Back to Journals » Clinical Ophthalmology » Volume 17
Comparison of the 1st Generation and 3rd Generation Wavefront-Guided LASIK for the Treatment of Myopia and Myopic Astigmatism
Authors Hannan SJ, Teenan D , Venter JA, Hettinger KA, Berry CW, Hannan NC, Kiss HJ, Raju D, Schallhorn JM
Received 5 August 2023
Accepted for publication 6 November 2023
Published 22 November 2023 Volume 2023:17 Pages 3579—3590
DOI https://doi.org/10.2147/OPTH.S434037
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Stephen J Hannan,1 David Teenan,1 Jan A Venter,1 Keith A Hettinger,1 Colin W Berry,1 Noelle C Hannan,1 Huba J Kiss,1 Dasi Raju,1 Julie M Schallhorn2,3
1Optical Express, Glasgow, United Kingdom; 2University of California, San Francisco, Department of Ophthalmology, San Francisco, CA, USA; 3F.I. Proctor Foundation, University of California, San Francisco, San Francisco, CA, USA
Correspondence: Stephen J Hannan, Clinical Services Department, Optical Express, 200 St Vincent St, Glasgow, G2 5SG, United Kingdom, Tel +44 7740592389, Email [email protected]
Purpose: To compare refractive, visual, and patient-reported outcomes associated with a 1st generation wavefront-guided (WFG) treatment with those associated with a 3rd generation WFG treatment.
Patients and Methods: This retrospective study included patients who underwent femtosecond laser-assisted in situ keratomileusis (LASIK) for myopia/myopic astigmatism. Two random stratified samples of patients who underwent either 1stgeneration (WaveScan, Johnson & Johnson Vision, Santa Ana, CA) or 3rd generation (iDesign 2.0, Johnson & Johnson Vision, Santa Ana, CA) treatment matched on preoperative refraction were compared (4290 eyes of 2145 patients in each group). One-month postoperative visual, refractive, and patient-reported outcomes were analyzed. Refractive and monocular visual acuity analyses were performed using one random eye of each patient.
Results: The percentage of eyes achieving 20/20 or better uncorrected vision was 91.3% (1958/2145) in the 1st generation group and 95.9% (2056/2145) in the 3rd generation group (p< 0.01). Binocularly, the percentage of patients with 20/20 or better UDVA was 97.0% (2080/2145) and 99.2% (2127/2145) in the 1st and 3rd generation groups, respectively (p< 0.01). The mean postoperative MSE was − 0.01 ± 0.33 D in the 1st generation group and +0.19 ± 0.33 D in the 3rd generation group (p< 0.01). Postoperative refractive astigmatism had a mean value of − 0.20 ± 0.26 D and − 0.18 ± 0.24 D in the 1st and 3rd generation groups, respectively (p< 0.01). The mean correction index of refractive astigmatism was 1.09 ± 0.53 in the 1st generation group and 1.02 ± 0.38 in the 3rd generation group, p< 0.01. The overall percentage of patients satisfied with vision was 92.8% (1991/2145 patients) in the 1st generation group and 97.3% (2087/2145 patients) in the 3rd generation group (p< 0.01).
Conclusion: For the majority of postoperative variables, there were significant differences between 1st and 3rd generation treatments. The 3rd generation treatments had better visual acuity outcomes and higher patient satisfaction.
Keywords: wavefront-guided LASIK, myopia, aberrometer, refractive outcomes, patient-reported outcomes
Introduction
Wavefront-guided (WFG) treatment of refractive error utilizing the excimer laser has been a cornerstone of refractive surgery for nearly a quarter of a century.1,2 Study after study has demonstrated the excellent safety and accuracy profile of these methods.1
The 1st generation WaveScan (J&J Vision, Santa Ana, CA) wavefront aberrometer introduced wavefront-guided technology and established the use of Hartmann-Shack aberrometry in developing custom ablation patterns.3,4 This technology, which facilitated 1st generation wavefront (CustomVue) procedures on the VISX Star S4 IR laser (J&J Vision, Santa Ana, CA), was followed by the 2nd generation iDesign Advanced WaveScan Studio System (J&J Vision, Santa Ana, CA).5,6 This 2nd generation device introduced a higher density Hartmann-Shack aberrometer which had approximately five times the resolution of the previous generation sensor.5 This device, when coupled with an updated software package for treatment planning as well as integration of corneal topography into the treatment planning, has yielded a 3rd generation of wavefront treatment in the iDesign Refractive Studio and iDesign 2.0 (J&J Vision, Santa Ana, CA).
