Back to Journals » Clinical Ophthalmology » Volume 16
Comparison of the Accuracy of Intraoperative Aberrometry in Intraocular Lens Implantation Between Myopic Eyes with Emmetropia and Myopia Targets
Authors Sakai D , Demoto S, Iwai Y, Hirami Y, Nakamura M , Kurimoto Y
Received 19 February 2022
Accepted for publication 11 April 2022
Published 16 April 2022 Volume 2022:16 Pages 1165—1171
DOI https://doi.org/10.2147/OPTH.S363228
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Daiki Sakai,1– 3 Sakiko Demoto,1,2 Yukako Iwai,1,2 Yasuhiko Hirami,1,2 Makoto Nakamura,3 Yasuo Kurimoto1,2
1Department of Ophthalmology, Kobe City Eye Hospital, Kobe, Japan; 2Department of Ophthalmology, Kobe City Medical Center General Hospital, Kobe, Japan; 3Department of Surgery, Division of Ophthalmology, Kobe University Graduate School of Medicine, Kobe, Japan
Correspondence: Daiki Sakai, Department of Ophthalmology, Kobe City Eye Hospital, 2-1-8 Minatojima Minamimachi, Chuo-ku, Kobe-shi, Hyogo, 650-0047, Japan, Tel +81-78-381-9876, Fax +81-78-381-9910, Email [email protected]
Purpose: To compare the accuracy of intraoperative aberrometry (IA) for predicting postoperative refraction between eyes with emmetropia and myopia targets.
Patients and Methods: This retrospective analysis included patients with axial myopia (axial length ≥ 25.0 mm) who underwent uncomplicated phacoemulsification cataract surgery with IA to achieve emmetropia (plano to − 0.5 D) or intentional myopia (− 2.5 D to − 5.0 D). Preoperative ocular biometry was performed in all eyes using an IOLMaster. Refractive prediction errors in IA were compared between eyes with emmetropia and myopia targets. Refractive prediction errors in IA for both groups were also compared with those predicted by intraocular lens power calculation formulas including the SRK/T, Holladay 1, Hoffer Q, Holladay 2, Haigis, and Barrett Universal II formulas.
Results: Thirty-nine eyes of 39 patients with a target of emmetropia and 22 eyes of 22 patients with a target of intentional myopia were included in the final analysis. The mean numerical error was significantly different from zero (myopic trend) in myopia-targeted eyes (− 0.37 ± 0.54 D, one-sample t-test, P = 0.004, 95% confidence interval: − 0.61 to − 0.14), while it was close to zero in emmetropia-targeted eyes. The mean absolute error was significantly smaller in emmetropia-targeted eyes (0.28 ± 0.27 D) than in myopia-targeted eyes (0.51 ± 0.41 D, P = 0.01). IA was revealed as the most accurate method for predicting postoperative refraction in eyes with emmetropia target, whereas Barrett Universal II formula was found to be the most accurate for eyes with myopia target.
Conclusion: In patients with axial myopia, the performance of IA was altered when targeting intentional myopia compared with emmetropia. Myopic shift in the refractive outcome should be considered when IA is used to target myopia.
Keywords: cataract surgery, postoperative refraction, intentional myopia, axial myopia, intraocular lens calculation formula
Introduction
Modern cataract surgery is a refractive procedure. In recent times, there has been an increased focus on the importance of postoperative refractive outcomes. The accuracy of predicting postoperative refraction has improved with improvements in the methods of ocular biometry and intraocular lens (IOL) power calculation. Using modern biometers and calculation formulas, approximately 90% of eyes are expected to achieve a prediction error within 0.50 D.1 However, in atypical eyes such as those with short or long axial length, or eyes with previous corneal refractive surgery, accurately predicting postoperative refraction is still considered to be challenging.
Intraoperative aberrometry (IA) is a newly developed technique for improving postoperative refractive outcomes. Wavefront measurement technology is used to perform ocular biometry after crystalline lens extraction, and the ideal IOL power is suggested based on aberrations in the aphakic eye. The Optiwave Refractive Analysis (ORA) system (Alcon Laboratories, Fort Worth, TX, USA), based on the Talbot–Moiré interferometry, is one of the most studied intraoperative aberrometers. Its usefulness was first reported in patients with previous corneal refractive surgery.2 More recently, Hill et al reported that the ORA performed better when compared with the various IOL power calculation formulas in patients with axial myopia,3 suggesting that axial myopia could be a second candidate benefiting from IA.
