Back to Journals » Clinical Interventions in Aging » Volume 13
Comparison of the acute effects of traditional versus high velocity resistance training on metabolic, cardiovascular, and psychophysiological responses in elderly hypertensive women
Authors Orsano VSM , de Moraes WMAM , Sousa NMF , de Moura FC , Tibana RA , Silva AO , Schwerz Funghetto S , Schoenfeld BJ, Prestes J
Received 30 January 2018
Accepted for publication 23 March 2018
Published 31 July 2018 Volume 2018:13 Pages 1331—1340
DOI https://doi.org/10.2147/CIA.S164108
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Vânia Silva Macedo Orsano,1,2 Wilson Max Almeida Monteiro de Moraes,2 Nuno Manuel Frade de Sousa,3 Felipe Carmo de Moura,2 Ramires Alsamir Tibana,4 Alessandro de Oliveira Silva,5 Silvana Schwerz Funghetto,6 Brad J Schoenfeld,7 Jonato Prestes2
1Department of Physical Education, Federal University of Piaui (UFPI), Piauí, Brazil; 2Post Graduation Program on Physical Education, Catholic University of Brasilia (UCB), Brasília, Brazil; 3Laboratory of Exercise Physiology, Faculty Estacio of Vitoria, Espirito Santo, Brazil; 4Department of Physical Education, Federal University of Mato Grosso (UFMT), Mato Grosso, Brazil; 5Faculty of Education and Health Sciences, University Center of Brasilia (UniCEUB), Brasília, Brazil; 6Graduation Program in Health Sciences, University of Brasilia (UnB), Brasília, Brazil; 7Department of Health Sciences, CUNY Lehman College, Bronx, NY, USA
Objectives: The aim of the present study was to compare the acute effects of traditional
resistance training (RT) versus high velocity RT (HVRT) on metabolic, cardiovascular, and psychophysiological responses in elderly hypertensive women.
Methods: Fifteen elderly women (mean age ± standard deviation, 67.1±6.9 years) classified as having hypertension stage 1 or 2 were randomly allocated to complete traditional RT or HVRT; 1 week later, subjects allocated to RT completed the HVRT session and vice-versa. Heart rate, blood pressure, affective response, perceived effort, and blood samples analyzing lactate, nitrate, nitrite, oxidative damage (thiobarbituric acid reactive substances [TBARS]), and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid equivalent antioxidant capacity (TEAC) collected before and after training sessions were assessed. Nutritional counseling was provided regarding nutrients that could affect cardiovascular and nitrate/nitrite analysis.
Results: Systolic blood pressure was not statistically different (p>0.05) between conditions at the beginning and during 30 minutes after sessions. Diastolic blood pressure, rate pressure product, and heart rate were not statistically different (p>0.05) between conditions at the beginning and during 45 minutes after sessions. Nitric oxide was significantly higher (p<0.0005) for HVRT compared to RT after 30 minutes of exercise. TBARS and TEAC were significantly higher (p<0.05) for HVRT compared with RT only immediately after exercise. There were no differences for psychophysiological variables between protocols.
Conclusion: The acute cardiovascular and metabolic responses, including oxidative stress, are transient and within normal values. Taken together with the positive affective responses, both HVRT and RT with this intensity and volume seem to be safe for elderly hypertensive women under medication.