In this study, we compare the visual, refractive, and patient-reported outcomes of the 1st generation wavefront-guided treatment (CustomVue with the WaveScan aberrometer) and the 3rd generation WFG technology (iDesign 2.0 with the iDesign Refractive Studio) in a series of femtosecond laser-assisted in situ keratomileusis (LASIK) patients.
Patients and Methods
This study was deemed exempt from review by the University of California, San Francisco Institutional Review Board because it used only retrospective, de-identified information. All patients provided full informed consent to undergo laser-assisted in situ keratomileusis (LASIK) as well as to have their de-identified information used for research purposes and statistical analysis.
The electronic medical record of Optical Express (Glasgow, United Kingdom) was searched to identify patients who underwent femtosecond LASIK using the 1st generation and the 3rd generation wavefront-guided (WFG) treatments with the following inclusion criteria: bilateral surgery, age between 18 and 40 years (to avoid inclusion of eyes that might have been targeted for monovision), completed one-month postoperative visit, and completed patient experience questionnaire.
Exclusion criteria for treatment were any active ophthalmic disease, history of ophthalmic disease, certain medical conditions that could interfere with corneal healing, abnormal corneal shape, and calculated postoperative corneal stromal bed thickness of less than 250 μm in each eye. Soft and rigid gas-permeable contact lens wearers were asked to discontinue their use at least 1 day prior to their initial preoperative consultation and then a minimum of 1 week and 4 weeks, respectively, before the final diagnostic measurement and scan capture used to obtain accurate preoperative data and treatment plans.
Preoperative examination included manifest and cycloplegic refraction, monocular and binocular uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA) using a calibrated projected eye chart, low-light pupil diameter, anterior eye health examination via slit lamp biomicroscopy, dilated fundus examination, tonometry, corneal topography and pachymetry, and wavefront aberration measurement. Uncorrected and corrected distance visual acuities were measured at 4 meters with a logarithmic acuity chart and refractions corrected to optical infinity based on the testing distance.
Postoperative examinations included UDVA and CDVA measurements, manifest refraction (except for day 1), anterior eye health assessment via slit lamp biomicroscopy, tonometry, and keratometry. At the postoperative visits, patients were required to complete a patient experience questionnaire, which facilitates patient-reported outcomes measurements (PROMS). The methodology of obtaining the questionnaire has been previously described.7
Surgical Technique
All WFG LASIK surgeries were performed by 34 experienced surgeons in 40 clinics across the United Kingdom. Corneal flaps were created using a femtosecond laser (iFS, J&J Vision, Santa Ana, CA). The diameter of the femtosecond flaps ranged from 8.0–9.2 mm, and the intended depth ranged from 100–120 μm. After flap lifting, the laser ablation calculated according to the 1st generation or the 3rd generation Hartmann-Shack aberrometer measurement (WaveScan or iDesign Refraction Studio) was applied using the Star S4IR excimer laser (J&J Vision, Santa Ana, CA). Treatments were programmed in all cases with a 6.0 mm optical zone and an outer blend zone meaning a total ablation zone between 8.0–9.0 mm.