In previous studies, IOL power calculation methods were mainly investigated on the accuracy when the target refraction was emmetropia. However, intentional myopia is another choice of target refraction selected in clinical settings. Patients with axial myopia often want to retain the myopia to maintain good vision at near-distance. Additionally, it compensates for the risk of hyperopic surprise.4
To date, there have been few reports on the accuracy of IOL power calculation methods for eyes with a target of intentional myopia. Altered performance of IOL calculation formulas has been suggested when targeting myopia compared with emmetropia, but this conclusion remains uncertain.5–8 Moreover, the accuracy of IA when targeting myopia has not been reported yet. The purpose of this study was to compare the accuracy of IA in predicting postoperative refraction between eyes with target refractions of emmetropia and intentional myopia.
Materials and Methods
This retrospective study was performed in accordance with the Declaration of Helsinki and was approved by the Medical Ethics Committee of Kobe City Medical Center General Hospital (Kobe, Japan). An opt-out method of informed consent was used for this observational study involving the use of medical records. Patients who refused consent were excluded from the study. We reviewed the medical records of patients with axial myopia (axial length ≥ 25.0 mm) who underwent uncomplicated phacoemulsification cataract surgery with IA from December 2017 to November 2020 at Kobe City Eye Hospital. This study included eyes with refractive targets of emmetropia (0 to −0.50 D) or intentional myopia (−2.00 to −5.00 D) regardless of IOL type. Preoperative ocular biometry was performed using an IOLMaster 700 (Carl Zeiss Meditec, Jena, Germany). Surgeries were performed by one of the nine surgeons in the Kobe City Eye Hospital. The manifest refraction at a follow-up visit between one week and two months postoperatively was recorded as the postoperative refraction. If there were multiple visits in this period, the latest one was selected. Exclusion criteria included previous corneal refractive surgery (n = 9), lack of pre- and postoperative data (n = 7), early postoperative complications (within two months) such as IOL dislocation (n = 1) or toric IOL axis rotation (n = 3), postoperative corrected distance visual acuity worse than 20/40, and previous ocular surgery (trabeculectomy: n = 4). Additionally, if both eyes of a patient were eligible (24 patients), one of them was randomly selected to account for inter-eye correlation.9 Finally, 39 eyes of 39 patients with a target of emmetropia and 22 eyes of 22 patients with a target of intentional myopia were included.
Preoperatively, IOL power was calculated using the surgeon’s preferred IOL calculation formula. Intraoperatively, after cortical cleanup, each eye was inflated using an ophthalmic viscosurgical device, and an intraocular pressure of 20 mmHg was verified using a Barraquer tonometer. IA using the ORA system was then performed to measure the refractive power of the aphakic eye, and a recommended IOL power was immediately obtained. When there was a discrepancy between the IOL power suggested by the ORA system and that selected preoperatively, each surgeon was allowed to choose either one or another power between them.
The predicted refraction (calculated with the power of the actually implanted IOL) obtained from IA and each calculation formula integrated into the IOLMaster (Sanders, Retzlaff, and Kraft/theoretical [SRK/T]; Holladay 1; Hoffer Q; Holladay 2; Haigis; and Barret Universal II formulas) were retrospectively recorded. A User Group for Laser Interference Biometry-optimized IOL constant was used. In one myopia-targeted eye, the predicted refraction using the Holladay 2 formula was unavailable due to data insufficiency. Prediction error was calculated by subtracting the predicted refraction from the postoperative refraction (numerical error), and absolute error was defined as the absolute value of the numerical error. A negative numerical error indicated a myopic error, and a positive numerical error indicated a hyperopic error. The accuracy of IA was compared between eyes with refractive targets of emmetropia and intentional myopia. Additionally, the accuracy of IA was compared with that of each IOL calculation formula.
All statistical analyses were performed using SPSS for Windows (version 25, SPSS Inc.). In both groups, categorical variables were compared using the chi-square test, and continuous variables were compared using the Mann–Whitney U-test. The one-sample t-test was used to determine whether the numerical error followed a normal distribution with a mean equal to zero for each group. Comparisons of the absolute error between IA and each IOL calculation formula were performed using the Friedman test followed by the Wilcoxon signed-rank test with Bonferroni correction. Statistical significance was set at p ≤ 0.05.
Results
A total of 61 eyes from 61 patients were included in the study. Thirty-four patients were female, and the mean (± standard deviation) age of all participants was 66.9 ± 9.9 years. Multifocal IOLs were implanted in 28 eyes, all of which had emmetropia as the target. Femtosecond laser-assisted cataract surgery was performed in four eyes with multifocal IOL implantation. Toric IOLs were implanted in 20 eyes, of which 12 were emmetropia-targeted and eight were myopia-targeted.