Keywords: aging, resistance training, power training, blood pressure, nitric oxide, oxidative stress
Introduction
The aging process increases the relative risk of developing several chronic diseases including cardiovascular disease, hypertension, type 2 diabetes, obesity, and some types of cancer, and thus increases mortality risk.1,2 Data from the National Center of Health Statistics1 indicates that the age-adjusted prevalence of hypertension (both diagnosed and undiagnosed) from 2003 to 2006 was 75% for older women and 65% for older men, demonstrating that, with age, women surpass men in the prevalence of hypertension. Elderly people also present a higher prevalence of degenerative musculoskeletal conditions such as osteoporosis, arthritis, and sarcopenia when compared with young adults.2 Sarcopenia is characterized by an age-related decrease in muscle mass accompanied by an impaired capacity to generate strength and power.3 Muscle strength and power are also predictors of cardiovascular mortality, independent of cardiovascular fitness.4 The American College of Sports Medicine recommends resistance training (RT) as a primary means to increase and maintain muscle mass, physical functional, and muscle strength and power.2 Moreover, high velocity RT (HVRT) with light to moderate loads and high velocities of movement are also recommended to restore and enhance functional capacity.2
Evidence indicates that muscle power shows a higher correlation with improved mobility and decreased fall risk as compared to maximal strength.5 Training with high velocity contractions is more effective to increase muscle power as compared to traditional RT with slow contractions, and such training is safe and well-tolerated, even in octogenarians and elderly with individuals limited mobility.6 Moreover, HVRT has the potential to induce postexercise hypotension (PEH) in normotensive young subjects.7,8 PEH is thought to promote cardiovascular protection because of a significant reduction in blood pressure (BP) during the recovery period.9 However, the acute metabolic and cardiovascular responses to HVRT in elderly hypertensive subjects have been neglected, including that of PEH and its mechanisms.
Sustained postexercise vasodilatation is one of the mechanisms associated with PEH, occurring largely within the vascular beds of active muscles and mediated by combined central neural mechanisms and local vasodilatory mechanisms.10 Among the vasodilatory mechanisms, nitric oxide (NO) is one of the released vasoactive substances that is most studied.11 Hypertensive subjects present endothelial dysfunction associated with decreased bioavailability of NO by several mechanisms such as a reduction in endothelial NO synthase, uncoupling of endothelial nitric oxide synthase enzymatic activity, or scavenging of NO by reactive oxygen species.12 Exercise training may counteract this decline in endothelial function during aging and reduce oxidative stress and arterial stiffness, which is a predictor of major cardiovascular events.13 The release of NO from endothelial cells induced by exercise is dependent on vascular shear stress.14 Thus, high velocity concentric muscle contractions may be beneficial in promoting an increase in NO bioavailability and inducing PEH.11 Moreover, exercise training induces adaptations that enhance antioxidant defenses and minimize oxidative stress in response to acute exercise conditions,15 as well as increase resistance to a subsequent nonexercise oxidative challenge,16 increasing the bioavailability of NO.
Although RT has potential health benefits, exercise adherence may depend, at least in part, on the prescribed initial intensity,17 which influences the affective response. High-intensity RT for detrained subjects results in an elevated ratings of perceived exertion (RPE) and unpleasant affective responses that impairs exercise adherence.18 There is evidence that low-intensity HVRT produces similar health benefits to traditional heavier load RT,19,20 although the affective responses have not been directly compared between these 2 types of training.
Thus, the aim of the present study was to compare the acute effects of traditional RT versus HVRT on metabolic, cardiovascular, and psychophysiological responses in elderly hypertensive women. We hypothesized that HVRT with lighter loads and higher concentric contraction velocities compared to traditional RT would produce a more pronounced shear stress accompanied by a higher NO bioavailability, thereby enhancing PEH. Moreover, we hypothesized that similar affective and subjective RPE would result from both training conditions, with a higher transitory metabolic stress noted for HVRT.
Methods
Subjects
Fifteen elderly women aged between 60 and 80 years were locally recruited through community posters and lectures to participate in the present study on a voluntary basis (eutrophic, n=3; overweigh, n=8; obese, n=4; by the body mass index classification). Prospective participants completed a thorough physical examination including medical history screening, resting and exercise electrocardiogram, manual BP measurement, and anthropometric and orthopedic evaluation prior to inclusion in the study. The inclusion criteria were as follows: hypertensive women above 60 years of age who had not undergone RT in the previous 6 months. All subjects were classified with hypertension stage 1 or 2 according to the “Seventh report of the joint national committee on prevention, detection, evaluation and treatment of high blood pressure”21 and were evaluated by an experienced physician. Participants were classified as sedentary and untrained according to the International Physical Activity Questionnaire22 and the American College of Sports Medicine guidelines.2 Subjects with physical disabilities, diagnosis of diabetes, cardiovascular diseases, hypertension (systolic BP [SBP] 180 mmHg and diastolic BP [DBP] 110 mmHg), musculoskeletal disease, or who smoked or abused drugs/alcohol were excluded from the trial. All subjects were informed of the procedures and provided a written informed consent to participate in the study. This study was approved by the Institutional Ethics Committee of the Federal University of Piaui (protocol number 1094736) and conformed to the Helsinki Declaration regarding the use of human subjects for research.
