Back to Journals » Clinical Ophthalmology » Volume 14
Complete and Early Vitrectomy for Endophthalmitis After Cataract Surgery: An Alternative Treatment Paradigm
Authors Dib B , Morris RE , Oltmanns MH , Sapp MR, Glover JP, Kuhn F
Received 5 May 2020
Accepted for publication 11 June 2020
Published 8 July 2020 Volume 2020:14 Pages 1945—1954
DOI https://doi.org/10.2147/OPTH.S253228
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Video abstract presented by Robert E Morris.
Views: 2909
Bernard Dib, 1– 4 Robert E Morris, 1– 4 Matthew H Oltmanns, 1– 4 Mathew R Sapp, 1– 4 Jay P Glover, 5 Ferenc Kuhn 2, 6, 7
1Retina Specialists of Alabama, Birmingham, AL, USA; 2Helen Keller Foundation for Research and Education, Birmingham, AL, USA; 3University of Alabama at Birmingham (UAB), Department of Ophthalmology, Birmingham, AL, USA; 4UAB Callahan Eye Hospital, Birmingham, AL, USA; 5Retina Consultants of Nashville, Nashville, TN, USA; 6Milos Eye Hospital, Belgrade, Serbia; 7Zagorskiego Eye Hospital, Krakow, Poland
Correspondence: Robert E Morris
Retina Specialists of Alabama, 2208 University Blvd, Ste. 101, Birmingham, AL 35233, USA
Email [email protected]
Purpose: In this study, we report the treatment outcomes of complete and early vitrectomy for endophthalmitis (CEVE) after cataract surgery as the predominate initial treatment, accompanied by systemic antibiotics and retreatment of persistent or recurrent purulence (CEVE+).
Patients and Methods: Clinical features and microbiological factors were retrospectively reviewed in 62 eyes of 62 patients who were treated for acute postcataract endophthalmitis (APCE) occurring within three weeks of cataract surgery at Retina Specialists of Alabama, between 2007 and 2017.
Results: Visual acuity on presentation included light perception (LP) in 18 eyes (29%) and hand motion (HM) in 23 eyes (37%). Initial treatment was maximum possible vitrectomy in 48 eyes (77%) and tap-and-inject in 14 eyes (23%), with 38 eyes (61%) receiving two or more treatments. Cultures for the first intervention were positive in 49 eyes (79%) and virulent in 18 eyes (29%). At a median follow-up time of five months, final visual acuity was ≥ 20/40 in 49 eyes (79%), between 20/50 and 5/200 in seven eyes (11%), and < 5/200 in six eyes (10%). Virulence was the strongest predictor of poor visual outcome. Retinal detachment occurred in four eyes (6%), likely from necrotic retinal defects in each case.
Conclusion: Complete and early vitrectomy is a safe and effective initial treatment for APCE. When accompanied by systemic antibiotics and retreatment (CEVE+) of recurrent media opacification, it improves recovery of 20/40 or better visual acuity by approximately 50% compared to a predominantly tap-and-inject treatment paradigm. We recommend CEVE for fundus-obscuring APCE (∼ 75% of all cases) whenever the view is inadequate to rule out macular distress.
Keywords: endophthalmitis, postcataract endophthalmitis, vitrectomy, tap and inject, TAP, VIT, EVS, APCE
A Letter to the Editor has been published for this article.
A Response to Letter has been published for this article.