Statistical Analysis
For each treatment type, a stratified sample of patients matched on preoperative sphere and cylinder was selected. The patient selection followed the stratified random sample methodology. Patients were assigned to stratification groups based on their preoperative refraction, then a series of random samples were taken from each stratum. The stratification ensured that the patient in the 1st generation group would also be eligible for treatment by the 3rd generation WFG LASIK and vice versa. One-month postoperative outcomes were then presented and compared using the standardized graphs for reporting outcomes of refractive surgery and astigmatic outcomes.8,9 To report refractive outcomes and monocular visual acuity outcomes, one random eye of each patient was selected for analysis to account for interrelatedness of the two eyes of the same patients. Binocular visual acuity analysis and patient-reported outcomes were performed on a per-patient basis. Independent t-test was used to compare one-month postoperative outcomes between the two groups and a Chi-square test was used to compare percentages and proportions. All data were analyzed using Microsoft Office Excel program (Microsoft Corp., Redmond, WA).
Results
The stratified sample included 4290 eyes (2145 patients) in each group. For the refractive outcomes and monocular visual acuity comparison, one random eye of each patient was included, resulting in a 2145 eyes per group sample. Table 1 shows the comparison of preoperative and postoperative clinical data. Both groups had comparable preoperative mean age and a similar male/female ratio. There was no statistically significant difference between preoperative refractive sphere, cylinder, manifest spherical equivalent, or CDVA (Table 1).
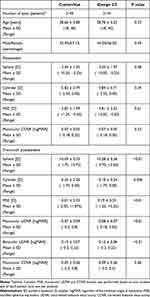 |
Table 1 Preoperative and One-Month Postoperative Outcomes |
Visual Outcomes
Figures 1A and B show cumulative monocular and binocular UDVA at the one month postoperative visit. The percentage of monocular eyes achieving 20/20 or better vision was 91.3% (1958/2145) in the 1st generation group and 95.9% (2056/2145) in the 3rd generation group (p<0.01). Binocularly, the percentage of patients with 20/20 or better UDVA was 97.0% (2080/2145) and 99.2% (2127/2145) in the 1st generation and 3rd generation groups, respectively (p<0.01).
 |
Figure 1 Cumulative monocular (A) and binocular (B) uncorrected distance visual acuity. (VA – visual acuity). |
Figure 2 depicts the difference between preoperative CDVA and postoperative UDVA. Of all eyes, 78.8% (1690/2145) in the 1st generation group and 84.8% (1818/2145) in the 3rd generation group had postoperative UDVA the same or better than preoperative CDVA (p<0.01). The percentage of eyes with postoperative UDVA within one line of preoperative CDVA was 92.4% (1983/2145) in the 1st generation group and 96.6% (2073/2145) in the 3rd generation group (p<0.01).
 |
Figure 2 The difference between preoperative corrected distance visual acuity (CDVA) and postoperative uncorrected distance visual acuity (UDVA). |
Figure 3 presents the difference between preoperative and postoperative CDVA. The difference in the distribution of percentages between the two groups was statistically significant (p<0.01). The percentage of eyes with postoperative CDVA unchanged or better compared with preoperative CDVA was higher in the 3rd generation group (1st generation: 85.2% or 1828/2145 eyes, 3rd generation: 90.0% or 1930/2145 eyes; p<0.01).
 |
Figure 3 The difference between preoperative and postoperative corrected distance visual acuity (CDVA). |
Refractive Outcomes
The scattergram of attempted vs achieved correction of the manifest spherical equivalent (MSE) is presented in Figures 4A and B. Both scattergrams show a very tight distribution of data points with a similar coefficient of determination (R2=0.9738 for both 1st and 3rd generation), but the regression line in the 3rd generation group shows a slight overcorrection of MSE.
 |
Figure 4 Attempted versus achieved correction of manifest spherical equivalent. (A) 1st generation treatment, (B) 3rd generation treatment. |
Likewise, the histogram in Figure 5 shows a postoperative distribution of MSE skewed slightly to the right (slight hyperopia) in the 3rd generation group. This might be expected for an early postoperative visit, considering a slight regression of MSE is likely to occur after the one-month visit. As a result of the slight overcorrection in the 3rd generation group, the percentage of eyes within ±0.50 D of emmetropia was higher in the 1st generation group (1st generation: 91.8% or 1969/2145 eyes, 3rd generation: 87.9% or 1886/2145 eyes; p<0.01). Both groups achieved similar percentages of eyes within ±1.00 D of emmetropia (1st generation: 99.0% or 2123/2145 eyes, 3rd generation: 98.7% or 2117/2145 eyes; p=0.39). One-month postoperative mean MSE was −0.01 ± 0.33 D in the 1st generation group and +0.19 ± 0.33 D in the 3rd generation group (p<0.01).
