Back to Journals » Clinical Ophthalmology » Volume 15
Comparison of Surgical Outcomes Between Ab Interno Suture Trabeculotomy and Ab Externo Metal Trabeculotomy in Adult Patients with Glaucoma
Authors Otori Y, Matsuoka T, Kumoi M, Tachibana E, Tsujino C, Matsuda S
Received 1 June 2021
Accepted for publication 15 July 2021
Published 30 July 2021 Volume 2021:15 Pages 3213—3220
DOI https://doi.org/10.2147/OPTH.S322166
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
Yasumasa Otori, Takanori Matsuoka, Miho Kumoi, Eri Tachibana, Chieko Tsujino, Satoshi Matsuda
Department of Ophthalmology, National Hospital Organization, Osaka National Hospital, Osaka, Japan
Correspondence: Yasumasa Otori Email [email protected]
Purpose: To compare the outcomes of ab interno suture trabeculotomy (AbI-TLO) and ab externo metal trabeculotomy (AbE-TLO) in adult patients with glaucoma aged over 40 years.
Patients and Methods: A retrospective chart review was conducted, including adult patients with glaucoma who underwent AbI-TLO or AbE-TLO between January 2015 and June 2019. A single surgeon (YO) performed all the operations. Eighty-one patients (81 eyes) were included in this study. Surgical success was defined as a postoperative intraocular pressure (IOP) of ≤ 18 mmHg and an IOP reduction of ≥ 20% from the preoperative IOP, without requiring additional glaucoma surgery. Success rates were assessed using Kaplan–Meier survival curves and log-rank (Mantel–Cox) tests, while risk factors were analyzed using the Cox proportional hazards model.
Results: Forty-nine patients who underwent AbI-TLO and 32 patients who underwent AbE-TLO were studied; the preoperative IOPs were 27.9 ± 7.3 (mean ± standard deviation) mmHg and 25.6 ± 8.1 mmHg in the AbI-TLO and AbE-TLO groups, respectively (p=0.217). The 12-month postoperative IOPs were 15.8 ± 4.0 mmHg and 16.3 ± 4.2 mmHg in the AbI-TLO and AbE-TLO groups, respectively (p=0.724). The surgical success rates at 12 months were 77.6% and 62.5% in the AbI-TLO and AbE-TLO groups, respectively (p=0.144). Postoperative hyphema with level formation and ocular hypertension over 30 mmHg were observed in 22.4% and 26.5% of patients in the AbI-TLO group and 18.8% and 12.5% of those in the AbE-TLO group, respectively. Stepwise multivariate Cox regression analysis showed that a longer axial length was a risk factor for surgical failure (hazard ratio: 2.030; p=0.042).
Conclusion: AbI-TLO and AbE-TLO had similar surgical outcomes and postoperative complications. A longer axial length was associated with an insufficient IOP reduction.
Keywords: axial length, gonio-assisted transluminal trabeculotomy, intraocular pressure, MIGS, outcomes, trabeculotomy
Introduction
Glaucoma is an optic neuropathy characterized by retinal ganglion cell death, leading to both optic nerve head damage and visual field loss. Currently, intraocular pressure (IOP) is the most important determinant for glaucoma treatment, to arrest the progression of visual field loss.1
Ab externo trabeculotomies have been performed since the 1960s,2,3 with the indications for this procedure believed to be congenital, infantile, and juvenile glaucoma.4,5 In 1977, Luntz et al reported that regardless of the reason, trabeculotomy was not the operation of choice for primary adult-onset open-angle glaucoma (OAG).6 Nagata et al expanded the indications for ab externo metal trabeculotomy (AbE-TLO) in Japan to include various types of glaucoma, including child,7,8 exfoliation,9 primary open-angle,9 steroid-induced,10 and primary angle-closure glaucoma (ACG).11,12
In the last ten years, minimally invasive glaucoma surgery (MIGS) for the Schlemm’s canal (canal-based MIGS), including ab interno trabeculotomy such as Trabectome,13 gonio-assisted transluminal trabeculotomy,14 the Kahook dual blade,15 and Tanito’s microhook16 procedures were developed with the aim of attenuating physiological aqueous outflow resistance. Trabectome was the first canal-based MIGS that removes a segment of the trabecular meshwork and the inner wall of Schlemm’s canal using microelectrocautery. The Kahook dual blade, a slicer-like two blade structure, can also be used for resection in a manner similar to that in Trabectome. On the other hand, gonio-assisted transluminal trabeculotomy and Tanito’s microhook can cut the trabecular meshwork and the inner wall of Schlemm’s canal in a relatively wider range.