Introduction
With its ability to restore quality of life to those affected, cataract extraction is one of the most successful and impactful surgeries of modern medicine. As technology improves, surgeons and patients alike expect better outcomes. Despite these advancements, acute postcataract endophthalmitis (APCE) remains one of the most feared complications of cataract surgery.1,2
APCE causes significant morbidity, with almost half of patients losing reading visual acuity (≥20/40) and a quarter becoming legally blind (20/200 or worse) in the Endophthalmitis Vitrectomy Study (EVS) of 1995.3 As the only prospective, randomized study for the treatment of APCE, the EVS established (core) vitrectomy (VIT) as equivalent to tap-and-inject (TAP) for the 75% of eyes that presented with hand motion or better vision. The EVS also found no benefit from the use of intravenous antibiotics. These two findings encouraged office treatment of endophthalmitis with substantial cost savings and increased convenience.4
Today, vitrectomy has become small-gauge and sutureless, employing increasingly precise suction control and ultra-high-speed cutting rates of 10,000 cuts per minute (cpm) compared to the 600 cpm available in the EVS era. Improved microscopes with panoramic viewing have also significantly enhanced the safety and efficiency of surgery. Despite these improvements, visual outcomes of APCE remain mostly unchanged or have even deteriorated since the EVS, with ≥20/40 visual acuity achieved in half of all cases at best, in virtually all the studies that have reported on the treatment of APCE.5–11
This situation is partly explained by the fact that the majority of APCE eyes are still treated with TAP based on the EVS recommendations and thus do not benefit from the extensive surgical advances made over the last two decades. In fact, some experts have questioned the applicability of the EVS to today’s treatments.12–14 The main limitation of the EVS is that its vitrectomy was strictly a core VIT, with removal of (cortical vitreous) purulence on the retinal surface explicitly discouraged for fear of causing iatrogenic retinal tears.15 The primary goal of this partial vitrectomy was to “obtain specimens for culture and inject intravitreal antibiotics”; removal of infectious material was “a secondary goal.”15
Today’s instrumentation allows the performance of complete vitrectomy without compromising safety.16 The main advantage of a complete VIT over a core VIT is the removal of purulence in the cortical vitreous and on the retinal surface where most of the visually significant damage from endophthalmitis likely occurs. In fact, the major cause of vision loss in the EVS study was maculopathy, accounting for close to half of cases with impaired final visual acuity (<20/40).
Therefore, in an effort to limit retinal injury caused by endophthalmitis (endophthalmitis retinopathy and endophthalmitis maculopathy),17 we prefer to perform Complete and Early Vitrectomy for Endophthalmitis (CEVE)16 for all fundus-obscuring infections. TAP is reserved for early cases in which a view of the fundus is relatively preserved. Regardless of initial treatment, we follow patients closely with a low threshold to retreat significant deterioration of intraocular media clarity.18 CEVE-treated eyes with deteriorating clarity receive additional vitrectomy lavage and/or antibiotic injection (CEVE+). In this study, we describe the characteristics and outcomes of 62 APCE eyes treated with such an approach in the modern era of small gauge vitrectomy.
Patients and Methods
Patients
The study included a review of clinical features and microbial factors in all patients treated for APCE between 2007 and 2017 at Retina Specialists of Alabama (RSA), Birmingham. APCE was defined by the appearance of clinical symptoms and signs of endophthalmitis within three weeks of cataract surgery, regardless of whether or not cultures were ultimately positive. Cases that had cataract surgery combined with other ocular surgeries were excluded, as were cases that were deemed to be sterile postoperative inflammation. Since our goal was to assess the ability of predominantly CEVE treatment to restore good visual acuity (like the EVS), we excluded eyes with pre-existing maculopathy or ocular comorbidities limiting visual acuity to <20/100, and eyes with severe corneal opacification precluding even core VIT.
Initial Treatment
Patients with light perception (LP) presenting visual acuity were counseled that VIT was the preferred treatment. For hand motion (HM) or better visual acuity, patients were counseled that either TAP or VIT were reasonable options, but that we preferred VIT for fundus-obscuring endophthalmitis. Patients subsequently gave informed consent prior to all treatments.