Table 1 shows the characteristics of the participants and the implanted IOLs in eyes with targets of emmetropia and intentional myopia. The participants with emmetropia-targeted eyes were significantly younger than those with myopia-targeted eyes. There were no significant between-group differences in preoperative biometric values (axial length, average keratometry, white to white), which were required for using IA.
 |
Table 1 Characteristics of the Patients and Implanted Intraocular Lenses |
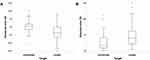
A box and whisker plot of the prediction error of IA is shown in Figure 1, and the between-group comparison of the prediction error is shown in Table 2. The mean numerical error of the myopia-targeted eyes (−0.37 ± 0.54 D) was significantly smaller (relatively myopic) than that of the emmetropia-targeted eyes (0.04 ± 0.39 D, P = 0.001). The mean numerical error of the emmetropia-targeted eyes was not significantly different from zero (one-sample t-test, P = 0.55, 95% confidence interval [CI]: −0.09 to 0.16). However, the mean numerical error of the myopia-targeted eyes was significantly different from zero (myopic trend) (one-sample t-test, P = 0.004, 95% CI: −0.61 to −0.14). The mean absolute error of the emmetropia-targeted eyes (0.28 ± 0.27 D) was significantly smaller than that of the myopia-targeted eyes (0.51 ± 0.41 D, P = 0.01).
 |
Table 2 Prediction Error of Intraoperative Aberrometry |
The comparisons of accuracy between IA and IOL calculation formulas for emmetropia-targeted eyes and myopia-targeted eyes are shown in Tables 3 and 4, respectively. In emmetropia-targeted eyes, the mean absolute errors varied significantly between the IOL calculation methods (P < 0.001, Friedman test). The mean absolute error of IA was significantly smaller than that of the Holladay 1, Hoffer Q, and Holladay 2 formulas (P < 0.001, < 0.001, and = 0.015, respectively). The mean absolute error of IA was also smaller than that of the SRK/T, Haigis, and Barrett Universal II formulas, although this difference was not statistically significant. In myopia-targeted eyes, the mean absolute errors were not significantly different between the different IOL calculation methods (P = 0.051, Friedman test). The mean absolute errors of the Holladay 2, Haigis, and Barrett Universal II formulas were smaller than that of IA, although these differences were not statistically significant.
 |
Table 3 Comparison Between Accuracy of Intraoperative Aberrometry and Intraocular Lens Calculation Formulas in 39 Eyes with Target of Emmetropia |
 |
Table 4 Comparison Between Accuracy of Intraoperative Aberrometry and Intraocular Lens Calculation Formulas in 22 Eyes with Target of Intentional Myopia |
Discussion
The use of IA is expected to improve the refractive outcome of patients with axial myopia.3,10 Previous reports on IA accuracy mainly focused on achieving emmetropia, although intentional myopia is another common choice for patients with axial myopia. In this study, we aimed to investigate the influence of intentional myopia on the refractive prediction of IA. We found that the performance of IA was altered when targeting intentional myopia compared with emmetropia.
There are limited studies in the available literature that have compared the accuracy of the IOL power calculation methods between emmetropia- and myopia-targeted eyes. However, some of these studies suggested that the IOL power calculation methods might be less accurate for targeting myopia compared with emmetropia. In a study conducted by Geggel et al, the Haigis formula was less accurate when targeting myopia (−1.0 D) than when targeting emmetropia.11 Turnbull et al studied patients planned for monovision and reported that IOL power calculation formulas such as the SRK/T, Holladay 1, Hoffer Q, Haigis, Barret Universal II, and Hill-RBF version 2.0 were all less accurate in eyes with a target of myopia (−1.25 D) than in eyes with a target of emmetropia.6 The present study is the first to compare the accuracy of IA between emmetropia- and myopia-targeted eyes. Our results suggested that similar to the IOL power calculation formulas, IA might also be less accurate when targeting myopia (−2.5 to −5.0 D) than when targeting emmetropia. When targeting emmetropia, IA was more accurate than any of the IOL power calculation formulas preinstalled on the IOLMaster, which is consistent with a previous report.3 However, when targeting intentional myopia, the superiority of IA was not found. Although there was no statistically significant difference in the accuracy of the IOL power calculation methods in myopia-targeted eyes, the Barrett Universal II formula was the most accurate.
We observed a significant myopic shift on refractive outcome using IA in myopia-targeted eyes. Behndig et al studied a large cohort of over 17,000 eyes and reported that eyes with a target of myopia (≤ −1.6 D) became more myopic than planned preoperatively.12 Although their study had a limitation of inclusion of various formulas of IOL power calculation, the findings were similar to our results. Cooke et al previously reported a myopic trend in the refractive outcome using SRK/T for targeting intentional myopia (≤ −1.5 D).7 Moreover, we recently reported that SRK/T, Holladay 1, Hoffer Q, and Holladay 2 formulas showed a relative myopic trend in the refractive outcome when targeting intentional myopia (−2.0 D to −3.0 D) as compared to emmetropia.8 Therefore, it might be helpful to take into consideration the relative myopic shift in the refractive outcome for accurate targeting of myopia.