The AbE-TLO procedure requires a conjunctival incision and scleral flap. Japanese glaucoma surgeons prefer making a scleral flap in the inferotemporal region to preserve the upper region for future filtering surgery, if required. According to a survey conducted involving the Japan Glaucoma Society specialists, in 2009, AbE-TLO combined with phacoemulsification was the most common procedure performed for mild open-angle glaucoma with cataract in 2009 (72.7%). However, the number of canal-based MIGS combined with phacoemulsification surgeries significantly increased in 2019 (79.0%), compared with number of AbE-TLO surgeries performed (4.6%).17
In this study, we therefore compared AbE-TLO and ab interno suture trabeculotomy (AbI-TLO) performed for the treatment of adult-onset glaucoma in patients aged over 40 years.
Patients and Methods
This retrospective, single-center, cohort study was approved by the Institutional Review Board of the National Hospital Organization Osaka National Hospital, Osaka, Japan (IRB No. 20121), and followed the tenets of the Declaration of Helsinki. Informed consent was obtained from all patients for inclusion in this study.
Subjects
Consecutive patients who underwent either AbE-TLO (between January 2015 and February 2017), or AbI-TLO (between February 2017 and June 2019) at the National Hospital Organization Osaka National Hospital were included. All included patients were followed up for at least 12 months. In the present study, the indication for TLO was medically uncontrolled glaucoma, including primary OAG, exfoliation glaucoma, and primary ACG. Patients aged <40 years were excluded.
Surgical Techniques
One surgeon (YO) performed all the surgeries. The surgical procedure for AbE-TLO was as follows: a 4×4 mm external scleral flap of approximately two-thirds of the scleral thickness was made on the inferotemporal sclera; a 3.5×3.5 mm internal scleral flap was then made to expose Schlemm’s canal. Two metal trabeculotomy probes (Nagata; Inami & Co., Ltd., Tokyo, Japan) were inserted into Schlemm’s canal and rotated by approximately 120 degrees, cutting the inner wall of the trabecular meshwork. Deep sclerectomy and sinusotomy were not performed simultaneously. The external scleral flap and conjunctiva were sutured using 10-0 nylon (MANI Inc., Tochigi, Japan) and 8-0 Vicryl (Ethicon Inc.) sutures, respectively.
AbI-TLO was performed according to Grover’s report;14 a temporal corneal and upper or lower side ports were created, and a high-molecular-weight viscoelastic material was injected into the anterior chamber through the temporal side port. The patient’s head and surgical microscope were oriented to allow suitable visualization of the nasal trabecular meshwork using a Hill open access surgical gonio lens (Ocular Instruments, Bellevue, Washington, United States). The inner wall of the trabecular meshwork was incised using a 27G needle, and a viscoelastic material was inserted into Schlemm’s canal to prevent blood reflux. Rounded tip 5-0 nylon sutures (Chin trabeculotomy sutures; Handaya Co., Ltd. Tokyo, Japan) were inserted into Schlemm’s canal from the upper or lower port using 25G forceps (Maxgrip; Alcon Grieshaber, Schaffhausen, Switzerland). After visualizing the nylon suture in Schlemm’s canal using a Mori upright surgical gonio lens (Ocular Instruments), the inner wall of Schlemm’s canal was gently incised using 25G Maxgrip forceps. The viscoelastic material was washed out with 10 mL of OPEGURD-MA intraocular irrigating solution (Senju Pharmaceutical Co., Ltd., Osaka, Japan) or BSS PLUS solution (Alcon Laboratories, Inc., Geneva, Switzerland) through the temporal side port.