 |
Figure 5 The distribution of postoperative manifest spherical equivalent (MSE). |
Astigmatic Outcomes
Preoperatively, both groups had a comparable amount of refractive astigmatism (Table 1). At the one-month visit, the mean refractive astigmatism was −0.20 ± 0.26 D in the 1st generation group and −0.18 ± 0.24 D in the 3rd generation group (p = 0.006). Although there was a statistically significant difference between the two means due to the large sample size of the dataset, this small difference (0.02 D) likely lacks clinical significance. The histogram in Figure 6 compares the distribution of residual refractive astigmatism between the two groups. The 3rd generation group had a higher percentage of eyes with the absolute value of residual refractive cylinder ≤0.50 D (1st generation: 92.5% or 1984/2145 eyes, 3rd generation: 94.5% or 2028/2145 eyes; p<0.01), but the percentage of eyes with residual astigmatism ≤1.00 D was comparable between the two groups (1st generation: 99.3% or 2131/2145 eyes, 3rd generation: 99.4% or 2132/2145 eyes; p=0.85).
 |
Figure 6 The distribution of postoperative residual refractive astigmatism (absolute values). |
Figures 7 and 8 present the outcomes of vector analysis of refractive astigmatism. Figures 7A (1st generation) and 7B (3rd generation) show the scattergram of target induced (TIA) vs surgically induced (SIA) astigmatism vector. Both graphs show an undercorrection of astigmatism with increasing amounts of target induced astigmatism. Both regression lines had a very similar coefficient of determination (1st generation: R2=0.9078, 3rd generation: R2=0.9021). The 1st generation group shows a more apparent overcorrection of lower values of postoperative astigmatism, whereas the 3rd generation group shows a more noticeable undercorrection of astigmatism for higher amounts of TIA. However, the number of eyes with high TIA was quite small in this dataset. For all astigmatic eyes, the mean correction index (ratio of SIA to TIA; the value of 1.0 indicated ideal correction, >1.0 represents overall overcorrection, <1.0 represents overall undercorrection) was 1.09 ± 0.53 in the 1st generation group and 1.02 ± 0.38 in the 3rd generation group, p<0.01.
 |
Figure 7 The scattergram of target induced (TIA) versus surgically induced (SIA) astigmatism vector. (A) 1st generation treatment, (B) 3rd generation treatment. |
 |
Figure 8 The distribution of angle of error (cc/wise – counterclockwise, c/wise – clockwise). (A) 1st generation treatment, (B) 3rd generation treatment. |
Figures 8A and B present the distribution of the angle of error (the angle between the axis of the SIA and the axis of the TIA). In both cases, the mean value of the angle of error was small (group A: −0.6 ± 15.5°, group B: −0.7 ± 12.7°; p=0.87), indicating that the treatment was applied at the correct axis.
Patient-Reported Outcomes
Table 2 summarizes the outcomes of the patient experience questionnaire. The overall distribution of percentages of patients satisfied/dissatisfied with postoperative vision was significantly different in favor of the 3rd generation group. The differences in percentages were the most apparent at the “very satisfied” level, where 58.5% (1254/2145 patients) of patients in the 1st generation group and 71.8% (1540/2145 patients) of patients in the 3rd generation group reported being “very satisfied” with vision (p<0.01). The overall percentage of patients satisfied with vision (the sum of patients “very satisfied” or “satisfied”) was 92.8% (1991/2145 patients) in the 1st generation group and 97.3% (2087/2145 patients) in the 3rd generation group (p<0.01).