As a result, CEVE was usually performed for anything but early endophthalmitis – defined by a preserved red reflex and a posterior view adequate to rule out retinal distress particularly in the macula (hemorrhages, vasculitis, retinal surface purulence).16,18 In such early APCE cases, TAP was typically recommended, and cultures were obtained by needle aspiration of the vitreous or anterior chamber. Vitrectomy was predominately 25-gauge (G) with occasional use of 23G or 27G vitrectomy. The primary goal of CEVE was to remove as much purulence as safely possible, particularly from the surface of the macula (macular hypopyon).17 If needed, CEVE included the creation of a posterior vitreous detachment and/or debridement of macular surface purulence (Supplementary Video 1). As the initial intravitreal treatment, 100% of patients received vancomycin (1 mg), 95% received ceftazidime (2.25 mg), 31% received dexamethasone (0.4mg), and only one patient received amikacin (0.4mg). At the conclusion of vitrectomy, 18% of eyes received 0.75 µg of tPA intravitreal for fibrinolysis to aid removal of macular hypopyon (Supplementary Video 1).
Patients were admitted as inpatients (typically for 48–72h) in all cases of CEVE and in 9 of 14 TAP cases. Most patients received frequent topical treatment with fortified vancomycin, tobramycin, and prednisolone acetate while avoiding the supine position. Systemic moxifloxacin or vancomycin was given in most cases to achieve protective retinal and uveal tissue levels against gram-positive bacteria (94% of all cultured bacteria in the EVS) aided by expected breakdown of the blood-ocular barrier.19 Inpatients were typically examined twice daily with the patient held NPO pending each examination result.
Retreatment
Both the decision to retreat and the form of retreatment were determined by the severity of retinopathy uncovered at the initial vitrectomy, gram stains and cultures, and the ability of an eye to maintain or improve the media clarity noted at presentation or established by the initial vitrectomy. Recurrent or increasing media opacity despite treatment was viewed as an indicator that inflammation and/or infection were inadequately controlled, and the eye was retreated accordingly with VIT or TAP. Antibiotics instilled during subsequent treatments were within safe doses20 and tailored to available culture results and clinical suspicion.
Visual Outcome Analysis
Final visual acuities were measured using Snellen charts, with a minimum of three months follow-up required, unless visual acuity of ≥20/40 was achieved earlier, or irreversible vision loss was deemed to have occurred. A Pearson’s chi-square test with one degree of freedom was used to compare success rates in achieving ≥20/40 visual acuity across different groups within this study, and between this study and the EVS.
Results
Sixty-nine eyes of 69 consecutive patients were identified that fulfilled the criteria for APCE as defined above. Similar to the EVS, a total of seven eyes were excluded: one based on severe corneal opacification precluding even core VIT (bacillus, with phthisical outcome); two due to lack of sufficient follow-up (both 20/100 at <2weeks); and four based on a pre-existing visual acuity-limiting ocular comorbidity. The latter four excluded eyes achieved stable and clear status in the early postoperative period. Thus, 62 APCE patients remained and were analyzed in this series.
Table 1 summarizes the baseline characteristics and management features in this study as compared to the EVS. Visual acuity on presentation had a distribution that was similar to the EVS. Initial treatment was with as complete a VIT as possible in 77% of cases and with TAP in 23% of cases. Of the 62 eyes, 89% required at least one VIT (including 7 of 14 eyes initially treated with TAP), and 61% needed at least two treatments (9% in the EVS).
 |
Table 1 Baseline Characteristics |
First culture results (Table 2) were more likely to be positive in LP/HM eyes (90%) compared to counting fingers (CF) or better eyes (43%, p=0.002). In 8 of 29 eyes (28%) that were recultured at an average time of 1.8 days after initial treatment, the second culture results were positive. Presentation within two days of cataract surgery was a predictor of virulent growth (gram-negative bacteria, coagulase-positive Staphylococcus, and Streptococcus/Enterococcus), with 50% of these eyes growing virulent bacteria compared to 24% (p=0.07) of eyes that presented three or more days after cataract surgery. Still, 44% of all virulent infections presented five or more days after cataract surgery.