After this initial screening, subjects were provided with 2 familiarization training sessions consisting of 1 exercise for each major muscle group, employing 2 sets of 15 submaximal repetitions. Seven days following strength and anthropometric testing, subjects were randomly allocated to complete traditional RT or HVRT. One week later, subjects crossed over so that those allocated to RT completed the HVRT session and vice-versa (Figure 1). Heart rate (HR), BP, affective response, and RPE were assessed, and blood samples to analyze lactate, nitrate, nitrite, and pro- and antioxidant capacity were collected before and after training sessions. Subjects received nutritional counseling regarding nutrients that could affect cardiovascular and nitrate/nitrite analysis (beet, melon, and processed meat) and were advised to avoid them during the study period. Table 1 presents the subjects’ basic characteristics.
Ten repetition maximum test
Three days after the familiarization sessions, the 10 repetition maximum (10 RM) test was employed to evaluate strength in the following exercises: barbell bench press, horizontal leg press, front lat pull-down, leg extension, military press, leg curl, cable elbow extension, standing calf raise, standing elbow flexion, and dorsiflexion (Physicus, Auriflama, Sao Paulo, Brazil). The tests were completed on the same day with 10 minutes between exercises and were repeated 5 days later to determine test–retest reproducibility with a mean R=0.98 for all exercises. We adopted the protocol used in a previous study from our research group.23 The test was terminated at the moment subjects were unable to perform the complete movement with proper form or when voluntary concentric failure (inability to perform the concentric phase of the movement) occurred. The following strategies were adopted to reduce test variations: 1) standard instructions were given before the test so that the volunteer was aware of the entire routine to be performed during data collection; 2) the subject was provided technical instruction on proper exercise performance; 3) the evaluator was aware of the position adopted by the practitioner during the test, since slight variations in the positioning of the joints involved in the movement could trigger other muscles, leading to misinterpretation of the obtained scores; 4) the subjects were verbally encouraged with the purpose of keeping their motivation level high; 5) the additional load used in the study was previously measured on a precision scale. Rest intervals between trials in each exercise during the 10 RM test were set between 3 and 5 minutes, with 3 attempts and initial load determined in the familiarization sessions. The subjects were instructed to refrain from consuming any stimulant (caffeine or alcohol) and not perform physical activity during the week prior to experimental testing. The 10 RM tests were scheduled between 4 and 6 pm and were performed under standardized controlled room temperature.
Resistance exercise sessions
Training sessions were composed of 10 exercises performed at 70% of 10 RM, involving 3 sets of 10 repetitions with 1 minute of rest between sets and exercises. The resistance exercises for both traditional RT and HVRT were as follows: barbell bench press, horizontal leg press, front lat pull-down, leg extension, military press, leg curl, cable elbow extension, standing calf raise, standing elbow flexion, and dorsiflexion (Physicus). Before both sessions, subjects were allowed to warm-up for 10 minutes on a treadmill (model PRO 150; Movement, Sao Paulo, Brazil) at 65%–75% of HR reserve. During traditional RT, subjects were advised to complete the concentric and eccentric phase of the movement at a moderate pace (2–3 seconds each), while during HVRT they completed the concentric phase as fast as possible and the eccentric phase with a 2–3 seconds cadence. Cardiovascular measures (HR and BP) were controlled during sessions to avoid any abnormal response.
HR and BP
SBP and DBP were measured with an oscillometric device (Microlife 3AC1-1, Widnau, Switzerland) according to the recommendations of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. The cuff size was adapted to the arm circumference of each participant according to the manufacturer’s instructions. All BP measures were assessed in triplicate (measurements separated by 1 minute) with the mean value used for analysis. BP and HR measurements were performed after 15 minutes of seated rest (0) and at 5, 15, 30, and 45 minutes after the resistance exercise sessions. HR was measured by an HR monitor (Polar S810i, Polar Electo Oy, Kempele, Finland). Rate pressure product (RPP) was evaluated as RPP = HR × SBP. During BP measurements, participants remained seated quietly under a controlled room temperature.
