Back to Journals » International Journal of General Medicine » Volume 15
Complete Blood Count and Myocardial Markers Combination with Sequential Organ Failure Assessment Score Can Effectively Predict the Mortality in Sepsis: A Derivation and Validation Study
Authors Wen K , Du H, Tang B, Xiong B, Zhang A, Wang P
Received 16 November 2021
Accepted for publication 8 March 2022
Published 23 March 2022 Volume 2022:15 Pages 3265—3280
DOI https://doi.org/10.2147/IJGM.S349751
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Keli Wen, Hu Du, Binfei Tang, Bin Xiong, An Zhang,* Pengfei Wang*
Department of Critical Care Medicine, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, 400010, People’s Republic of China
*These authors contributed equally to this work
Correspondence: An Zhang; Pengfei Wang, Department of Critical Care Medicine, The Second Affiliated Hospital of Chongqing Medical University, No. 74 Linjiang Road, Yuzhong District, Chongqing, 400010, People’s Republic of China, Tel +86 23-63693452, Email [email protected]; [email protected]
Purpose: The purpose of our study was to explore the prognostic value of complete blood count and myocardial markers combination with Sequential Organ Failure Assessment (SOFA) score in predicting the 28-day mortality among sepsis patients.
Patients and methods: A retrospective observational cohort study was performed. Three hundred and nineteen sepsis patients who were hospitalized at the Second Affiliated Hospital of Chongqing Medical University, China, from January 2019 to September 2021 were included. The clinical and laboratory data, the Acute Physiological and Chronic Health Evaluation II (APACHE II) score and SOFA score at the time of the initial sepsis diagnosis were collected, and the predictive values of the single and combination variables for 28-day mortality were compared.
Results: The derivation cohort consisted of 221 patients and included 59 (26.7%) died. The area under the curve (AUC) [95% confidence interval (CI)] of RDW and cTnT were 0.735 (0.663– 0.807) and 0.753 (0.678– 0.827) for mortality, and the cut-off value were 14.05% and 0.039 ng/mL, respectively. The combination of RDW, cTnT and the SOFA score showed a better performance for the prediction of mortality, and the AUC was significantly higher than that of the SOFA score (0.791 vs 0.726, DeLong test: P=0.032). Multivariate Cox analysis identified that the combination of RDW, cTnT and the SOFA score (HR=6.133, P=0.004) and APACHE II score (HR=1.093, P< 0.001) were independent detrimental factors for 28-day mortality. The validation cohort consisted of 98 patients and included 23 (23.5%) died. Similarly, the AUC of the RDW, cTnT and the SOFA score combination is significantly higher than that of the SOFA score (0.821 vs 0.739, DeLong test: P=0.035).
Conclusion: RDW and cTnT showed good performance in predicting 28-day mortality rates among patients with sepsis. Combined RDW and cTnT with the SOFA score can significantly improve the predictive value of SOFA score for the prognosis of sepsis.
Keywords: sepsis, septic shock, Sequential Organ Failure Assessment score, red blood cell distribution width, cardiac troponin T, marker
Introduction
Sepsis is considered as a life-threatening systemic clinical syndrome caused by infection, involving activation of inflammation, coagulation, immune and endocrine, and ultimately leads to dysfunction of one or more organs.1–3 In-hospital mortality for sepsis was slightly declined in recent years due to the significant advances in critical care and treatment, but hospitalization rate was still increased. The 30-day and 180-day readmission rates among survivors of sepsis also increased to 26% and 48%, respectively, resulting in a heavy financial burden and severely impaired in quality of life.2–4
There is currently no specific therapy for sepsis. Earlier recognition of risk factors, timely intervention and more effective infection control are still the keys to improve the prognosis of sepsis. Sequential Organ Failure Assessment (SOFA) score, a widely used and well-proven scoring system for assessing organ dysfunction, is also a validity method to predict in-hospital mortality in patients with suspected infection. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) consent to the use of SOFA score for diagnosis of Sepsis.1,5 However, not all target organs that may be damaged by sepsis are included in the SOFA scoring system. Using it alone may result in delayed or missed diagnosis of sepsis. It is reasonable to assume that SOFA score combined with biomarkers may increase the sensitivity of sepsis diagnosis. There is growing evidence that cardiovascular dysfunction occurs frequently in patients with sepsis, called septic cardiomyopathy (SCM), and may contribute to circulatory failure and worse multiple organ dysfunction. We can detect abnormal heart movements utilizing echocardiography and to guide therapeutic decisions. The manifestations were systolic or diastolic dysfunction, left or right ventricular dysfunction, global hypokinesia or local ventricular wall movement abnormalities, or even mixed.6 However, sometimes echocardiography may not be immediately available due to the lack of trained technicians. With the popularity of Point-of-Care Testing, serum myocardial markers have been widely used in sepsis and may represent a potential diagnostic avenue for SCM.6 A complex and persistent set of functional and phenotypic changes in innate and adaptive immune cells play a central role in the septic inflammatory response, and leukocytosis or leukopenia can be observed at an early stage.7 Excessive release of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-17 and bacterial products up-regulated the expression of granulocyte-macrophage-colony stimulating factor (GM-CSF), can stimulate the production of circulating neutrophils.8 Studies of septic patients and animal sepsis models showed that lymphocyte apoptosis was an important pathogenesis of sepsis immunoparalysis, evidenced by accelerated apoptosis of lymphocytes only observed in patients with sepsis.9 A decrease in platelet (PLT) and hemoglobin (Hb) counts is not unusual in patients with severe sepsis. Thrombocytopenia and anaemia occurring during sepsis are considered as a marker of disease severity and a higher rate of death.10–12 The neutrophil-to-lymphocyte ratio (NLR) and red cell distribution width (RDW) as neotype inflammatory markers have recently been shown to be associated with poor prognosis.13,14
In this research, we analyzed the clinical data and outcomes of 319 septic patients to discuss whether combining complete blood count and myocardial markers into SOFA score can help to improve its accuracy and practicability.
