fluorouracil along with irinotecan, cetuximab, and vemurafenib. We observed complete recession of metastases in our patients, which may be due to the additive cytotoxic effects of 5-fluorouracil. The rationale behind including 5-fluorouracil is the reported augmentation of cytotoxic activity of irinotecan in previous studies.26 The combination of fluorouracil with cetuximab may also result in reversion of fluorouracil resistance, which is similar to reversion of irinotecan resistance. Also, it is evident that the combination of two cytotoxic drugs with cetuximab has been effective in converting unresectable liver metastases into resectable form.27 Also, a post hoc analysis of the primary cancer site from the Cancer and Leukemia B and SWOG 80405 trial comparing cetuximab and bevacizumab in combination with chemotherapy revealed left-sided primary cancer to have relatively higher OS compared with right-sided primary carcinoma.28 Contrary to the previous publication, we observed complete recession of mCRC with right-sided primary carcinoma in our case study. Hence, we suggest that this treatment regimen could be explored as a first-line treatment for unresectable CRCs.
Furthermore, hepatitis B in patients with CRC is reported to reduce the risk of liver metastasis with simultaneous increase in extrahepatic metastasis.29 Furthermore, chemotherapy is reported to reactivate hepatitis B in patients with CRC, who were HBsAg-negative previously.30 Hence, telbivudine (600 mg qd) was administered throughout the chemotherapeutic period to obtain the optimal effects of the treatment.
The presence of only minor adverse events indicates that the drug combination is well tolerated in our case. Although we could not ascertain the long-term recurrence of mCRC and safety in our patient, the drug combination could be an option as first-line therapy for treating similar patients.
To conclude, this case report suggests that the combination of 5-fluorouracil, vemurafenib, cetuximab, and irinotecan therapy may be an option for BRAFV600E mutations in patients with mCRC, which could be further explored in larger prospective studies to vindicate our claim.
Ethics approval and consent to participate
The study was approved by the institutional ethical committee and the patient signed an informed consent to participate in the study.
Consent for publication
Written informed consent for publication of clinical details and clinical images was obtained from the patient. A copy of the consent form is available for review by the editor of this journal.
Acknowledgments
The authors acknowledge Dr Kaushik Subramanian G (PhD) and Dr Anuradha Nalli (PhD) from Indegene Pvt. Ltd., Bangalore, India, for their assistance in medical writing and critical evaluation of the supporting literature while drafting this manuscript.
Author contributions
Zhan Wang and Wei-Ping Dai contributed to the concept and design, acquisition, analysis and interpretation of data. All authors contributed equally in data analysis, drafting and revising the article and gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Tárraga López PJ, Albero JS, Rodríguez-Montes JA. Primary and secondary prevention of colorectal cancer. Clin Med Insights Gastroenterol. 2014;7:33–46. | ||
Gu MJ, Huang QC, Bao CZ, et al. Attributable causes of colorectal cancer in China. BMC Cancer. 2018;18(1):38. | ||
Kudryavtseva AV, Lipatova AV, Zaretsky AR, et al. Important molecular genetic markers of colorectal cancer. Oncotarget. 2016;7(33):53959–53983. | ||
Mccain J. The MAPK (ERK) Pathway. P T. 2013;38(2):96–108. | ||
Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38(7):787–793. | ||
Ruzzo A, Graziano F, Canestrari E, Magnani M. Molecular predictors of efficacy to anti-EGFR agents in colorectal cancer patients. Curr Cancer Drug Targets. 2010;10(1):68–79. | ||