Blood analysis
Blood samples were collected before and at 3, 15, and 30 minutes after the training sessions in BD Vacutainer® Fluoret/EDTA tubes (Becton Dickinson, Franklin Lakes, NJ, USA) to measure plasma lactate, nitrite and nitrate, total antioxidant capacity (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid [Trolox] method), and lipid peroxidation (thiobarbituric acid reactive substances [TBARS] method). Plasma was separated immediately after the blood was collected and samples for nitrite/nitrate, TBARS, and Trolox were centrifuged at 2,500 rpm for 15 minutes. Lactate samples were centrifuged at 3,000 rpm for 5 minutes. The plasma was stored at −80°C for analysis.
Lipid peroxidation estimation
The lipid peroxidation (malondialdehyde estimated) in plasma was estimated by using the TBARS method,24 and malondialdehyde concentration was calculated using a molar extinction coefficient of 1.56×105 mol−1.
Nitric oxide bioavailability
The method consisted of simultaneous evaluation of nitrate and nitrite concentrations. The principle of this assay is reduction of nitrate by vanadium (III) combined with detection by the acidic Griess reaction. This assay is sensitive to 0.5 μM NO3− and is useful in plasma. After deproteinization, 100 μL of supernatants were distributed to 96-well plates in triplicate and 100 μL of Griess reagent (1% sulfanilamide, 0.1% N-(1-Naphthyl)ethylenediamine dihydrochloride and 2.5% H3PO4) (Sigma-Aldrich, St Louis, MO, USA) was added. The plates were incubated at room temperature and optical density was read at 540 nm. The NO concentration was calculated from a standard curve generated with NaNO2 ranging from 0 to 200 μM.25
Trolox equivalent antioxidant capacity (TEAC)
Plasma antioxidant capacity was determined by a modified TEAC assay (Sigma Kit, Sigma-Aldrich, St Louis, MO, USA) and expressed as Trolox equivalent, as described in the study by Rohn et al.26
Lactate
Plasma lactate levels were determined using a standard kit (Biotecnica ref. 10.018.00) by spectrophotometric readings in EZ Read 400 Microplate Reader (Biochrom, Cambridge, UK).
Affective response and rating of perceived exertion
The affective responses of pleasure and displeasure following exercise were measured after the last set of each resistance exercise, with subjects seated and waiting for the orientation. The instrument used to assess ratings was the sensation scale with a bipolar classification of 11 points, varying from +5 to −5; the anchor was zero (neutral), and all odd integers ranged from “very well” (+5) to “very bad” (−5).27 The RPE was measured by the Borg scale of 10 points with anchors varying from 0 (“extremely easy”) to 10 (“extremely hard”). The session RPE was determined 30 minutes after the sessions as follows: “How much effort did you feel in your whole body during this training session?” This procedure of memory anchoring, in which the subject reflects on her routine, was followed according to the protocol recommended by Foster et al.28
Nutritional counseling
The diet control was carried out by a nutritionist, who counseled subjects about nutrients that could interfere with blood and cardiovascular analyses (beet, melon, and processed meat), and thus should not be ingested during the study period. The nutritionist individually gave subjects a list of foods that could be consumed.
Statistical analysis
The data are expressed as mean value ± standard deviation (SD). Shapiro–Wilk test was applied to check for normal distribution of study variables. In case of nonnormal distribution, logarithmic transformation was performed. RPE and affective response presented nonnormal distribution, so nonparametric tests were used. A 2-way repeated-measures analysis of variance was used to compare the hemodynamic, metabolic, and oxidative stress variables between strength and HVRT sessions. Compound sphericity was verified by the Mauchley test. When the assumption of sphericity was not met, the significance of F-ratios was adjusted according to the Greenhouse–Geisser procedure. Tukey’s post hoc test with Bonferroni adjustment was applied in the event of significance. The power of the sample size was determined using G*Power version 3.1.3 (Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany), based on the interaction between the interventions and time for SBP and DBP, nitrate, and TBARS. Considering the sample size of this study, an effect size of 0.814 for SBP, 0.577 for DBP, 2.317 for nitrate, and 1.800 for TBARS was achieved, and an α error of 0.05 and power (1 − β) of 0.998 for SBP, 0.966 for DBP, and 1.0 for nitrate and TBARS was achieved. The level of significance was p≤0.05, and SPSS version 20.0 (IBM Corporation, Armonk, NY, USA) software was used for the analysis.