Methods
Study Population
This retrospective cohort study was conducted at the Second Affiliated Hospital of Chongqing Medical University in China from January 2019 to September 2021. All adult patients (≥18 years old) were hospitalized in the intensive care unit (ICU) for sepsis and/or septic shock. All cases met the criteria for the diagnosis of sepsis 3.0.1 Sepsis was defined as an exact infection accompanied by an increase of 2 points or more in the SOFA score. Septic shock was diagnosed if serum lactate level was >2 mmol/L or vasopressors were required to maintain a mean arterial pressure ≥65 mmHg after adequate fluid resuscitation. The exclusion criteria were as follows: patients with hematological disease, chronic renal insufficiency, liver cirrhosis, pregnant females, or received medication that may induce morphologic changes in hemocyte (such as erythropoietin, recombinant human granulocyte stimulating factor), radiotherapy and/or chemotherapy-induced myelosuppression, acute coronary syndrome, chronic heart failure, consciousness disorder due to factors other than sepsis and age younger than 18 years. All enrolled survivors were followed up for 28 days.
Data Collection
Laboratory parameters including oxygenation index, Hb, RDW, white blood cell count (WBC), neutrophil percentage (NEU), lymphocyte count (LYM), NLR, PLT, lactates (LACs), C-reactive protein (CRP), myohemoglobin (MYO), creatine kinase-MB (CK-MB), B type brain natriuretic peptide precursor (Pro-BNP) and cardiac troponin T (cTnT) were collected at the time of diagnosis. The SOFA score and the Acute Physiological and Chronic Health Evaluation II (APACHE II) score were assessed after enrolment. For all subjects, demographic characteristics, comorbid diseases, organ failure, whether received blood transfusion or continuous renal replacement therapy (CRRT) treatment, modes of breathing support, hospitalization costs and time to administration of antibiotics were also recorded. Time to administration of antibiotics, hereafter this text will be abbreviated as time-to-antibiotics, was defined as the time from diagnosis of sepsis to antibiotic administration.
Laboratory Measurements
Hb, RDW, WBC, NEU, LYM and PLT were determined by Japan Sysmex XT-4000i Automated Hematology System analyzer. The kit was a hemolytic agent for blood cell analysis, provided by Sysmex Corporation (Wuxi). CRP was measured using a dry immunofluorescence quantitative method. The kit was provided by Zhonghan Shengtai Biotechnology, and the instrument was Hangzhou Zhonghan Shengtai Jet.istar 3000. MYO, CK-MB, Pro-BNP and cTnT were measured using the double antibody sandwich method by Germany Roche Cobas e601 Automated Electrochemiluminescence Immunoanalyzer. The kit was provided by Roche Diagnostics (Shanghai).
Statistical Analysis
Normally distributed measurement data were expressed as means and standard deviation (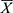 ±S), and the measurement data of the skewed distribution was represented as the medians (interquartile range) (M [P25, P75]). Based on the data distribution, numeric data were analyzed using Student’s t-test or the Mann–Whitney U-test respectively. Categorical variables were displayed as frequencies and percentages (n [%]), and the Chi-square test and Fisher’s exact test were used to analyze relationships between groups. Receiver-operating characteristic curve (ROC) analysis was performed to compare the ability of each variable to predict the mortality, and the Hosmer–Lemeshow test was applied for goodness-of-fit test of the combination variables. Cox proportional hazards model was used to determine risk factors for death. If P value of the variable was less than 0.05 after univariate Cox analysis, multivariate Cox analysis model was included. P ≤ 0.05 was considered significant; P > 0.05 confirmed that the combination variable was a good fit, implying no difference between the expected and observed outcomes. All data were performed using SPSS 22.0 statistical software (SPSS Inc., Chicago, IL, USA). The area under the curve (AUC) was compared using the MedCalc 19.5.6 Software.
±S), and the measurement data of the skewed distribution was represented as the medians (interquartile range) (M [P25, P75]). Based on the data distribution, numeric data were analyzed using Student’s t-test or the Mann–Whitney U-test respectively. Categorical variables were displayed as frequencies and percentages (n [%]), and the Chi-square test and Fisher’s exact test were used to analyze relationships between groups. Receiver-operating characteristic curve (ROC) analysis was performed to compare the ability of each variable to predict the mortality, and the Hosmer–Lemeshow test was applied for goodness-of-fit test of the combination variables. Cox proportional hazards model was used to determine risk factors for death. If P value of the variable was less than 0.05 after univariate Cox analysis, multivariate Cox analysis model was included. P ≤ 0.05 was considered significant; P > 0.05 confirmed that the combination variable was a good fit, implying no difference between the expected and observed outcomes. All data were performed using SPSS 22.0 statistical software (SPSS Inc., Chicago, IL, USA). The area under the curve (AUC) was compared using the MedCalc 19.5.6 Software.
Results
Characteristics of Patients
Baseline and outcome characteristics of all derivation and validation populations are presented in Table 1. Between January 2019 and September 2021, a total of 319 patients with sepsis were enrolled in the study, among which 108 had septic shock. The 319 patients were divided into two study populations: the 221 forming the derivation cohort and the remaining 98 forming the validation cohort. The baseline characteristics of the two cohorts were similar.