Morikawa T, Inada R, Nagasaka T, et al. BRAF V600E mutation is a predictive indicator of upfront chemotherapy for stage IV colorectal cancer. Oncol Lett. 2018;15(2):2195–2201. | ||
Bahrami A, Hesari A, Khazaei M, Hassanian SM, Ferns GA, Avan A. The therapeutic potential of targeting the BRAF mutation in patients with colorectal cancer. J Cell Physiol. 2018;233(3):2162–2169. | ||
Morkel M, Riemer P, Bläker H, Sers C. Similar but different: distinct roles for KRAS and BRAF oncogenes in colorectal cancer development and therapy resistance. Oncotarget. 2015;6(25):20785–20800. | ||
Kopetz S, Mcdonough SL, Lenz H-J, et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406). J Clin Oncol. 2017;35(15 Suppl):3505. | ||
Cohen R, Cervera P, Svrcek M, et al. BRAF-Mutated Colorectal Cancer: What Is the Optimal Strategy for Treatment? Curr Treat Options Oncol. 2017;18(2):9. | ||
Sueda T, Sakai D, Kawamoto K, et al. BRAFV600E inhibition stimulates AMP-activated protein kinase-mediated autophagy in colorectal cancer cells. Sci Rep. 2016;6(1):18949. | ||
Strickler JH, Wu C, Bekaii-Saab T. Targeting BRAF in metastatic colorectal cancer: maximizing molecular approaches. Cancer Treat Rev. 2017;60:109–119. | ||
Tampellini M, di Maio M, Baratelli C, et al. Treatment of Patients with Metastatic Colorectal Cancer in a Real-World Scenario: Probability of Receiving Second and Further Lines of Therapy and Description of Clinical Benefit. Clin Colorectal Cancer. 2017;16(4):372–376. | ||
Mcquade RM, Stojanovska V, Bornstein JC, Nurgali K. Colorectal Cancer Chemotherapy: The Evolution of Treatment and New Approaches. Curr Med Chem. 2017;24(15):1537–1557. | ||
Lucas AS, O’Neil BH, Goldberg RM. A decade of advances in cytotoxic chemotherapy for metastatic colorectal cancer. Clin Colorectal Cancer. 2011;10(4):238–244. | ||
Gustavsson B, Carlsson G, Machover D, et al. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer. 2015;14(1):1–10. | ||
van Cutsem E, Cuyle P-J, Huijberts S. BEACON CRC study safety lead-in (SLI) in patients with BRAFV600E metastatic colorectal cancer (mCRC): Efficacy and tumor markers. JCO. 2018;36(4 Suppl):627–627. | ||
Cantwell-Dorris ER, O’Leary JJ, Sheils OM. BRAFV600E: implications for carcinogenesis and molecular therapy. Mol Cancer Ther. 2011;10(3):385–394. | ||
Yaeger R, Cercek A, O’Reilly EM, et al. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Clin Cancer Res. 2015;21(6):1313–1320. | ||
Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. | ||
Hong DS, Morris VK, El Osta B, et al. Phase IB Study of Vemurafenib in Combination with Irinotecan and Cetuximab in Patients with Metastatic Colorectal Cancer with BRAFV600E Mutation. Cancer Discov. 2016;6(12):1352–1365. | ||
Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N Engl J Med. 2015;373(8):726–736. | ||
Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27(12):2091–2096. | ||
Rodriguez J, Zarate R, Bandres E, et al. Combining chemotherapy and targeted therapies in metastatic colorectal cancer. World J Gastroenterol. 2007;13(44):5867–5876. | ||
Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355(9209):1041–1047. | ||
Van Cutsem E, Oliveira J, On behalf of the ESMO Guidelines Working Group. Advanced colorectal cancer: ESMO Clinical Recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl 4):iv61–iv63. | ||
Venook AP, Niedzwiecki D, Innocenti F, et al. Impact of primary (1o) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance). JCO. 2016;34(15 Suppl):3504–3504. | ||
Qiu HB, Zhang LY, Zeng ZL, et al. HBV infection decreases risk of liver metastasis in patients with colorectal cancer: a cohort study. World J Gastroenterol. 2011;17(6):804–808. | ||
Okagawa Y, Takada K, Hisai H, et al. Successful treatment with entecavir for reactivation of hepatitis B virus following systemic chemotherapy in a hepatitis B surface antigen-negative patient with colorectal cancer. Intern Med. 2014;53(16):1759–1762. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.