Why is the performance of IA altered when targeting myopia? We found no significant differences in ocular biometric parameters between emmetropia-targeted eyes and myopia-targeted eyes. One suggested hypothesis is that the higher IOL power required for targeting myopia enhances the effect of any inaccuracy in the calculation of predicted postoperative refraction.6 To date, the detailed process by which the ORA system calculates the predicted postoperative refraction is undisclosed. Further studies in this regard are required to explain the results of this study.
A major limitation of this study is that the implanted IOLs were not uniform. Different types of IOLs (monofocal, multifocal, and toric) were used in this study. In particular, the selection of multifocal IOL was biased, which was inevitable because multifocal IOL implantation is generally performed targeting emmetropia. Other limitations include the retrospective design, relatively small sample size, and short follow-up time. Future studies with larger samples and uniformity in IOL implantation are needed to validate the findings of this study.
Conclusion
The performance of IA was altered in eyes with a refractive target of intentional myopia compared with those with a target of emmetropia. When IA is used to target myopia, myopic shift should be considered, and newer IOL power calculation formulas, such as Barrett Universal II are recommended to be used concomitantly to achieve improved refractive outcomes.
Abbreviations
CI, confidence interval; IA, intraoperative aberrometry; IOL, intraocular lens; ORA, optiwave refractive analysis; SRK/T, Sanders, Retzlaff, and Kraft/theoretical.
Funding
There is no funding to report.
Disclosure
Dr Yasuo Kurimoto reports grants and/or personal fees from Astellas, Santen, Tomey, Nikon, Sumitomo Dainippon Pharma, AMO Japan, Senju, Kowa, Alkon Japan, Bayer, Pfizer, Novartis, and Viatris, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Savini G, Hoffer KJ, Balducci N, Barboni P, Schiano-Lomoriello D. Comparison of formula accuracy for intraocular lens power calculation based on measurements by a swept-source optical coherence tomography optical biometer. J Cataract Refract Surg. 2020;46(1):27–33. doi:10.1016/j.jcrs.2019.08.044
2. Ianchulev T, Hoffer KJ, Yoo SH, et al. Intraoperative refractive biometry for predicting intraocular lens power calculation after prior myopic refractive surgery. Ophthalmology. 2014;121(1):56–60. doi:10.1016/j.ophtha.2013.08.041
3. Hill DC, Sudhakar S, Hill CS, et al. Intraoperative aberrometry versus preoperative biometry for intraocular lens power selection in axial myopia. J Cataract Refract Surg. 2017;43(4):505–510. doi:10.1016/j.jcrs.2017.01.014
4. Yokoi T, Moriyama M, Hayashi K, Shimada N, Ohno-Matsui K. Evaluation of refractive error after cataract surgery in highly myopic eyes. Int Ophthalmol. 2013;33(4):343–348. doi:10.1007/s10792-012-9690-6
5. Dalto RF, Ferreira MA, Queiroz W, Coelho RP, Paula JS, Messias A. Haigis and SRKT formulae accuracy for intentional myopic overcorrection. Int Ophthalmol. 2018;38(4):1459–1463. doi:10.1007/s10792-017-0607-2
6. Turnbull AMJ, Hill WE, Barrett GD. Accuracy of intraocular lens power calculation methods when targeting low myopia in monovision. J Cataract Refract Surg. 2020;46(6):862–866. doi:10.1097/j.jcrs.0000000000000187
7. Cooke DL, Huie T, Pletcher J. IOL power formula accuracy for intentional myopic overcorrection. J Cataract Refract Surg. 2021;47(9):1237–1238. doi:10.1097/j.jcrs.0000000000000456
8. Sakai D, Hirami Y, Nakamura M, Kurimoto Y. Accuracy of intraocular lens power calculation formulas in myopic eyes with target refractions of emmetropia and intentional myopia. Clin Ophthalmol. 2021;15:4535–4541. doi:10.2147/OPTH.S342392
9. Armstrong RA. Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol Opt. 2013;33(1):7–14. doi:10.1111/opo.12009
10. Christopher KL, Patnaik JL, Ifantides C, et al. Time utilization and refractive prediction enhancement associated with intraoperative aberrometry use during cataract surgery. Clin Ophthalmol. 2021;15:531–539. doi:10.2147/OPTH.S287573
11. Geggel HS. Comparison of formulas and methods for high myopia patients requiring intraocular lens powers less than six diopters. Int Ophthalmol. 2018;38(4):1497–1504. doi:10.1007/s10792-017-0611-6
12. Behndig A, Montan P, Stenevi U, Kugelberg M, Zetterström C, Lundström M. Aiming for emmetropia after cataract surgery: Swedish National Cataract Register study. J Cataract Refract Surg. 2012;38(7):1181–1186. doi:10.1016/j.jcrs.2012.02.035
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.