 |
Table 2 Microbiology Results |
Table 3 presents an analysis of the final visual acuities of all eyes. Of the 62 eyes, 79% achieved final visual acuity of ≥20/40 (53% in the EVS, p=0.0001). Those who presented early (<5 days after cataract surgery) had better outcomes than those who presented later (≥5 days), with 90% of the early group (n=31) achieving ≥20/40 visual acuity compared to 68% of the late group (n=31, p = 0.29). Although there was a similar prevalence of virulence in each group (32% vs 26%, respectively), the patients in the late group were twice as likely to present with LP vision (39% versus 19%) and waited an average of 1.8 days between symptom onset and presentation as opposed to 0.5 days for the early group.
 |
Table 3 Final Visual Acuity |
Profound vision loss (<5/200) occurred in 26% (5/19) of eyes with virulent growth; 16% (5/31) of late presenters (≥5 days); and 11% (2/18) of eyes with initial LP vision. Of the six eyes that suffered profound vision loss, five harbored virulent organisms, five presented late, while only two presented with LP vision. Virulence was hence the strongest predictor of profound visual loss, followed by delay in presentation and LP visual acuity on presentation.
As outlined in Table 4, vision loss <20/40 occurred in 13 eyes (21%), and the main reason was maculopathy in six eyes (10%), retinal detachment (RD) in four eyes (6%) and phthisis in three eyes (5%). Maculopathy mainly included foveal atrophy and chronic macular edema, but also epimacular proliferation and one TAP-treated case of residual vitreomacular traction (VMT) for which the patient declined treatment, accepting 20/50 visual acuity.
 |
Table 4 Reason for Vision Loss (<20/40) |
All four cases of RD occurred in LP/HM eyes that underwent VIT, of which two were core VIT limited by keratopathy, and two were complete VIT that uncovered severe existent endophthalmitis retinopathy. All four cases of RD were thought to be secondary to necrotic defects, which were visualized in two cases. Three eyes (5%) became phthisic with poor final visual acuity <5/200, all of which harbored virulent organisms.
Discussion
The EVS significantly enhanced our understanding of postcataract endophthalmitis and continues to provide clinicians with information that directly impacts clinical practice. Outcomes for APCE have not improved, however, and have arguably deteriorated over the last two decades, even as vitrectomy technology has rapidly advanced. In fact, all retrospective series published to date (except one limited report using CEVE)12 report a final visual acuity of ≥20/40 in half of all cases at best (53% overall in the EVS).3,5–11
Enabled by the EVS recommendations, TAP remains the primary treatment for most APCE cases, particularly in the United States. This preference is evident in a recent survey,21 but also in several retrospective studies,5,6,8-10 three of which show VIT rates as low as 10% of all cases. Interestingly, this applied even to eyes presenting with LP vision, of which only 12%,9 16%,6 and less than 50%5 received a primary VIT despite the EVS recommendation for VIT treatment of all LP eyes. Thus, the trend towards TAP appears to have accelerated beyond the EVS guidelines, encouraged by the convenience and routine of office-based injection.