If cataract surgery was simultaneously required, continuous circular capsulorhexis was performed first, followed by AbE-TLO or AbI-TLO, and cataract surgery. The time taken for the surgery was defined as the duration from the first cut to the removal of the surgical drape. All the patients were administered retrobulbar or peribulbar anesthesia. Subsequently, the patients received both 1.5% topical levofloxacin (NIPRO, Osaka, Japan) and 0.1% preservative-free betamethasone sodium phosphate eye drops (Nitten, Nagoya, Japan); postoperative eye drops were discontinued 2–4 weeks postoperatively. All the glaucoma drugs were discontinued after surgery and were resumed if the IOP increased. We followed up each patient from one week until one month after surgery, and subsequently at three months postoperatively.
Other Data Collection
Best-corrected decimal visual acuity (VA) was measured using a Landolt ring chart and was converted to the logarithm of minimal angle resolution (logMAR) for statistical analysis. Data for the IOP was collected at one and seven days; one, two, three, six, nine, and twelve months after the surgery; and at the last visit. The IOP reduction rate was calculated as follows: (preoperative IOP – postoperative IOP at 12 months/preoperative IOP) x 100. The axial length was measured using the IOLMaster 500 (Carl Zeiss Meditec, Germany). The central corneal thickness (CCT) was measured using a specular microscope, TOMEY EM-3000 (TOMEY corporation, Nagoya, Japan).
Definitions of Success
Surgical success was defined as a postoperative IOP of ≤18 mmHg and ≥20% reduction from the preoperative IOP, without the requirement for additional glaucoma surgery; the IOP was measured using a Goldmann applanation tonometer (Haag-Streit, Koeniz-Berne, Switzerland).
The glaucoma drug scores were as follows: (1) the use of glaucoma eye drops contributed one point, while (2) a fixed combination and the use of an oral carbonic anhydrase inhibitor contributed two points.18
Statistical Analysis
Success rates were assessed using Kaplan-Meier survival curves and Log-rank (Mantel–Cox) tests; if the IOP went beyond the success range twice in a row, the first event was defined a surgical failure. Other quantifiable parameters were compared using the Mann–Whitney U-test. The chi-square test was used for noncontinuous variables with n≥5; Fisher’s exact test was used for noncontinuous variables with n<5. Kaplan-Meier survival curves and Log rank tests were performed using Cox proportional hazard analyses. Factors with P-values <0.1 in the univariate Cox regression analysis underwent stepwise multivariate Cox regression analysis. All statistical analyses were performed using SYSTAT (version 13.1; HULINKS, Tokyo, Japan). The level of statistical significance was set at p<0.05.
Results
The demographic characteristics of the patients are presented in Table 1. Forty-nine and 32 patients who underwent AbI-TLO and AbE-TLO, respectively, were studied. The preoperative IOP and glaucoma drug scores, sex, CCT, and axial length were not significantly different between the AbI-TLO group and the AbE-TLO group; however, the age of the patients in the AbI-TLO group was significantly higher than that of those in the AbE-TLO group. Additionally, the rates of ACG combined with cataract extraction procedure were significantly higher in the AbE-TLO group than in the AbI-TLO group. The prevalence of pseudophakic eyes was significantly higher in the AbI-TLO group than in the AbE-TLO group. The preoperative logMAR VA of the AbE-TLO group was significantly better than that of the AbI-TLO group. The total follow-up periods in the AbE-TLO group were significantly longer than those of the AbI-TLO group. It was determined that 93.8% (76/81) of the patients had pseudophakic eyes. No patients had previously undergone selective laser trabeculoplasty (SLT) in the present study.