Results
Hemodynamic variables
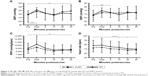
BP, HR, and RPP prior to and during 45 minutes after strength and HVRT sessions are presented in Figure 2. There was a statistically significant 2-way interaction between intervention and time on SBP, F(5,70)=7.168, p<0.0005. SBP was significantly higher (p=0.049) for HVRT session compared with RT only after 45 minutes of exercise. The SBP was also significantly higher (p<0.05) 30 and 45 minutes after the HVRT session compared to rest values. The SBP was significantly higher (p<0.05) post-0 only after the RT session as compared to rest values (Figure 2A).
There was also a statistically significant 2-way interaction between intervention and time on DBP, F(5,70)=4.663, p=0.001. DBP was not statistically different (p>0.05) in the RT session compared to HVRT session. DBP was significantly higher (p<0.05) post-0 and post-5 minutes only after the RT session compared to resting values (Figure 2B).
A statistically significant 2-way interaction between intervention and time was observed for RPP, F(5,70)=6.328, p<0.0005. RPP was significantly higher (p=0.009) post-0 and significantly lower (p=0.046) post-45 minutes only after RT compared to resting values. No statistically significant differences (p>0.05) were observed on RPP after power sessions compared to resting values (Figure 2C).
There was no 2-way interaction between intervention and time on HR, F(5,70)=0.809, p=0.547. HR was significantly lower (p<0.05) post-15, post-30, and post-45 minutes for both sessions compared to rest values (Figure 2D).
Metabolic variables
A significant 2-way interaction between intervention and time was observed for NO, F(2,24)=64.192, p<0.0005. NO was statistically significantly higher (p<0.0005) for HVRT compared to RT only after 30 minutes of exercise. NO was significantly higher post-30 compared to post-0 and pre for both interventions (Figure 3A).
There was also a significant 2-way interaction between intervention and time with regard to blood lactate concentrations, F(3,39)=8.671, p<0.0005. Blood lactate concentrations were significantly higher (p=0.039) for RT compared to HVRT after 30 minutes of exercise (Figure 3B).
Oxidative stress variables
TBARS, Trolox, and Trolox/TBARS ratio prior to and during 30 minutes after RT and HVRT sessions are presented in Figure 4. A significant 2-way interaction between intervention and time was observed on TBARS, F(2,24)=38.886, p<0.0005 and Trolox, F(2,24)=37.609, p<0.0005. TBARS and Trolox were significantly higher (p<0.05) for HVRT when compared with RT only immediately after exercise. TBARS was significantly higher (p<0.05) post-0 and post-30 minutes after RT session compared to resting values and only post-0 after HVRT session compared to resting values. Trolox was significantly higher (p<0.05) post-0 after both sessions compared to resting values and significantly lower (p<0.05) post-30 minutes compared to resting values.
There was no 2-way interaction between intervention and time noted for TBARS/Trolox ratio, F(2,24)=0.809, p=0.477. TBARS/Trolox ratio was not statistically different (p>0.05) after exercise for both sessions compared to resting values. TBARS/Trolox ratio was not statistically different (p=0.093) in the RT session compared to HVRT session at the beginning and 30 minutes after sessions (Figure 4C).
RPE and affective response
RPE were not statistically different (p=0.351) in the RT session (3.5±1.9) compared to HVRT session (3.0±2.3) after exercise. The affective responses after each exercise of HVRT and RT are presented in Table 2. Affective responses were significantly different (p=0.027) only after leg extension exercise.
  | Table 2 Affective responses after each exercise of strength and HVRT sessions (median [Q1 – Q2]) |
Discussion
The present study compared the acute effect of HVRT versus traditional RT (RT – moderate velocity) on metabolic, cardiovascular and psychophysiological responses in hypertensive elderly women. The main results revealed that HVRT induced a higher NO bioavailability, while oxidative stress and cardiovascular responses were transiently increased and returned to pre-exercise values up to 30–45 minutes after exercise in both training sessions. There were no differences for psychophysiological variables between protocols.