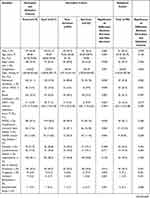 |  |  |
Table 1 Comparisons of Clinical and Laboratory Variables in Patients with Sepsis |
The overall mortality was 25.7% (82/319) during follow-up. The mean age of the patients was 69.00 (57.00,78.00) years old, and there were 197 males (61.8%) and 122 females (38.2%). Main common selection complications were coronary heart disease (17.6%), chronic obstructive pulmonary disease (COPD)/chronic lung disease (12.2%), diabetes (21.9%), cerebrovascular disease (17.2%) and hypertension (40.1%). Infectious pathogens were identified as gram-positive bacteria, gram-negative bacteria, fungus, virus and mixed or culture negative (8.8%, 33.9%, 5.6%, 0.9% and 52.7%, respectively). The two most frequent primary sites of infection were intra-abdominal (41.7%) and pulmonary (39.5%). Other sites of infection included urogenital (6.0%), bone, skin or soft tissue (3.4%), catheter or bacteremia (2.2%), and central nervous system (1.9%). Besides, mixed infection and the source of infection in 5.6% of patients were unknown. Mean APACHE II score and mean SOFA score were 14.00 (10.00, 20.00) and 5.00 (3.00, 7.00), respectively.
Derivation Study
We included 221 patients who had been admitted between 1 January 2019 and 1 May 2021 to the ICU within the derivation cohort, 59 died. The frequency of pulmonary (32 [54.2%] vs 58 [35.8%], P=0.014), catheter and bacteremia (5 [8.5%] vs 1 [0.6%], P=0.001) infection was higher in non-survivors than in survivors, however, that of intra-abdominal (14 [23.7%] vs 75 [46.3%], P=0.002) and urogenital (0 [0.0%] vs 12 [7.4%], P=0.032) infection was lower in non-survivors than in survivors. The following parameters were significantly increased in non-survivors compared to survivors: age, septic shock rate, mechanical ventilation rate, proportion of patients requiring blood transfusion, the incidence of multiple organ dysfunction syndrome (MODS), APACHE II score, SOFA score, RDW, MYO, CK-MB, Pro-BNP and cTnT. Non-survivors had significant lower oxygenation index, Hb and PLT than survivors. There was no significant difference between survivors and non-survivors in terms of gender, usage rate of noninvasive ventilation (NIV) or high flow nasal cannula (HFNC), usage rate of CRRT, time-to-antibiotics, select comorbidities, WBC, NEU, NLR, LYM and CRP (Table 1).
Diagnostic Performance of Variables and Survival Analysis
The ROC curves used for the analysis of variables between the non-survivors and survivors were plotted (Figures 1–3). The AUCs and the optimal cut-off values of the different variables are shown in Tables 2 and 3. The results showed that WBC, NEU, NLR, LYM and CRP had no predictive value for death (all P>0.05). Of the complete blood count variables, the largest AUC (95% confidence interval [CI]) was 0.735 (0.663–0.807) for RDW. The optimal cut-off point was 14.05% with a sensitivity of 83.1% and a specificity of 57.4%. The AUCs (95% CI) of Hb and PLT were 0.603 (0.518–0.688) and 0.603 (0.516–0.690), respectively. The cut-off value of Hb and PLT were 101.50g/L (64.4% sensitivity and 58.4% specificity) and 71.50x109/L (32.2% sensitivity and 88.8% specificity).
 |
Table 2 Diagnostic Information for the Prediction of 28-Day Mortality According to the Complete Blood Count and Myocardial Markers |
 |
Table 3 Diagnostic Information for the Prediction of 28-Day Mortality According to the APACHE II Score, The SOFA Score and the Combination Variables |
 |
Figure 1 Receiver-operating characteristic curve of the Hb, RDW, WBC, NEU, LYM, NLR and PLT for the prediction of 28-day mortality. |
 |
Figure 2 Receiver-operating characteristic curve of the CRP, MYO, CK-MB, Pro-BNP and cTnT for the prediction of 28-day mortality. |
 |
Figure 3 Receiver-operating characteristic curve of the APACHE II score, the SOFA score and the combination variables for the prediction of 28-day mortality. |
The cTnT had the greatest AUC (95% CI) among the myocardial markers, which is 0.753 (0.678–0.827). The optimal cut-off point was 0.039 ng/mL with a sensitivity of 75.0% and a specificity of 61.7%. The AUCs (95% CI) of Pro-BNP, MYO and CK-MB were 0.751 (0.674–0.828), 0.627 (0.524–0.729) and 0.604 (0.513–0.694), at the cut-off value of 1119.81 pg/mL, 414.70 ng/mL and 1.82 ng/mL, respectively.
The AUC of SOFA score was 0.726 with the 95% CI from 0.649 to 0.802, the sensitivity and the specificity were 72.9% and 63.0%, respectively. The AUC of APACHE II score was 0.782 with the 95% CI from 0.720 to 0.845, the sensitivity and the specificity were 93.2% and 50.6%, respectively. We also plotted combined ROC curves for RDW, cTNT and SOFA score. The results showed that the AUCs of the combined variables (two variables or three variables) were higher than that of the single SOFA score. The AUC (95% CI) of the RDW, cTnT and the SOFA score combination was 0.791 (0.722–0.861), significantly higher than SOFA score (DeLong test: P=0.032), resulting in the sensitivity of 76.8% and the specificity of 75.8%. The combination of RDW, cTnT and the SOFA score also showed an excellent goodness-of-fit (Hosmer–Lemeshow test: χ2=13.414, P=0.098).