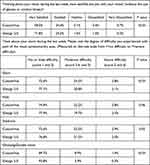 |
Table 2 Satisfaction and Visual Phenomena |
Table 2 also shows the outcomes of postoperative visual phenomena scores. Patients in the 3rd generation group generally had better scores for visual phenomena, and a higher percentage of patients reported not having any difficulty or only little difficulty with postoperative glare, halo, starburst, ghosting, or double vision. The differences in percentages reached statistical significance for glare, halo, and ghosting/double vision (in favor of the 3rd generation treatments) and were on the borderline of statistical significance for halo (Table 2).
Discussion
The outcomes of this study indicate that the new generation WFG treatment resulted in better visual acuity compared with the 1st generation. A higher percentage of eyes achieved 20/20 or better uncorrected visual acuity compared with the 1st generation treatment (95.9% vs 91.3%, p<0.01). Potentially, a slightly more hyperopic postoperative MSE in the 3rd generation group could have been somewhat responsible for the better uncorrected acuity, but the outcomes were also superior in terms of corrected distance visual acuity. A higher percentage of eyes in the 3rd generation group had postoperative uncorrected vision comparable to or better than preoperative corrected vision (84.8% vs 78.8%, p<0.01). The loss of corrected distance visual acuity one month postoperatively was also slightly lower in the 3rd generation group (Figure 3).
The distribution of postoperative manifest spherical equivalent, however, showed a very slight overcorrection of refractive error for the 3rd generation treatments, resulting in a slightly hyperopic mean MSE (+0.19 ± 0.33 D). Considering the study reports one-month postoperative outcomes, regression of MSE could be expected over time, potentially moving the mean manifest spherical equivalent closer to plano and increasing the percentage of eyes achieving postoperative emmetropia. The US Food and Drug Administration premarket approval studies of the 2nd generation device demonstrated that refractive stability is normally achieved around the six-months visit,10,11 and a slight regression of MSE is expected following myopic laser vision correction.10 The reported refractive predictability of wavefront-guided laser vision correction varies in the literature, mainly because of different inclusion criteria of the studies, range of treated manifest spherical equivalent, follow-up time, or authors’ nomogram implementation. For the predecessors of the 3rd generation device, the reported range of eyes within 0.50 D was 69–100%.5,12–18 Our outcome of 91.8% (1st generation) and 87.9% (3rd generation) within ±0.50 D emmetropia is expected for one-month follow-up in a large group of patients with a wide range of included refractive errors and will likely improve in the 3rd generation group with a slight regression over time.
The patient-reported outcomes were greatly in favor of the 3rd generation device in the current study. The difference was evident in the percentage of patients being “very satisfied” with postoperative vision and in the proportion of patients not experiencing postoperative visual phenomena, which was higher in the 3rd generation group. It is difficult to elucidate whether the improved quality of vision could be related to other parameters, such as a lower induction of higher-order aberrations or an improved contrast sensitivity with the new-generation WFG treatment. Unfortunately, this retrospective study was not designed to measure these variables. A study by Jung et al12 compared 1st generation WFG (WaveScan) treatments with the 2nd generation WFG treatments (iDesign Advanced WaveScan Studio System) and found higher mesopic contrast sensitivity and a significantly lower change in root mean square values for spherical aberration in the iDesign group.12 Other studies of the 2nd generation WFG (iDesign Advanced WaveScan Studio System) found non-significant change or only a mild increase of higher order aberrations postoperatively.15,18,19 Since the 2nd generation iDesign Advanced WaveScan Studio System and the 3rd generation iDesign Refractive Studio share the same accuracy of micro-refraction capture but the 3rd generation aberrometer has some improved features, it is possible that the correction of higher order aberrations is similar or superior, but this will have to be investigated in further comparative studies.
Vector analysis of the refractive cylinder showed a relatively accurate correction of astigmatism in both groups with a tendency to undercorrection for higher values of intended astigmatism correction, which has been commonly observed in numerous laser vision correction studies.13,18,20,21 The angle of error was relatively small in both groups (Figure 8), indicating the treatment was applied at the correct axis. From the comparison in this study, it is difficult to conclude whether one treatment type was superior to the other in terms of astigmatic correction. Although there was an indication that some postoperative parameters were better for the 3rd generation device (e.g., a higher percentage of eyes with postoperative astigmatism ≤0.50 D or a more favorable correction index), both groups had relatively low amounts of TIA and some vector parameters might not be reliable in eyes with low preoperative astigmatism.22,23 A further study comparing astigmatic correction in a larger group of patients with higher preoperative astigmatism would be necessary to address the astigmatic correction of the two treatment types fully.