There was no significant decrease in SBP and DBP following traditional RT and HVRT in elderly hypertensive women. These results are in contrast to the findings of Coelho-Júnior et al11 who compared the effects of HVRT and RT and found a hypotensive effect on SBP for HVRT in hypertensive and normotensive elderly women. The study also demonstrated a decrease in HR and DBP (0–60 minutes) following RT and only at 60 minutes for HVRT, without differences in NO changes between conditions.11 It seems that alternative mechanisms other than an NO increase would be necessary to induce a decrease in SBP following RT and HVRT in hypertensive elderly women. Other studies have failed to demonstrate significant acute alterations in HR, DBP, and RPP following moderate RT29,30 in elderly normotensive and hypertensive women. The volume of the training sessions and number of exercises (10 in total) used in the present study might also have affected the cardiovascular responses, possibly inducing a metabolic stress that would require more time for postexercise recovery of the cardiovascular system. The use of different medications and a comparison with a control session without exercise could help to provide further insights into the findings.
Moreover, BP increased with RT duration. This finding suggests an important effect of training session duration, conceivably due to the accumulation of several metabolic substances responsible for an increase in BP.31 Another study using 4 exercises with explosive contractions failed to find alterations in BP and HR;32 thus, 8 exercises might be an interesting number and a longer recovery time may be required for reduction in blood pressure following 8 exercises. Of note, values of BP, HR, and RPP were considered to be within normal, safe, and well-tolerated limits by the hypertensive women. The RPP is considered to be a valid predictor of cardiovascular risk,33 which reinforces that neither RT nor HVRT negatively affected the cardiovascular response.
HVRT resulted in a higher NO bioavailability 30 minutes after exercise when compared with the RT session, while the RT session induced a higher blood lactate over the same time period. Considering that NO and lactate are both vasodilators, this might help to explain the absence of difference in BP between protocols. A previous study compared the hypotensive effects of treadmill exercise versus dance performed at the same HR intensity, and demonstrated a greater drop in BP for the dance group accompanied by a higher lactate concentration and no difference in nitrite production (NO2−).34 Furthermore, lactate may also stimulate the bioavailability of other vasodilators.35
Another result of note was the higher damage oxidative marker (TBARS) values immediately after HVRT when compared with RT, while values returned to normal within 30 minutes postexercise. Interestingly, this transient increase in TBARS was followed for increase in Trolox, with no significant changes in TBARS/Trolox ratio. Since plasma total antioxidant reflects a combination of various antioxidant defenses,36 our findings suggest that acute HVRT probably promoted increased in activity of antioxidant enzymes. Whether this transient oxidative stress in response to HVRT can be considered beneficial has not yet been clarified. However, knowing that reactive species of oxygen induced by acute exercise are necessary for promoting adaptation, such as antioxidant enzyme upregulation,37 exercise training protocols that promote some level of oxidative stress acutely could provide increased resistance to an oxidative insult.15,16 This is particularly interesting since aging is associated with diminished or even abolished ability to increase reactive oxygen species from acute exercise and lowering of resistance to oxidative stress.16,32 Moreover, hypertension and elevated body fat are also responsible for increased oxidative stress, which may also influence the results of the present study.
Another aim of the present study was to evaluate possible alterations in affective response resultant to different contraction velocities. According to the double-mode theory, the affective responses change with variations in exercise intensity. Exercises performed below anaerobic threshold promote feelings of pleasure due to a decreased perturbation of organic systems and cellular homeostasis. Moderate intensity exercise increases cognitive processes, which result in a variety of sensation responses, sometimes of pleasure other times of displeasure or discomfort. Exercise performed above anaerobic threshold normally results in unpleasable feelings.38 Our results revealed that both HVRT and RT were within moderate intensity levels as determined by the RPE, with no negative affective responses in elderly hypertensive women. Thus, the positive affective response to RT and HVRT may contribute to motivation and continuation of exercise training for longer periods.39 Other studies observed similar affective responses to RT in elderly sedentary women, trained young women, and sedentary men.18,40,41 The initial intensity selected to start an RT program may be very important to training adherence, especially in elderly people with hypertension.