In the univariate Cox proportional hazard regression analyses, the 28-day mortality was significantly associated with the APACHE II score, the SOFA score, RDW combined with the SOFA score, TnT combined with the SOFA score, RDW combined with cTnT, RDW combined with cTnT and the SOFA score. In the multivariate analyses, the APACHE II score (HR: 1.093; P<0.001) and RDW combined with cTnT and the SOFA score (HR: 6.133; P=0.004) were independent prognostic factors for the 28-day mortality (Table 4).
 |
Table 4 Univariate and Multivariate Cox Proportional Hazards Analysis for 28-Day Mortality |
Subgroup Analysis
We subdivided the patients into two groups using RDW values 14.05% (the optimal cut-off point of ROC curve of RDW for 28-day mortality): RDW elevated group (RDW>14.05%) and RDW non-elevated group (RDW≤14.05%). One hundred and eighteen patients (53.4%) in the study population had elevated RDW and 103 patients (46.6%) had non-elevated RDW at presentation. RDW elevated group had significantly higher mortality (49 [41.5%] vs 10 [9.7%], P<0.001). These patients also had significantly higher proportion of patients with septic shock (52 [44.1%] vs 23 [22.3%], P=0.001), MODS (84 [71.2%] vs 45 [43.7%], P<0.001). Daily hospitalization costs, APACHE II score, SOFA score, lactate levels, proportion of patients requiring blood transfusion and mechanical ventilation were significantly higher in RDW elevated patients compared to those in non-elevated patients (all P<0.001). Further details have been provided in Tables 5.
 |
Table 5 Clinical Characteristics of Patients with RDW Elevated and RDW Non-Elevated, cTnT Elevated and cTnT Non-Elevated |
Likewise, the study cohort was further divided into two groups based on cTnT values 0.039 ng/mL (the optimal cut-off point of ROC curve of cTnT for 28-day mortality): 100 patients (45.2%) in cTnT elevated group (cTnT>0.039 ng/mL) and 121 patients (54.8%) in cTnT non-elevated group (cTnT≤0.039 ng/mL). Across the two study groups, cTnT elevated showed a significant association with higher severity of illness as evidenced by higher proportion of patients requiring blood transfusion and mechanical ventilation, with septic shock and MODS, daily hospitalization costs, APACHE II score, SOFA score and lactate levels (all P<0.001). Patients with positive blood culture were higher in cTnT elevated group although it did not reach the statistical significance (18 [18.0%] vs 12 [9.9%], P=0.081). 28-day mortality was significantly higher in cTnT elevated group (42 [42.0%] vs 17 [14.0%], P<0.001). Further details have been provided in Tables 5.
Validation Study
Ninety-eight patients including 23 deaths admitted to the ICU between 2 May 2021 and 30 September 2021 were included in the validation cohort. The mortality, the septic shock rate, proportion of patients requiring blood transfusion and CRRT, time-to-antibiotics, the comorbidities, the source of sepsis and pathogenic microorganisms were not significantly different from that in the derivation group and described in Table 1. The ROC analysis for the validation data is shown in Table 6 and Figure 4. The AUCs (95% CI) of RDW, cTnT, SOFA score and APACHE II score were 0.771 (0.667–0.876), 0.786 (0.693–0.878), 0.739 (0.614–0.863) and 0.812 (0.713–0.911), respectively. The cut-off values of parameters above were 14.15%, 0.039 ng/mL, 5.5 and 14.5, respectively. The AUC (95% CI) of the RDW, cTnT and the SOFA score combination was 0.821 (0.721–0.920), significantly higher than SOFA score (DeLong test: P=0.035), also higher sensitivity (73.9% vs 69.6%) and specificity (80.0% vs 70.7%). The Hosmer–Lemeshow χ2 statistic was 9.525 (P=0.300), again confirmed the combination variable was a good calibration.
 |
Table 6 Diagnostic Information for the Prediction of 28-Day Mortality According to the Validation Data |
 |
Figure 4 Receiver-operating characteristic curve of the validation data for the prediction of 28-day mortality. |
Discussion
In the derivation study, we illustrated the significant differences in Hb, RDW, PLT, MYO, CK-MB, Pro-BNP and cTnT levels between death and survival groups of patients with sepsis. The performance of RDW, Pro-BNP and cTnT to predict mortality for septic patients was found to be better than the SOFA score. Furthermore, the APACHE II score and a combination of RDW, CTNT and SOFA score were observed to be independent predictors of 28-day mortality in this sample of septic patients.