This study has some limitations. It is retrospective in nature and, as such, subject to follow-up bias. The analysis does not address some important variables necessary to evaluate the performance of a wavefront-guided treatment, such as higher-order aberrations or contrast sensitivity. These were not routinely performed on postoperative visits. A longer-term assessment would also be useful, as slight changes in refractive error are still likely to occur beyond the one-month follow-up. Unfortunately, due to a shift in clinical care surrounding the covid-19 pandemic, the follow-up rates for patients receiving the 3rd generation were substantially decreased compared with the 1st generation treatments, and thus did not yield data acceptable for analysis. Hence, long-term evaluation is necessary to assess refractive stability and the stability of astigmatic correction.
Conclusion
Despite some limitations of the study, wavefront-guided LASIK using the 3rd generation resulted in comparable and, in some parameters, better outcomes than a WFG treatment with the 1st generation aberrometer.
Disclosure
Stephen Hannan, David Teenan, Jan Venter, Colin Berry, Noelle Hannan, Huba Kiss, and Dasi Raju are employees of Optical Express. Julie Schallhorn received personal fees from Carl Zeiss Meditec, Allergan, Long Bridge, and Forsight V6 and has a financial interest in Journey 1, Neurotrigger, and Novus Vision. The authors report no other conflicts of interest in this work.
References
1. Manche E, Roe J. Recent advances in wavefront-guided LASIK. Curr Opin Ophthalmol. 2018;29(4):286–291. doi:10.1097/ICU.0000000000000488
2. Kim A, Chuck RS. Wavefront-guided customized corneal ablation. Curr Opin Ophthalmol. 2008;19(4):314–320. doi:10.1097/ICU.0b013e328302ccae
3. Jabbur NS, Kraff C, Visx Wavefront Study G. Wavefront-guided laser in situ keratomileusis using the WaveScan system for correction of low to moderate myopia with astigmatism: 6-month results in 277 eyes. J Cataract Refract Surg. 2005;31(8):1493–1501. doi:10.1016/j.jcrs.2004.12.050
4. Partal AE, Manche EE. CustomVue laser in situ keratomileusis for myopia and myopic astigmatism using the Visx S4 excimer laser: efficacy, predictability, and safety. J Cataract Refract Surg. 2006;32(3):475–479. doi:10.1016/j.jcrs.2005.12.128
5. Schallhorn S, Brown M, Venter J, Teenan D, Hettinger K, Yamamoto H. Early clinical outcomes of wavefront-guided myopic LASIK treatments using a new-generation Hartmann-Shack aberrometer. J Refract Surg. 2014;30(1):14–21. doi:10.3928/1081597x-20131029-02
6. Smadja D, Santhiago MR, Tellouck J, et al. Safety and efficacy of wavefront-guided myopic laser in situ keratomileusis using a new wavefront sensor technology: first 100 cases. J Cataract Refract Surg. 2015;41(8):1588–1593. doi:10.1016/j.jcrs.2014.11.053
7. Schallhorn SC, Venter JA, Hannan SJ, Hettinger KA. Outcomes of wavefront-guided laser in situ keratomileusis using a new-generation Hartmann-Shack aberrometer in patients with high myopia. J Cataract Refract Surg. 2015;41(9):1810–1819. doi:10.1016/j.jcrs.2015.10.007
8. Reinstein DZ, Archer TJ, Randleman JB. JRS standard for reporting astigmatism outcomes of refractive surgery. J Refract Surg. 2014;30(10):654–659. doi:10.3928/1081597x-20140903-01
9. Waring GO, Reinstein DZ, Dupps WJ, et al. Standardized graphs and terms for refractive surgery results. J Refract Surg. 2011;27(1):7–9. doi:10.3928/1081597x-20101116-01
10. Summary of safety and effectiveness data: STAR S4 IRTM excimer laser system iDesign advanced wavescan studioTM system. Date of FDA Notice of Approval: 6th May, 2015. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf/P930016S044B.pdf.