 |
Table 1 Patient Demographic Data |
Postoperative outcomes and adverse effects are presented in Table 2. The duration of surgery of the AbI-TLO group (34.9 ± 7.8, n=31) was significantly shorter than that of the AbE-TLO group (50.4 ± 8.0, n=28; p<0.0001). The cut ratio for Schlemm’s canal was 212.2 ± 65.6 (120–360) degrees in the AbI-TLO group and 116.3 ± 14.8 (60–120) degrees in the AbE group; the preoperative IOP was 27.9 ± 7.3 mmHg in the AbI-TLO group and 25.6 ± 8.1 mmHg in the AbE-TLO group (p=0.217). The postoperative IOP at 12 months of follow-up was 15.8 ± 4.0 mmHg in the AbI-TLO group and 16.3 ± 4.2 mmHg in the AbE-TLO group (p=0.724). The IOP reduction rate at 12 months was 39.7 ± 20.3% in the AbI-TLO group and 33.6 ± 17.8% in the AbE-TLO group (p= 0.067). Further, the postoperative glaucoma drug score at the final visit was 3.4 ± 1.5 in the AbI-TLO group and 2.9 ± 1.4 in the AbE-TLO group (p=0.223). The postoperative glaucoma drug score was significantly decreased in both the AbI-TLO group (p=0.007, paired t-test) and the AbE-TLO group (p=0.004, paired t-test) compared with the respective preoperative glaucoma drug scores. The postoperative logMAR VA in the AbI-TLO group was significantly better than the preoperative logMAR VA (p=0.007, paired t-test); however, no significant changes were observed in the postoperative logMAR VA of AbE-TLO as compared to preoperative logMAR VA (p=0.175, paired t-test).
 |
Table 2 Postoperative Outcomes and Adverse Effects |
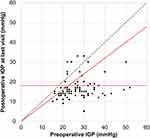
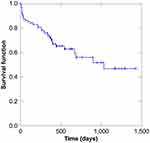
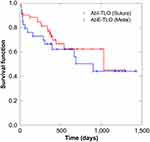
The patients’ postoperative IOPs reduced significantly after TLO (Figures 1 and 2). The surgical success rate according to Kaplan-Meier analysis was 47.1% at 34 months after TLO (Figure 3), whereas the surgical success rates at 12 months postoperatively were 77.6% and 62.5% in the AbI-TLO and AbE-TLO groups, respectively (p=0.144). According to the Kaplan-Meier survival curves, the success rates of AbI-TLO and AbE-TLO were not significant (Figure 4, Log-rank test; p=0.875); moreover, the success rates of ACG (n=38) and OAG (n=43) were not significant (Log-rank test; p=0.299). Postoperative hyphema with level formation and transient ocular hypertension over 30 mmHg were observed in 22.4% and 26.5% of patients in the AbI-TLO group, and 18.8% and 12.5% of those in the AbE-TLO group, respectively.
Stepwise multivariate Cox regression analysis showed that a longer axial length was a risk factor for surgical failure (hazard ratio: 2.030; p=0.042, Table 3); eyes with an axial length <24.0 mm thus exhibited significantly better outcomes than those with an axial length ≥24.0 mm (Figure 5, Log-rank test; p=0.031).
 |
Table 3 Results of Univariate and Multivariate Analyses |
Discussion
Our study revealed that the success rates and postoperative complications between AbI-TLO and AbE-TLO were not significantly different. We also found that a longer axial length was associated with insufficient IOP reduction for TLO.