According to current consensus, sepsis is defined as a dysregulated host response to infection causing dysfunction of at least one organ.1 The septic organ dysfunction is often interdependent, especially in the presence of cardiovascular failure, which generates hypoperfusion, thus worsening cellular hypoxia, tissue and organ out of order. This shows that SCM plays a key role in septic multiple organ dysfunction; however, there is no unified definition, diagnostic criteria, and treatment strategy for SCM. SCM was originally described as reversible significant myocardial suppression during sepsis, characterized by a decline in left ventricular ejection fraction (LVEF) and ventricular dilation, which can be recovered within 7–10 days.15 Evaluation of cardiac function by echocardiography is the primary basis for the diagnosis of SCM nowadays, but several studies analyzing the LVEF and mortality indicated that LVEF was not an ideal predictor of mortality in patients with sepsis.16,17
Measurement of serum myocardial markers can be used as a supplement to echocardiography. Myocardial markers can be broadly divided into three categories, representing damage or necrosis of cardiomyocytes, cardiac insufficiency or circulatory disturbance, and inflammation of cardiac bistiocyte, respectively. Commonly used clinical myocardial markers include MYO, CK-MB, cTnT, BNP and CRP. Serum cardiac troponin is a specific marker of myocardial damage or necrosis with a sensitivity and specificity up to 90% and 95%.18 Previous studies have shown that cTnT elevation is common in critical illness patients. In a prospective study of patients admitted for non-cardiac reasons, although the cTnT levels 84% (121/144) of patients were equal or greater than 15 ng/L at least once, about half of patients had no electrocardiograph changes. Moreover, only 27% of them had a suspicious myocardial infarction and 14% of them had a definite myocardial infarction.19 Further study by Ostermann et al20 found that cTnT was significantly associated with IL-6 and procalcitonin, and sepsis was the first common cause of for cTnT raised in patients admitted for non-cardiac reasons, with a whopping 71%. Landesberg et al21 described a correlation between raised troponin levels and left ventricular diastolic dysfunction and right ventricular dilatation in patients with sepsis and septic shock. Another American study analyzed 37 septic shock patients and measured cardiac troponin I (cTnI), which is another subtype of troponin found that patients with elevated cTnI levels (≥1.0ng/mL) had higher need for inotropic/vasopressor support (94% vs 53%, P=0.018), higher APACHE II score (28 vs 20, p=0.004) and higher mortality (56% vs 24%, P=0.04) compared to those with normal cTnI (< 1.0 ng/mL). The AUC of cTnI as a predictor of death was 0.742 with the 95% CI from 0.550 to 0.934 (P=0.019).22 In our study, also found that those with elevated cTnT levels had a higher rate of septic shock (46% vs 24%, P=0.001), higher APACHE II score (18 vs 12, P<0.001) and higher mortality (42% vs 14%, P<0.001) compared to those with non-elevated cTnT levels. Similarly, the AUCs (95% CI) of cTnT in the derivation and validation cohorts were 0.753 (0.678–0.827) and 0.771 (0.667–0.876), respectively. A recent prospective study of 93 patients with septic shock indicated a significant negative correlation between elevated plasma BNP levels and depressed left ventricular ejection fraction.23 The reason for high serum BNP and Pro-BNP levels observed in septic shock patients is not entirely clear. It is generally believed that BNP is released as a result of heart chamber ventricular volume expansion or ventricular wall stretch increases, but it is not the only reason. Excessive inflammatory response to sepsis may also be a potential factor.24 A single center observational study concluded that BNP above 1000 pg/mL was a strong predictor of mortality in patients with sepsis.25 We also found a similar BNP cut-off level. In our study, a cut-off level of Pro-BNP of 1119.81pg/mL showed the greatest discrimination for 28-day mortality with AUC (95% CI) of 0.751 (0.674–0.828). However, other studies have used lower cut-off values.26 This may be due to the heterogeneity of the included patients. We found that elevated MYO and CK-MB concentration in non-survivors compared to survivors. The prognostic values of elevated MYO and CK-MB levels regarding 28-day mortality have also been established by performing ROC analysis, but their AUCs were significantly lower than that of cTnT and BNP (0.627 and 0.604, respectively). Conversely, our study has failed to show a correlation between CRP elevated and mortality.
Many studies have reported an association between complete blood count and sepsis. As the main cells of the innate immune system, neutrophil is the first line of defense in protecting the host from infection, and delayed apoptosis of neutrophil has been observed in sepsis.27 It was reported that delta neutrophil index was significantly higher in non-survivors and it was an independent predictor for 28-day mortality in patients with sepsis.28 Lymphopenia has been described in both septic patients and animal sepsis models.9 A Taiwan cohort study of septic patients in the emergency department illustrated patients who died had higher WBC levels, but lower LYM levels.29 However, a Korea study of severe sepsis and septic shock found that WBC was not different between survivors and non-survivors.30 WBC, NEU and LYM did not show any predictive value in terms of 28-day mortality in this study. Whether these variables affect the mortality of sepsis remains uncertain, and much more clinical studies are needed. In a recent Mate analysis, NLR has been identified as a helpful infection marker, and high NLR is a potential parameter for predicting mortality.31 We also found NLR higher in non-survivors but it did not reach the statistical significance (22.05[12.67,31.61] vs 16.06[9.10,28.46], P=0.568), which might be related to insufficient sample size.