Some limitations of the present study are the lack of measures from blood lipids, absence of taking into account the prevalence of metabolic syndrome, the acute nature of the results (which may not reflect chronic adaptations), and the absence of additional metabolic measures to improve the mechanistic approach. Moreover, the findings are specific to elderly hypertensive women and thus not necessarily generalizable to other populations. Future studies should seek to investigate longitudinal responses to HVRT and RT in the target population so we can develop a better understanding of the responses to these respective programs over time.
Conclusion
In conclusion, the acute cardiovascular and metabolic responses, including the oxidative stress, are transient and within normal values. Taken together with the positive affective responses, both HVRT and RT with this intensity and volume seem to be safe for elderly hypertensive women under medication.
Disclosure
The authors report no conflicts of interest in this work.
References
Alhawassi TM, Krass I, Pont LG. Hypertension in older persons: a systematic review of national and international treatment guidelines. J Clin Hypertens. 2015;17(6):486–492. | ||
American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687–708. | ||
Coelho-Júnior HJ, Aguiar SS, Gonçalves IO, et al. Sarcopenia is associated with high pulse pressure in older women. J Aging Res. 2015;2015:982–988. | ||
Ruiz JR, Sui X, Lobelo F, et al. Association between muscular strength and mortality in men: prospective cohort study. BMJ. 2008;337:a439. | ||
Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L. A comparison of leg power and leg strength within the study: which influences mobility more? J Gerontol A Biol Sci Med Sci. 2003;58(8):728–733. | ||
Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. 2012;40(1):4–12. | ||
Arazi H, Asadi A, Rahimzadeh M, Moradkhani AH. Post-plyometric exercise hypotension and heart rate in normotensive individuals: influence of exercise intensity. Asian J Sports Med. 2013;4(4):235–240. | ||
Ramírez-Campillo R, Abad-Colil F, Vera M, et al. Men and women exhibit similar acute hypotensive responses after low, moderate, or high-intensity plyometric training. J Strength Cond Res. 2016;30(1):93–101. | ||
Tibana RA, Pereira GB, de Souza JC, et al. Resistance training decreases 24-hour blood pressure in women with metabolic syndrome. Diabet Metabol Syndr. 2013;5:27. | ||
Halliwill JR, Taylor JA, Eckberg DL. Impaired sympathetic vascular regulation in humans after acute dynamic exercise. J Physiol. 1996;15;495(Pt 1):279–288. | ||
Coelho-Júnior HJ, Irigoyen MC, Aguiar SDS, et al. Acute effects of power and resistance exercises on hemodynamic measurements of older women. Clin Interv Aging. 2017;12:1103–1114. | ||
Vanhoutte PM, Shimokawa H, Tanq EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol. 2009;196(2):193–222. | ||
Taddei GF, Virdis A, Ghiadoni L, et al. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101(25):2896–2901. | ||
Juffer P, Bakker AD, Klein-Nulend J, Jaspers RT. Mechanical loading by fluid shear stress of myotube glycocalyx stimulates growth factor expression and nitric oxide production. Cell Biochem Biophys. 2014;69(3):411–419. | ||
Ceci R, Beltran Valls MR, Duranti G, et al. Oxidative stress responses to a graded maximal exercise test in older adults following explosive-type resistance training. Redox Biol. 2014;2:65–72. | ||
Nordin TC, Done AJ, Traustadóttir T. Acute exercise increases resistance to oxidative stress in young but not older adults. Age (Dordr). 2014;36(6):9727. | ||
Ekkekakis P, Lind E, Joens-Matre RR. Can self-reported preference for exercise intensity predict physiologically defined self-selected exercise intensity? Rev Q Exerc Sport. 2006;77(1):81–90. | ||
Alves RC, Ferreira SS, Benites ML, Krinski K, Follador L, Silva SG. Exercícios com pesos sobre as respostas afetivas e perceptuais. Rev Bras Med Esporte. 2015;21(3):200–205. | ||