Kinoshita-Nakano et al reported that at 12 months postoperatively, the IOP reduction rate was higher for AbE-TLO combined with deep sclerectomy/sinusotomy (30.9%. Preoperative IOP 24.2 ± 6.6 mmHg, preoperative glaucoma drug score 3.2 ± 0.8) than for AbI-TLO using a Trabectome (23.0%. Preoperative IOP 22.6 ± 7.4 mmHg, preoperative glaucoma drug score 3.5 ± 1.0).19 They also showed that neither combined cataract surgery nor the type of surgery were significantly related to surgical success based on the definition of success used in this study, suggesting that successful surgical outcomes following Trabectome use and AbE-TLO combined with deep sclerectomy/sinusotomy were not significantly different after 36 months of follow-up. Mori et al reported that the success rates of AbE-TLO combined with deep sclerectomy and AbI-TLO using a microhook were not significantly different at the 12-month follow-up.18 In their study, the definition of surgical success was a postoperative IOP ≤21 mmHg and ≥20% reduction in IOP. They reported that the IOP reduction rate for AbI-TLO using a microhook (28.4 ± 7.8 mmHg, 4.9 ± 1.1) and AbE-TLO with deep sclerectomy (preoperative IOP 32.5 ± 11.2 mmHg, preoperative glaucoma drug score 4.3 ±1.4) was 34.4% and 40.5% at 12 months after surgery, respectively. In this study, the IOP reduction rate for AbI-TLO (27.9 ± 7.3 mmHg, 4.2 ± 1.5) and AbE-TLO (preoperative IOP 25.6 ± 8.1 mmHg, preoperative glaucoma drug score 3.7 ± 1.2) was 39.7% and 33.6% at 12 months after surgery, respectively. The IOP reduction rate at 12 months of AbI-TLO using 5–0 nylon suture seems to be better than that of AbI-TLO using a Trabectome or a microhook. In summary, the efficacy of AbI-TLO and AbE-TLO were equivalent in terms of IOP reduction.
Ahuja et al reported that when the definition of surgical success was a postoperative IOP ≤21 mmHg, or ≥20% reduction of IOP, cases of AbI-TLO by Trabectome use combined with cataract surgery had a higher rate of success after surgery than cases of AbI-TLO by Trabectome use only.20 However, if the definition of surgical success was a postoperative IOP ≤18 mmHg and ≥20% reduction (the same criteria as in this study), the success rates decreased, along with the differences in the success rates between AbI-TLO combined with cataract surgery and AbI-TLO alone. In this study, the rate of combined cataract surgery was 72% and thus unfavorably influenced surgical success; conversely, the efficacy of ACG and OAG for TLO was equivalent. We compared the success rate of TLO combined with cataract surgery (n=59) and TLO alone for pseudophakia (n=17) and phakia (n=5). The TLO-only group included several patients with pseudophakic eyes. The outcomes of TLO combined with cataract surgery were similar to those of TLO alone. These findings suggest that AbI- or AbE-TLO might have similar IOP-lowering effects for phakic and pseudophakic eyes.
In the present study, the cut ratio of the inner wall of Schlemm’s canal in the AbI-TLO group was wider than that in the AbE-TLO group; nevertheless, the success rates were not significantly different between the two groups. Manabe et al reported that during AbE-TLO (suture), incisions in Schlemm’s canal of ≥150 degrees did not affect the reduction in IOP at 12 months postoperatively.21 Sato et al also reported that incisions in different locations and ranges of Schlemm’s canal during AbI-TLO did not affect surgical outcomes at the 12-month follow-up.22 These findings suggest that incisions in Schlemm’s canal of >120 degrees may be sufficient to reduce IOP during short-term follow-up periods.
We also demonstrated that a longer axial length was related to insufficient IOP reduction for TLO. Kuusniemi et al reported that an axial length >23.82 mm might reduce Trabectome procedure success;22 however, the mechanism of IOP elevation after TLO in patients with a longer axial length remains unclear. Canal-based MIGS is expected to increase the outflow pathway from the trabecular meshwork to the collector channel. If the main outflow resistance were distal to the collector channel, canal-based MIGS may be ineffective; thus, a longer axial length may cause elongation of the aqueous vein, thereby increasing aqueous venous pressure. Kuusniemi et al reported that a history of SLT before canal-based MIGS was a risk factor for surgical failure,23,24 and while it is easy to recognize that canal-based MIGS might not be effective in patients resistant to SLT, no studies have reported a longer axial length to be a risk factor for SLT failure; therefore, further investigation is required.