PLT has been shown to play a crucial role in the pathogenesis of non-hemostatic illness, including immune, inflammation and malignant diseases. In addition to participating in hemostatic process, PLT can also affect immune responses by directly binding to microorganisms, synthesizing immunomodulatory molecules and activating T cells in septic patients, and may be one of the first cell types to be activated.32,33 The vast majority of patients with severe sepsis usually develop thrombocytopenia, with a reported incidence of up to 55%.34 Azkarate et al35 conducted a prospective, observational study over a period of 6 years using multivariate analysis and found that thrombocytopenia was significantly correlated to mortality in 1136 patients with severe sepsis and septic shock. The odds ratio (OR) was 2.74 with the 95% CI from 1.77 to 4.22. It is well known that PLT is also included in SOFA score. Anemia seems to be more common than thrombocytopenia in sepsis. A previous study that enrolled 821 critically ill patients found that lower Hb was associated with worsening respiratory SOFA score the following day, and increasing Hb can reduce the probability of deteriorating respiratory dysfunction scores, with a OR (95% CI) of 0.64 (0.53–0.77).12 RDW is an indicator to measure the heterogeneity of circulating red blood cells (RBCs), which has been utilized in various disorders, such as inflammatory bowel disease, cardiac-cerebral vascular disease, pulmonary embolism, tumors, sepsis or septic shock, other than traditionally for interpretation of hematological system diseases.36,37 Some studies suggested that the inhibition of RBCs maturation by abundant inflammatory cytokines, leading to the release of immature erythrocyte into peripheral circulation, is a possible cause for RDW rise.38,39 Chun-Kuei Chen et al29 analyzed 6973 septic patients and revealed that the death group had higher RDW levels than the survival group (15.7% vs 13.8%), and the AUC of RDW to predict in-hospital mortality was 0.75 (95% CI: 0.72–0.77). Jo et al.30 Jandial et al40 confirmed that RDW showed a hierarchical association with 28-day or 30-day mortality in patients with severe sepsis or septic shock. In the derivation study, we also found that the performance of PLT, Hb and RDW in predicting mortality was good for septic patients. The AUC of RDW in predicting 28-day mortality was 0.735 (95% CI: 0.663–0.807), which was significantly higher than that of PLT (0.603) and Hb (0.603). The RDW of the death and survival groups was 15.4% and 13.9%, respectively. The above results are highly consistent with the research results of Chun-Kuei’s.29 The present study found that patients with elevated RDW tended to have higher APACHE II score and SOFA score (all P<0.05), which is consistent with Jo’s study. Besides, Jo et al30 observed that the 28-day mortality rate of patients with RDW > 14.0% was 37.5%, while in our study a higher 28-day mortality rate was shown (RDW > 14.05%; mortality, 41.5%). The AUC for RDW was 0.771 (95% CI: 0.667–0.876) in our validation study, again implying RDW performed well in predicting mortality.
Multiple clinical scores were developed for severity and outcome prediction in patients with sepsis and septic shock. The most prominent scores are the APACHE II score and SOFA score, but neither included RDW and cTnT. Khwannimit et al41 conducted a study on 2350 patients with sepsis in a middle-income country and concluded that the SOFA score presented a better prognosis accuracy for in-hospital mortality than qSOFA and SIRS criteria. A recent meta-regression analysis also confirmed that SOFA score was significantly associated with mortality (slope=0.49, 95% CI:0.17–0.82).42 However, regarding the association between SOFA score and mortality, studies differed in SOFA score cut-off levels and correlated mortality. In this study, the SOFA score also presented a good discrimination for 28-day mortality, but it was still lower than APACHE II Score. In this study, we proved that integration of RDW and cTnT into SOFA score could increase the performance of this score easily, with well sensitivity (76.8% and 73.9%) and specificity (75.8% and 80.0%). From the Cox analysis, we found that the combination of RDW, cTnT and the SOFA score was independent detrimental factors for 28-day mortality. To the best of our knowledge, this is the first study to assess the predictive power of the combination of the RDW, cTnT and SOFA score for mortality in patients with sepsis.
In our study, we also found a similar initial oxygenation index (226.83 [164.50, 297.50] vs 220.63 [160.00, 303.17], P=0.822) but a lower rate of intubation (37 [37.8%] vs 126 [57.0%], P=0.002) in the validation cohort compared with derivation cohort. Perhaps because we prefer to give patients with acute hypoxemic respiratory failure the opportunity to try NIV or HFNC prior to intubation in recent years. Indeed, validation cohort had a higher usage rate of NIV or HFNC than derivation cohort (24 [24.5%] vs 22 [10.0%], P=0.001).
There are several limitations we must admit. First, this is a retrospective study, involving patients from one medical center in China with a limited sample size. Hence, there may be some potential sampling error, and some treatments may be influenced by the subjective consciousness of physician. Second, most of the cases were patients with pulmonary and intra-abdominal infections (81.2%), and there were few urogenital, catheter, central nervous system, bone, skin or soft tissue infections, so subgroup analysis based on the site of infection could not be performed. Third, the follow-up period was not long enough to assess long-term prognosis. Multi-center prospective studies that have sufficient follow-up time should be conducted in the future.
Conclusion
RDW and cTnT are relatively easy to obtain as frequently used laboratory tests and had higher predictive value of 28-day mortality compared to other complete blood count and myocardial markers. The combination of RDW, cTnT and SOFA score can significantly improve the predictive value of SOFA score for the prognosis of sepsis. APACHE II score and combination of RDW, cTnT and SOFA score at diagnosis are independent predictors of mortality in patients with sepsis.
Abbreviations
SOFA, sequential organ failure assessment; APACHE II, acute physiological and chronic health evaluation II; ROC, receiver operating characteristic; RDW, red blood cell distribution width; cTnT, cardiac troponin T; CI, confidence interval; SCM, septic cardiomyopathy; TNF, tumor necrosis factor; IL, interleukin; GM-CSF, granulocyte-macrophage-colony stimulating factor; PLT, platelet; Hb, hemoglobin; NLR, neutrophil-to-lymphocyte ratio; ICU, intensive care unit; WBC, white blood cell count; NEU, neutrophil percentage; LYM, lymphocyte count; LAC, lactate; CRP, C-reactive protein; MYO, myohemoglobin; CK-MB, creatine kinase-MB; Pro-BNP, B type brain natriuretic peptide precursor; CRRT, continuous renal replacement therapy; AUC, area under the curve; COPD, chronic obstructive pulmonary disease; MODS, multiple organ dysfunction syndrome; NIV, noninvasive ventilation; HFNC, high flow nasal cannula; LVEF, left ventricular ejection fraction; cTnI, cardiac troponin I; OR, odds ratio; RBCs, red blood cells.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
This study was carried out in accordance with the recommendations of the Declaration of Helsinki, and approved by the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University [IRB number: (2021) 643]. Since this was a retrospective observational study with no potential harm to the included cases, the Ethics Committee approved us to waive informed consent. We confirm that the patient data was maintained with confidentiality.