With complete VIT becoming considerably safer and less morbid than in the EVS era, a complete and early clean-up is now not only possible, but it also presents several potential advantages based on available experimental data. For instance, injecting rabbit eyes with bacterial culture fluid, even with the bacteria removed, resulted in substantially more severe and rapid toxicity than injecting live bacteria, extinguishing the electroretinogram within six hours.22 In another study, the inflammatory response induced by injecting live bacteria into rabbit eyes continued even after the bacteria reached an undetectable level within the eye.23 These experiments and others show that retinal damage is mostly due to toxin production and the host inflammatory response, and it can occur very rapidly.24,25 Therefore, early and thorough clearance of purulence and toxins is likely the most critical advantage of complete VIT over TAP, an advantage that was not fully realized by the EVS’s explicitly partial VIT.15
Enabled by technological improvements, the CEVE/CEVE+ paradigm improved visual outcomes, with 79% of eyes in this study achieving ≥20/40 visual acuity compared to 53% in the EVS (p=0.0001); 56% had VIT been used in all LP eyes in the EVS (p=0.0001); and an aggregated average of 44% (range 25–52%) in seven subsequent retrospective studies that mostly followed EVS guidelines.5–11
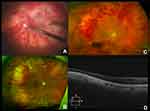
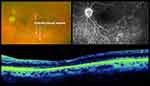
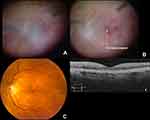
The CEVE/CEVE+ approach is intended to reduce the rate of maculopathy, the most common (~50%) cause of vision loss <20/40 in both the EVS and in this study. The term “endophthalmitis maculopathy” was introduced by Morris, Kuhn et al in 1995,17 but OCT technology has improved our understanding of this concept, highlighting both reversible (edema, epimacular proliferation) and irreversible (atrophic) macular pathology long after endophthalmitis resolution.26 This is not surprising, as complete vitrectomy frequently reveals pus and inflammatory debris adhering to the surface of the macula (macular hypopyon, Supplementary Video 1).17 Further studies are needed to better characterize endophthalmitis maculopathy, both anatomically and functionally, with OCT, angiography, microperimetry, and electroretinography. Representative macular and retinal abnormalities noted in our patients are shown in Figures 1–3.
The rate of RD was 8% overall in the EVS and 6.4% in our study. Non-randomized studies both before27,28 and after29 the EVS have fostered speculation about the causative role of vitrectomy in endophthalmitis-associated RDs. Indeed, the EVS cited possible iatrogenic RD as its rational for limiting VIT. But this has no support in the EVS final data. In fact, 7.8% of EVS patients in the VIT group suffered an RD vs. 9% in the TAP group, a difference that was not significant (p=0.66).30
Importantly, the EVS found other significant associations with a higher rate of RD, namely virulent growth (23% RD rate in virulent gram-positives), LP presenting vision (16.4% RD rate), and foregoing systemic antibiotics (11.2% vs. 5.3% RD rate). These associations indicate that endophthalmitis-associated RDs more likely occur as a result of necrotic retinal defects rather than iatrogenic tears. We have seen cases in which a completely necrotic, “moth-eaten” retina (Figure 4 and Supplementary Video 2) was found inferiorly in the areas where pus had settled by gravity. Complete VIT and adequate intraretinal antibiotic levels would logically tend to reduce necrotic defects secondary to such preretinal purulence.
 |
Figure 4 Retinal detachment resulting from large necrotic holes seen inferiorly two weeks after resolved Streptococcal endophthalmitis. This is sometimes seen inferiorly where preretinal pus settles by gravity in the upright position (ocular hypopyon). See Video 2 for more details. |
All 13 eyes with final visual acuity <20/40 either harbored virulent organisms (9 eyes) and/or presented >5 days after cataract surgery (10 eyes). In all 12 of these eyes treated with initial vitrectomy, there was already either advanced endophthalmitis maculopathy/retinopathy uncovered (6 eyes) or keratopathy (usually corneal edema) precluding complete VIT (6 eyes). No eye treated with CEVE/CEVE+ failed to recover at least 20/40 vision if significant endophthalmitis maculopathy/retinopathy was not already present at the time of initial vitrectomy. This underscores the crucial role of the cataract surgeon in educating patients about the symptoms of endophthalmitis to avoid delayed presentations.
This encouraging outcome also highlights the importance of appropriate retreatment, which occurred in 61% of all patients at an average of 1.8 days after initial treatment, for both TAP and VIT retreatment. Eyes with substantial recurrent media opacification were retreated with complete VIT, while eyes with relatively maintained media clarity received TAP if infection control was still in doubt. Confirmation of the need to retreat inadequately responsive eyes is apparent from the fact that 27.5% of such recultured eyes in this study and 42% in the EVS remained culture positive at retreatment.31 Only 9% of eyes in the EVS had retreatment within one week of initial treatment.