AbI-TLO requires a clear corneal incision, which is beneficial for future filtering surgery; however, AbE-TLO requires a conjunctival incision and scleral flap. Thus, the duration of AbI-TLO is significantly shorter than that of AbE-TLO. AbI-TLO was performed to directly observe the gonio using a surgical gonio lens. First, we identified the scleral spur, subsequently making an incision on the borderline of the pigmented and non-pigmented trabecular meshwork; if a white band—representative of the underlying sclera—could be seen, it was easy to set a 5–0 nylon suture into Schlemm’s canal. Nylon sutures may not hurt the outer wall of Schlemm’s canal; therefore, the risk of intraoperative complications during the AbE-TLO procedure—such as early perforation of the trabeculotome probe, cyclodialysis, iridodialysis, and Descemet’s membrane rupture—may be lower in the AbI-TLO procedure. Thus, the AbI-TLO procedure reduces the duration of the surgery and is minimally invasive when compared with AbE-TLO.
The limitations of this study include its retrospective nature, the possibility of selection bias, relatively short-term follow-up, and limited sample size. Canal-based MIGS is believed to be safer than glaucoma filtering surgery; however, the postoperative IOP in canal-based MIGS is higher than that of glaucoma filtering surgery. Ophthalmologists should, therefore, reconsider the indications for canal-based MIGS, even in patients with early stage glaucoma; a larger study may identify more preoperative factors influencing the outcomes of canal-based MIGS.
Conclusions
In conclusion, AbI-TLO reduces the duration of surgery and is minimally invasive when compared with AbE-TLO, and the success rates and postoperative complications between AbI-TLO and AbE-TLO were equivalent throughout the short-term follow-up periods. Additionally, a longer axial length was associated with insufficient IOP reduction in TLO.
Abbreviations
AbE-TLO, ab externo metal trabeculotomy; AbI-TLO, ab interno suture trabeculotomy; ACG, angle-closure glaucoma; IOP, intraocular pressure; OAG, open-angle glaucoma; SLT, Selective laser trabeculoplasty; canal-based MIGS, minimally invasive glaucoma surgery for Schlemm’s canal.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding
The authors received no funding for the preparation of this manuscript.
Disclosure
The authors report no conflicts of interest in the conduct of this work.
References
1. Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363(9422):1711–1720. doi:10.1016/S0140-6736(04)16257-0
2. Burian HM. A case of Marfan’s syndrome with bilateral glaucoma. With description of a new type of operation for developmental glaucoma (trabeculotomy ab externo). Am J Ophthalmol. 1960;50:1187–1192. doi:10.1016/0002-9394(60)91007-2
3. Smith R. A New technique for opening the canal of schlemm: preliminary report. Br J Ophthalmol. 1960;44(6):370–373. doi:10.1136/bjo.44.6.370