Funding
This study was supported by Novel Coronavirus Pneumonia Epidemic Emergency Project of Chongqing Science and Technology Bureau (No. cstc2020jscx-fyzxX0012) and Emergency Research Project for Novel Coronavirus Pneumonia Prevention and Control of Chongqing Health Commission (No. 2020NCPZX04).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287.
2. Meyer N, Harhay MO, Small DS, et al. Temporal trends in incidence, sepsis-related mortality, and hospital-based acute care after sepsis. Crit Care Med. 2018;46(3):354–360. doi:10.1097/CCM.0000000000002872.
3. Goodwin AJ, Rice DA, Simpson KN, Ford DW. Frequency, cost, and risk factors of readmissions among severe sepsis survivors. Crit Care Med. 2015;43(4):738–746. doi:10.1097/CCM.0000000000000859.
4. Wei JIANG, Bin DU. Epidemiology of sepsis in China. J Postgrad Med. 2019;32(1):5–8. doi:10.16571/j.cnki.1008-8199.2019.01.002.
5. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):762–774. Erratum in: JAMA. 2016 May 24–31;315(20):2237. doi:10.1001/jama.2016.0288.
6. Sanfilippo F, Corredor C, Arcadipane A, et al. Tissue Doppler assessment of diastolic function and relationship with mortality in critically ill septic patients: a systematic review and meta-analysis. Br J Anaesth. 2017;119(4):583–594. doi:10.1093/bja/aex254.
7. Rimmelé T, Payen D, Cantaluppi V, et al.; ADQI XIV workgroup. Immune cell phenotype and function in sepsis. Shock. 2016;45(3):282–291. doi:10.1097/SHK.0000000000000495.
8. Delano MJ, Ward PA. The immune system’s role in sepsis progression, resolution, and long-term outcome. Immunol Rev. 2016;274(1):330–353. doi:10.1111/imr.12499.
9. Lang JD, Matute-Bello G. Lymphocytes, apoptosis and sepsis: making the jump from mice to humans. Crit Care. 2009;13(1):109. doi:10.1186/cc7144.
10. Larkin CM, Santos-Martinez MJ, Ryan T, Radomski MW. Sepsis-associated thrombocytopenia. Thromb Res. 2016;141:11–16. doi:10.1016/j.thromres.2016.02.022.
11. Qi D, Peng M. Early hemoglobin status as a predictor of long-term mortality for sepsis patients in intensive care units. Shock. 2021;55(2):215–223. doi:10.1097/SHK.0000000000001612.
12. Hemauer SJ, Kingeter AJ, Han X, Shotwell MS, Pandharipande PP, Weavind LM. Daily lowest hemoglobin and risk of organ dysfunctions in critically ill patients. Crit Care Med. 2017;45(5):e479–e484. doi:10.1097/CCM.0000000000002288.
13. Lorente L, Martín MM, Ortiz-López R, et al. Association between neutrophil-to-lymphocyte ratio in the first seven days of sepsis and mortality. Enferm Infecc Microbiol Clin. 2020. English, Spanish. doi:10.1016/j.eimc.2020.11.004.
14. Sadaka F, O’Brien J, Prakash S. Red cell distribution width and outcome in patients with septic shock. J Intensive Care Med. 2013;28(5):307–313. doi:10.1177/0885066612452838.
15. Parker MM, Shelhamer JH, Bacharach SL, et al. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984;100(4):483–490. doi:10.7326/0003-4819-100-4-483.
16. Sato R, Kuriyama A, Takada T, Nasu M, Luthe SK. Prevalence and risk factors of sepsis-induced cardiomyopathy: a retrospective cohort study. Medicine. 2016;95(39):e5031. doi:10.1097/MD.0000000000005031.
17. Sevilla Berrios RA, O’Horo JC, Velagapudi V, Pulido JN. Correlation of left ventricular systolic dysfunction determined by low ejection fraction and 30-day mortality in patients with severe sepsis and septic shock: a systematic review and meta-analysis. J Crit Care. 2014;29(4):495–499. doi:10.1016/j.jcrc.2014.03.007.
18. Tiwari RP, Jain A, Khan Z, et al. Cardiac troponins I and T: molecular markers for early diagnosis, prognosis, and accurate triaging of patients with acute myocardial infarction [J]. Mol Diagn Ther. 2012;16(6):371–381. doi:10.1007/s40291-012-0011-6.
19. Ostermann M, Lo J, Toolan M, et al. A prospective study of the impact of serial troponin measurements on the diagnosis of myocardial infarction and hospital and six-month mortality in patients admitted to ICU with non-cardiac diagnoses. Crit Care. 2014;18(2):R62. doi:10.1186/cc13818.
20. Ostermann M, Ayis S, Tuddenham E, et al. Cardiac troponin release is associated with biomarkers of inflammation and ventricular dilatation during critical illness. Shock. 2017;47(6):702–708. doi:10.1097/SHK.0000000000000811.