Beyond initial toxin clearance, this represents a second significant advantage of primary CEVE: it restores clarity of the media, providing an extremely useful control parameter on which timely retreatment decisions can be based. In comparison, when TAP is performed on an eye with substantial media opacification, the decision to retreat is somewhat arbitrary since opacity and visual acuity do not typically improve within the next 24–48 hours, even if the infection is subsiding.
There are practical limitations to our approach. The CEVE+ paradigm often entails two or more vitrectomies in the same week, including after hours. This situation requires a team approach, a reliable setup for emergent surgery, and ideally an inpatient facility, all resources that have become increasingly scarce in ophthalmology. Nevertheless, we hope that the improved visual outcomes presented in this report will catalyze additional investigations and attainable practice pattern adjustments in treating this iatrogenic and frequently devastating disease.
Although our presenting patient cohort was similar to that of the EVS, the size, retrospective nature, and differing pharmacologics used in this report limit its comparability to the EVS and our ability to attribute the very substantial visual acuity improvements relative to the EVS solely to the use of CEVE/CEVE+. Nevertheless, we believe preferential use of CEVE (77%), frequent retreatment (61%), and use of appropriate systemic antibiotics (to achieve intraretinal levels protective against bacteria at the vitreoretinal interface) account for the majority of the observed improvement.
Based on these encouraging results and the substantial advances seen in vitreoretinal surgery since the EVS, we are currently planning a prospective clinical trial of CEVE/CEVE+ that will allow a closer comparison to the EVS and its conclusions that remain widely followed standards of care.
Conclusion
This study is the first detailed report of improved visual outcomes for APCE since the EVS 25 years ago. Akin to treating infections elsewhere in the body with abscess drainage,32 recurrent lavage, and sustained antibiotic dosing, we believe CEVE/CEVE+ rapidly restores and maintains a sterile, nontoxic intraocular environment, limiting further damage to the retina and uveal tract. We recommend CEVE as the primary treatment for all fundus obscuring APCE (~75% of cases), which includes all LP/HM eyes and approximately half of eyes with CF or better visual acuity. We reserve TAP for CF or better eyes in which the fundus view is adequate to rule out macular distress.
In cases where CEVE is planned but will be delayed, immediate in-office tap/inject can be considered. Regardless of initial therapy, clarity of the intraocular media should be closely followed with a low threshold to retreat with lack of improvement. In the era of small gauge vitrectomy, we believe the CEVE/CEVE+ paradigm provides the best chance of promptly halting and reversing this potentially devastating complication of cataract surgery.
Ethics Statement
This retrospective study was approved by the Western Institutional Review Board and adhered to the tenets of the Declaration of Helsinki for research involving human subjects.
Acknowledgments
We gratefully acknowledge the following: Jessica Haynes and Christina Sullivan for their extensive work in compiling data, images, and videos for this article; and the following fellowship physicians who helped care for the patients included in this study: Anthony Correnti, MD, Jeffrey L. Shere, MD, Brett D. Gerwin, MD, Alexander V. Talalight, MD, Wright B. Lauten, MD, Andrew D. Hsia, MD, Eric M. Zavaleta, MD, Charles L. Clark, III, MD, Daniel K. Bennett, MD, Nicholas H. Tosi, MD, Vikram T. Saini, MD, Daniel T. Kasuga, MD. Partial funding was provided by the Helen Keller Foundation for Research and Education through a grant from the Pete Hanna Charitable Trust and donations from the Kent Companies, Midland, Texas.
Author Contributions
Kuhn and Morris developed the CEVE paradigm.12,16-18 Morris, Sapp and Oltmanns developed the CEVE+ concept and treated the patients in this study. Dib compiled and performed the first analysis of the data and authored the first manuscript draft. Morris and Dib revised the manuscript through subsequent drafts. Morris performed three vitrectomies shown in supplemental videos and figures. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work, financial or otherwise.