4. Harms H, Dannheim R. Trabeculotomy—results and problems. Bibl Ophthalmol. 1970;81:121–131.
5. McPherson SD
6. Luntz MH, Livingston DG. Trabeculotomy ab externo and trabeculectomy in congenital and adult-onset glaucoma. Am J Ophthalmol. 1977;83(2):174–179. doi:10.1016/0002-9394(77)90612-2
7. Akimoto M, Tanihara H, Negi A, Nagata N. Surgical results of trabeculotomy ab externo for developmental glaucoma. Arch Ophthalmol. 1994;112(12):1540–1544. doi:10.1001/archopht.1994.01090240046024
8. Ikeda H, Ishigooka H, Muto T, Tanihara H, Nagata M. Long-term outcome of trabeculotomy for the treatment of developmental glaucoma. Arch Ophthalmol. 2004;122(8):1122–1128. doi:10.1001/archopht.122.8.1122
9. Tanihara H, Negi A, Akimoto M, et al. Surgical effects of trabeculotomy ab externo on adult eyes with primary open angle glaucoma and pseudoexfoliation syndrome. Arch Ophthalmol. 1993;111(12):1653–1661. doi:10.1001/archopht.1993.01090120075025
10. Honjo M, Tanihara H, Inatani M, Honda Y. External trabeculotomy for the treatment of steroid-induced glaucoma. J Glaucoma. 2000;9(6):483–485. doi:10.1097/00061198-200012000-00011
11. Tanihara H, Negi A, Akimoto M, Nagata M. Long-term results of non-filtering surgery for the treatment of primary angle-closure glaucoma. Graefes Arch Clin Exp Ophthalmol. 1995;233(9):563–567. doi:10.1007/BF00404707
12. Kiuchi Y, Tsujino C, Nakamura T, Otori Y, Mochizuki H. Phacoemulsification and trabeculotomy combined with goniosynechialysis for uncontrollable chronic angle-closure glaucoma. Ophthalmic Surg Lasers Imaging. 2010;41(3):348–354. doi:10.3928/15428877-20100430-09
13. Minckler DS, Baerveldt G, Alfaro MR, Francis BA. Clinical results with the trabectome for treatment of open-angle glaucoma. Ophthalmology. 2005;112(6):962–967. doi:10.1016/j.ophtha.2004.12.043
14. Grover DS, Godfrey DG, Smith O, Feuer WJ, Montes de Oca I, Fellman RL. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: technique report and preliminary results. Ophthalmology. 2014;121(4):855–861. doi:10.1016/j.ophtha.2013.11.001
15. Seibold LK, Soohoo JR, Ammar DA, Kahook MY. Preclinical investigation of ab interno trabeculectomy using a novel dual-blade device. Am J Ophtahlmol. 2013;155(3):524–529. doi:10.1016/j.ajo.2012.09.023
16. Tanito M. Microhook ab interno trabeculotomy, a novel minimally invasive glaucoma surgery. Clin Ophthalmol. 2017;12:43–48. doi:10.2147/OPTH.S152406
17. Iwasaki K, Arimura S, Takamura Y, Inatani M. Clinical practice preferences for glaucoma surgery in Japan: a survey of Japan Glaucoma Society specialists. Jpn J Ophthalmol. 2020;64(4):385–391. doi:10.1007/s10384-020-00749-w
18. Mori S, Murai Y, Ueda K, et al. A comparison of the 1-year surgical outcomes of ab externo trabeculotomy and microhook ab interno trabeculotomy using propensity score analysis. BMJ Open Ophthalmol. 2020;5(1):e000446. doi:10.1136/bmjophth-2020-000446
19. Kinoshita-Nakano E, Nakanishi H, Ohashi-Ikeda H, Morooka S, Akagi T. Comparative outcomes of trabeculotomy ab externo versus trabecular ablation ab interno for open angle glaucoma. Jpn J Ophthalmol. 2018;62(2):201–208. doi:10.1007/s10384-017-0559-0
20. Ahuja Y, Ma Khin Pyi S, Malihi M, Hodge DO, Sit AJ. Clinical results of ab interno trabeculotomy using the trabectome for open-angle glaucoma: the Mayo Clinic series in Rochester, Minnesota. Am J Ophthalmol. 2013;156(5):927–935.
21. Manabe SI, Sawaguchi S, Hayashi K. The effect of the extent of the incision in the schlemm canal on the surgical outcomes of suture trabeculotomy for open-angle glaucoma. Jpn J Ophthalmol. 2017;61(1):99–104. doi:10.1007/s10384-016-0487-4
22. Sato T, Kawaji T. 12-month randomised trial of 360° and 180° Schlemm’s canal incisions in suture trabeculotomy ab interno for open-angle glaucoma. Br J Ophthalmol. 2021;105(8):1094–1098.
23. Kuusniemi AM, Lindbohm N, Allinen P, Koskinen M, Harju M. Ab interno trabeculotomy: key prognostic factors. J Glaucoma. 2020;29(3):211–216. doi:10.1097/IJG.0000000000001432
24. Kasahara M, Shoji N. Effectiveness and limitations of minimally invasive glaucoma surgery targeting schlemm’s canal. Jpn J Ophthalmol. 2021;65(1):6–22. doi:10.1007/s10384-020-00781-w
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.