Lamas L, Ugrinowitsch C, Campos GER, et al. Treinamento de força máxima x treinamento de potência: alterações no desempenho e adaptações morfológicas. Rev Bras Educ Fis Esporte. 2007;21(4):331–340. | ||
Wallerstein LF, Tricoli V, Barroso R, et al. Effects of strength and power training on neuromuscular variables in older adults. J Aging Phys Act. 2012;20(2):171–185. | ||
Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–1252. | ||
Mazo GZ, Benedetti TRB. Adaptação do questionário internacional de atividade física para idosos. Rev Bras Cineantropom Desempenho Hum. 2010;12(6):480–484. | ||
Nascimento Dda C, Navalta JW, Durigan JL, et al. Acute eccentric resistance exercise decreases matrix metalloproteinase activity in obese elderly women. Clin Physiol Funct Imaging. 2016;36(2):139–145. | ||
Bernheim F, Bernheim ML, Wilbur KM. The reaction between thiobarbituric acid and the oxidation products of certain lipides. J Biol Chem. 1948;174(1):257–264. | ||
Green IC, Wagner DA, Glowski J, Skipper PL, Wishnok JS, Tannenbaum SB. Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. Anal Biochem. 1982;126(1):131–138. | ||
Rohn S, Rawel HM, Kroll J. Antioxidant activity of protein-bound quercetin. J Agric Food Chem. 2004;52(15):4725–4729. | ||
Hardy CJ, Rejeski WJ. Not what, but how one feels: measurement of affect during exercise. J Sport Exerc Psychol. 1989;11(3):304–317. | ||
Foster C, Florhaug A, Franklin J, et al. A new approach to monitoring exercise training. J Strength Cond Res. 2001;15(1):109–115. | ||
Mota MR, de Oliveira RJ, Dutra MT, et al. Acute and chronic effects of resistive exercise on blood pressure in hypertensive elderly women. J Strength Cond Res. 2001;27(12):3475–3480. | ||
Gerage AM, Ritti-Dias RM, do Nascimento MA, et al. Chronic resistance training does not affect post-exercise blood pressure in normotensive older women: a randomized controlled trial. Age (Dordr). 2015;37(3):63. | ||
Gjøvaag TF, Mirtaheri P, Simon K, et al. Hemodynamic responses to resistance exercise in patients with coronary artery disease. Med Sci Sports Exerc. 2016;48(4):581–588. | ||
Beltran Valls MR, Dimauro I, Brunelli A, et al. Explosive type of moderate-resistance training induces functional, cardiovascular, and molecular adaptations in the elderly. Age (Dordr). 2014;36(2):759–772. | ||
Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352(19):1951–1958. | ||
Sousa FEV, Nascimento EF, Souza LHR, et al. Dancing is more effective than treadmill walking for blood pressure reduction in hypertensive elderly women. J Exerc Physiol Online. 2016;19(1):124–134. | ||
Alhawassi TM, Krass I, Pont LG. Hypertension in older persons: a systematic review of national and international treatment guidelines. J Clin Hypertens. 2015;17(6):486–492. | ||
Fisher-Wellman K, Bloomer RJ. Acute exercise and oxidative stress: a 30 year history. Dyn Med. 2009;8:1. | ||
Ji LL, Kang C, Zhang Y. Exercise-induced hormesis and skeletal muscle health. Free Radic Biol Med. 2016;98:113–122. | ||
Ekkekakis P, Parfitt G, Petruzzello SJ. The pleasure and displeasure people feel when they exercise at different intensities: Decennial update and progress towards a tripartite rationale for exercise intensity prescription. Sports Med. 2011;41(8):641–671. | ||
Kwan BM, Bryan AD. Affective response to exercise as a component of exercise motivation: attitudes, norms, self-efficacy, and temporal stability of intentions. Psychol Sport Exerc. 2010;11(1):71–79. | ||
Focht BC, Garver MJ, Cotter JA, Devor ST, Lucas AR, Fairman CM. Affective responses to acute resistance exercise performed at self-selected and imposed loads in trained women. J Strength Cond Res. 2015;29(11):3067–3074. | ||
Elsangedy HM, Krinski K, Machado DG, Agrícola PM, Okano AH, Gregório da Silva S. Self-selected intensity, ratings of perceived exertion, and affective responses in sedentary male subjects during resistance training. J Phys Ther Sci. 2016;28(6):1795–1800. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.