References
1. West ES, Behrens A, McDonnell PJ, Tielsch JM, Schein OD. The incidence of endophthalmitis after cataract surgery among the U.S. medicare population increased between 1994 and 2001. Ophthalmology. 2005;112(8):1388–1394. doi:10.1016/j.ophtha.2005.02.028
2. Taban M, Behrens A, Newcomb RL, et al. Acute endophthalmitis following cataract surgery. Arch Ophthalmol. 2005;123(5):613. doi:10.1001/archopht.123.5.613
3. Doft B. Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Endophthalmitis Vitrectomy Study Group. Arch Ophthalmol. 1995;113(12):1479–1496.
4. Wisniewski SR, Hammer ME, Grizzard WS. An investigation of the hospital charges related to the treatment of endophthalmitis in the endophthalmitis vitrectomy study. Ophthalmology. 1997;104(5):739–745. doi:10.10.16/S0161-6420(97(30239-5
5. Yannuzzi NA, Si N, Relhan N, et al. Endophthalmitis after clear corneal cataract surgery: outcomes over two decades. Am J Ophthalmol. 2017;174:155–159. doi:10.1016/j.ajo.2016.11.006
6. Pijl BJ, Theelen T, Tilanus MAD, Rentenaar R, Crama N. Acute endophthalmitis after cataract surgery: 250 consecutive cases treated at a tertiary referral center in the Netherlands. Am J Ophthalmol. 2010;149(3):482–487.e2. doi:10.1016/j.ajo.2009.09.021
7. Mason LB, Mason JOI, Friedman DA, Mason JOI. Postoperative bacterial endophthalmitis: tap/inject versus sutureless vitrectomy. Med Res Archives. 2017. Available from: https://journals.ke-i.org/mra/article/view/999.
8. Lalwani GA, Flynn HW, Scott IU, et al. Acute-onset endophthalmitis after clear corneal cataract surgery (1996–2005). Clinical features, causative organisms, and visual acuity outcomes. Ophthalmology. 2008;115(3):473–476. doi:10.1016/j.ophtha.2007.06.006
9. Kamalarajah S, Silvestri G, Sharma N, et al. Surveillance of endophthalmitis following cataract surgery in the UK. Eye (Lond). 2004;18(6):580–587. doi:10.1038/sj.eye.6700645
10. Gower EW, Keay LJ, Stare DE, et al. Characteristics of endophthalmitis after cataract surgery in the United States medicare population. Ophthalmol. 2015;122(8):1625–1632. doi:10.1016/j.ophtha.2015.04.036
11. Combey de Lambert A. Baseline factors predictive of visual prognosis in acute postoperative bacterial endophthalmitis in patients undergoing cataract surgery. JAMA Ophthalmol. 2013;131(9):1159. doi:10.1001/jamaophthalmol.2013.4242
12. Kuhn F, Gini G. Ten years after … are findings of the Endophthalmitis Vitrectomy Study still relevant today? Graefes Arch Clin Exp Ophthalmol. 2005;243:1197–1199. doi:10.1007/s00417-005-9982-8
13. Clarke B, Williamson TH, Gini G, Gupta B. Management of bacterial postoperative endophthalmitis and the role of vitrectomy. Surv Ophthalmol. 2018;63(5):677–693. doi:10.1016/j.survophthal.2018.02.003
14. Maguire JI. Postoperative endophthalmitis: optimal management and the role and timing of vitrectomy surgery. Eye (Lond). 2008;22(10):1290–1300. doi:10.1038/eye.2008.51
15. Doft B. Endophthalmitis Vitrectomy Study. Manual of Operations. National Eye Institute.; 1990.
16. Kuhn F. Complete and Early Vitrectomy for Endophthalmitis (CEVE) as today’s alternative to the Endophthalmitis Vitrectomy Study. In: Kirchhof B, Wong D, editors. Essentials in Ophthalmology, Vitreo-Retinal Surgery. Berlin, Heidelberg: Springer-Verlag;2005:53–68. doi:10.1007/978-3-540-33670-9_5
17. Morris R, Witherspoon CD, Kuhn F, Byrne JB. Endophthalmitis. In: FH R, editor. Master Tech Ophthalmic Surgery. Philadelphia: Jaypee Brothers Medical Publishers; 1995:560–572.