21. Landesberg G, Jaffe AS, Gilon D, et al. Troponin elevation in severe sepsis and septic shock: the role of left ventricular diastolic dysfunction and right ventricular dilatation. Crit Care Med. 2014;42(4):790–800. doi:10.1097/CCM.0000000000000107.
22. Mehta NJ, Khan IA, Gupta V, Jani K, Gowda RM, Smith PR. Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol. 2004;95(1):13–17. doi:10.1016/j.ijcard.2003.02.005.
23. Post F, Weilemann LS, Messow CM, Sinning C, Münzel T. B-type natriuretic peptide as a marker for sepsis-induced myocardial depression in intensive care patients. Crit Care Med. 2008;36(11):3030–3037. doi:10.1097/CCM.0b013e31818b9153.
24. Witthaut R, Busch C, Fraunberger P, et al. Plasma atrial natriuretic peptide and brain natriuretic peptide are increased in septic shock: impact of interleukin-6 and sepsis-associated left ventricular dysfunction. Intensive Care Med. 2003;29(10):1696–1702. doi:10.1007/s00134-003-1910-0.
25. Khoury J, Arow M, Elias A, et al. The prognostic value of brain natriuretic peptide (BNP) in non-cardiac patients with sepsis, ultra-long follow-up. J Crit Care. 2017;42:117–122. doi:10.1016/j.jcrc.2017.07.009.
26. Perman SM, Chang AM, Hollander JE, et al. Relationship between B-type natriuretic peptide and adverse outcome in patients with clinical evidence of sepsis presenting to the emergency department. Acad Emerg Med. 2011;18(2):219–222. doi:10.1111/j.1553-2712.2010.00968.x.
27. Shen XF, Cao K, Jiang JP, Guan WX, Du JF. Neutrophil dysregulation during sepsis: an overview and update. J Cell Mol Med. 2017;21(9):1687–1697. doi:10.1111/jcmm.13112.
28. Seok Y, Choi JR, Kim J, et al. Delta neutrophil index: a promising diagnostic and prognostic marker for sepsis. Shock. 2012;37(3):242–246. doi:10.1097/SHK.0b013e3182454acf.
29. Chen CK, Lin SC, Wu CC, Chen LM, Tzeng IS, Chen KF. STARD-compliant article: the utility of red cell distribution width to predict mortality for septic patients visiting the emergency department. Medicine. 2016;95(24):e3692. doi:10.1097/MD.0000000000003692.
30. Jo YH, Kim K, Lee JH, et al. Red cell distribution width is a prognostic factor in severe sepsis and septic shock. Am J Emerg Med. 2013;31(3):545–548. doi:10.1016/j.ajem.2012.10.017.
31. Huang Z, Fu Z, Huang W, Huang K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: a meta-analysis. Am J Emerg Med. 2020;38(3):641–647. doi:10.1016/j.ajem.2019.10.023.
32. Semple JW, Italiano JE
33. McFadyen JD, Kaplan ZS. Platelets are not just for clots. Transfus Med Rev. 2015;29(2):110–119. doi:10.1016/j.tmrv.2014.11.006.
34. Sharma B, Sharma M, Majumder M, Steier W, Sangal A, Kalawar M. Thrombocytopenia in septic shock patients–a prospective observational study of incidence, risk factors and correlation with clinical outcome. Anaesth Intensive Care. 2007;35(6):874–880. doi:10.1177/0310057X0703500604.
35. Azkárate I, Choperena G, Salas E, et al. Epidemiology and prognostic factors in severe sepsis/ septic shock. Evolution over six years. Med Intensiva. 2016;40(1):18–25. English, Spanish. doi:10.1016/j.medin.2015.01.006.
36. Jurin I, Trkulja V, Ajduk M, Letilović T, Hadžibegović I. Red cell distribution width in acute pulmonary embolism patients: a simple aid for improvement of the 30-day mortality risk stratification based on the pulmonary embolism severity index. Heart Lung. 2019;48(5):436–445. doi:10.1016/j.hrtlng.2019.02.006.
37. Yousefi B, Sanaie S, Ghamari AA, Soleimanpour H, Karimian A, Mahmoodpoor A. Red cell distribution width as a novel prognostic marker in multiple clinical studies. Indian J Crit Care Med. 2020;24(1):49–54. doi:10.5005/jp-journals-10071-23328.
38. Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011–1023. doi:10.1056/NEJMra041809.
39. Pierce CN, Larson DF. Inflammatory cytokine inhibition of erythropoiesis in patients implanted with a mechanical circulatory assist device. Perfusion. 2005;20(2):83–90. doi:10.1191/0267659105pf793oa.
40. Jandial A, Kumar S, Bhalla A, Sharma N, Varma N, Varma S. Elevated red cell distribution width as a prognostic marker in severe sepsis: a prospective observational study. Indian J Crit Care Med. 2017;21(9):552–562. doi:10.4103/ijccm.IJCCM_208_17.
41. Khwannimit B, Bhurayanontachai R, Vattanavanit V. Comparison of the performance of SOFA, qSOFA and SIRS for predicting mortality and organ failure among sepsis patients admitted to the intensive care unit in a middle-income country. J Crit Care. 2018;44:156–160. doi:10.1016/j.jcrc.2017.10.023.
42. de Grooth HJ, Geenen IL, Girbes AR, Vincent JL, Parienti JJ, Oudemans-van Straaten HM. SOFA and mortality endpoints in randomized controlled trials: a systematic review and meta-regression analysis. Crit Care. 2017;21(1):38. doi:10.1186/s13054-017-1609-1.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