11. Summary of safety and effectiveness data: iDESIGN® refractive studio and STAR S4 IR® excimer laser systems. Date of FDA Notice of Approval: 9th September, 2019. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf/P930016S057B.pdf.
12. Jung JW, Chung BH, Han SH, Kim EK, Seo KY, Kim TI. Comparison of measurements and clinical outcomes after wavefront-guided LASEK between iDesign and WaveScan. J Refract Surg. 2015;31(6):398–405. doi:10.3928/1081597X-20150521-06
13. Moshirfar M, Shah TJ, Skanchy DF, Linn SH, Kang P, Durrie DS. Comparison and analysis of FDA reported visual outcomes of the three latest platforms for LASIK: wavefront guided Visx iDesign, topography guided WaveLight Allegro Contoura, and topography guided Nidek EC-5000 CATz. Clin Ophthalmol. 2017;11:135–147. doi:10.2147/opth.s115270
14. Moussa S, Dexl A, Krall EM, et al. Comparison of short-term refractive surgery outcomes after wavefront-guided versus non-wavefront-guided LASIK. Eur J Ophthalmol. 2016;26(6):529–535. doi:10.5301/ejo.5000882
15. Prakash G, Srivastava D, Suhail M. Femtosecond laser-assisted wavefront-guided LASIK using a newer generation aberrometer: 1-year results. J Refract Surg. 2015;31(9):600–606. doi:10.3928/1081597X-20150820-05
16. Maloney RK, Kraff CR, Coleman SC. Wavefront-guided myopic laser in situ keratomileusis with a high-resolution Hartmann-Shack aberrometer and a new nomogram. J Cataract Refract Surg. 2021;47(7):847–854. doi:10.1097/j.jcrs.0000000000000539
17. Moussa S, Dexl AK, Krall EM, Arlt EM, Grabner G, Ruckhofer J. Visual, aberrometric, photic phenomena, and patient satisfaction after myopic wavefront-guided LASIK using a high-resolution aberrometer. Clin Ophthalmol. 2016;10:2489–2496. doi:10.2147/OPTH.S108002
18. Duran JA, Gutierrez E, Atienza R, Pinero DP. Vector analysis of astigmatic changes and optical quality outcomes after wavefront-guided laser in situ keratomileusis using a high-resolution aberrometer. J Cataract Refract Surg. 2017;43(12):1515–1522. doi:10.1016/j.jcrs.2017.08.020
19. Smadja D, De Castro T, Tellouck L, et al. Wavefront analysis after wavefront-guided myopic LASIK using a new generation aberrometer. J Refract Surg. 2014;30(9):610–615. doi:10.3928/1081597X-20140815-01
20. Schallhorn JM, Seifert S, Schallhorn SC. SMILE, topography-guided LASIK, and wavefront-guided LASIK: review of clinical outcomes in premarket approval FDA studies. J Refract Surg. 2019;35(11):690–698. doi:10.3928/1081597X-20190930-02
21. Schallhorn SC, Venter JA, Hannan SJ, Hettinger KA. Clinical outcomes of wavefront-guided laser in situ keratomileusis to treat moderate-to-high astigmatism. Clin Ophthalmol. 2015;9:1291–1298. doi:10.2147/OPTH.S87887
22. Eydelman MB, Drum B, Holladay J, et al. Standardized analyses of correction of astigmatism by laser systems that reshape the cornea. J Refract Surg. 2006;22(1):81–95. doi:10.3928/1081-597X-20060101-16
23. Bullimore MA, Spooner G, Sluyterman G, Dishler JG. Correction of low levels of astigmatism. J Cataract Refract Surg. 2015;41(8):1641–1649. doi:10.1016/j.jcrs.2014.12.060
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