18. Morris RE, Clark CL, Sapp MR, Oltmanns MH, Kuhn F. Endophthalmitis. In: Roy FH, editor. Master Tech Ophthalmic Surgery.
19. Brockhaus L, Goldblum D, Eggenschwiler L, et al. Revisiting systemic treatment of bacterial endophthalmitis: a review of intravitreal penetration of systemic antibiotics. Clin Microbiol Infect. 2019;25(11):1364–1369. doi:10.1016/j.cmi.2019.01.017
20. Plugfelder SC, Hernández E, Fliesler SJ, et al. Intravitreal vancomycin: retinal toxicity, clearance, and interaction with gentamicin. Arch Ophthalmol. 1987;105(6):831–837. doi:10.1001/archopht.1987.01060060117045
21. Fliney GD, Pecen PE, Cathcart JN, Palestine AG. Trends in treatment strategies for suspected bacterial endophthalmitis. Graefes Arch Clin Exp Ophthalmol. 2018;256(4):833–838. doi:10.1007/s00417-018-3910-3
22. Callegan MC, Booth MC, Jett BD, Gilmore MS, Tuomanen EI. Pathogenesis of gram-positive bacterial endophthalmitis. Infect Immun. 1999;67(7):3348–3356.
23. Kim IT, Park SK, Lim JH. Inflammatory response in experimental Staphylococcus and Pseudomonas endophthalmitis. Ophthalmologica. 1999;213(5):305–310. doi:10.1159/000027444
24. Forster RK. Experimental postoperative endophthalmitis. Trans Am Ophthalmol Soc. 1992;90:505–559. doi:10.1001/archinte.1934.00160110018002
25. Callegan MC, Guess S, Wheatley NR, et al. Efficacy of vitrectomy in improving the outcome of Bacillus cereus endophthalmitis. Retina. 2011;31(8):1518–1524. doi:10.1097/IAE.0b013e318206d176
26. Zhou T, Aptel F, Bron AM, et al. Longitudinal study of retinal status using optical coherence tomography after acute onset endophthalmitis following cataract surgery. Br J Ophthalmol. 2017;101(9):1211–1216. doi:10.1136/bjophthalmol-2016-309542
27. Olson JC, Flynn HW, Forster RK, Culbertson WW. Results in the treatment of postoperative endophthalmitis. Ophthalmology. 1983;90(6):692–699. doi:10.1016/S0161-6420(83)34511-5
28. Nelsen PT, Marcus DA, Bovino JA. Retinal detachment following endophthalmitis. Ophthalmology. 1985;92(8):1112–1117. doi:10.1016/S0161-6420(85)33916-7
29. Chiquet C, Aptel F, Combey-de Lambert A, et al. Occurrence and risk factors for retinal detachment after pars plana vitrectomy in acute postcataract bacterial endophthalmitis. Br J Ophthalmol. 2016;100(10):1388–1392. doi:10.1136/bjophthalmol-2015-307359
30. Doft BM, Kelsey SF, Wisniewski SR, et al. Retinal detachment in the endophthalmitis vitrectomy study. Arch Ophthalmol. 2000;118(12):1661. doi:10.1001/archopht.118.12.1661
31. Doft BH, Kelsey SF, Wisniewski SR. Additional procedures after the initial vitrectomy or tap-biopsy in the endophthalmitis vitrectomy study. Ophthalmology. 1998;105(4):707–716. doi:10.1016/S0161-6420(98)94028-3
32. Brouwer MC, Coutinho JM, Van De Beek D. Clinical characteristics and outcome of brain abscess: systematic review and meta-analysis. Neurology. 2014;82(9):806–813. doi:10.1212/WNL.0000000000000172
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.