Back to Journals » Patient Preference and Adherence » Volume 17
Comparison of Rare and Common Diseases in the Setting of Healthcare Priorities: Evidence of Social Preferences Based on a Systematic Review
Authors Gu Y, Wang A, Tang H, Wang H, Jiang Y, Jin C, Wang H
Received 7 April 2023
Accepted for publication 8 July 2023
Published 24 July 2023 Volume 2023:17 Pages 1783—1797
DOI https://doi.org/10.2147/PPA.S416226
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Yichun Gu,1,* Anqi Wang,2,* Huan Tang,3 Haode Wang,4 Yuji Jiang,5 Chunlin Jin,1 Haiyin Wang1
1Shanghai Health Development Research Center, Shanghai, People’s Republic of China; 2School of Public Health, Weifang Medical University, Weifang, Shandong, People’s Republic of China; 3School of Public Health, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China; 4School of Health and Related Research, University of Sheffield, Sheffield, UK; 5Business School, Imperial College London, London, UK
*These authors contributed equally to this work
Correspondence: Chunlin Jin; Haiyin Wang, Shanghai Health Development Research Center, Shanghai, People’s Republic of China, Tel +8613162429202 ; +8618917769216, Email [email protected]; [email protected]
Background: In light of the limited availability of healthcare resources, providing universal access to healthcare is a challenging task. As a result, prioritizing healthcare services has emerged as a crucial issue. This study aims to explore the preferences of the public regarding healthcare prioritization for rare and common diseases. By examining public attitudes, this study seeks to inform government decisions concerning resource allocation and distribution within healthcare.
Methods: “Social preference” and “rare disease” were searched as MeSH terms in the electronic databases of Ovid Medline, Web of Science, Embase, and Econlit for articles published since their establishment, and the information on the characteristics of the articles and the results of social preferences for rare diseases were analyzed and summarized.
Results: The public held predominantly neutral views on the setting of healthcare priorities for rare and common diseases. The results of the included studies showed that with all else being equal, no social preference for rarity was found, but when the public considered the proportional advantage of rare diseases or when the respondents were young, a social preference for rarity existed. In addition, the public weighed attributes such as the health benefits of treatments, the effectiveness of treatment options, the safety of treatment, equity, unmet needs, and disease severity in the process of setting of treatment priorities for rare diseases. Furthermore, in consideration of equity, the public showed a willingness to pay for rare diseases in spite of the high medical costs.
Conclusion: International studies on social preferences provide some evidence for the setting of healthcare priorities for rare diseases, and health policymakers should consider social preferences in an integrated manner in order to set healthcare priorities appropriately.
Keywords: rare diseases, common diseases, healthcare, priority, social preference, social willingness to pay
Introduction
The growing concern over the scarcity of healthcare resources has intensified the global conversation around the judicious and ideal distribution of these resources Rare diseases, which have unique characteristics, pose challenges such as costly and complex drug development, limited patient populations, inadequate access to healthcare, and difficulties in ensuring the efficacy of treatments. Scholars are increasingly debating how to ensure equitable healthcare rights for individuals with rare diseases and how to allocate health resources efficiently and fairly between those with rare and common illnesses.1,2 The potential social preference for rare disease health resource allocation has generated considerable debate among scholars, healthcare providers and policymakers, arguing around the rationality and plausibility behind the egalitarian viewpoint.3,4 Some perspectives argue for the equitable distribution of resources across rare and common diseases, focusing on the severity of the condition as the driving factor.5 This approach holds that treatments for severe rare diseases should not be disregarded when determining funding priorities. Conversely, other views contend that healthcare resource allocation should better comply to the formal equality stand and prioritize prevalence, given the greater public health impact of common diseases. These viewpoints tend to emphasize the importance of maximizing population-level health outcomes and ensuring justifiable allocation of limited resources.6,7 Hence, in this study we aim to focus on how the public appropriately allocates limited healthcare resources between rare and common diseases—in other words, the goal is to understand how the public sets healthcare priorities for rare and common diseases. Recently, there has been an increasing number of empirical studies exploring social preferences for rare diseases, but the findings significantly differ, and a consistent and standardized preference system has yet to emerge. Moreover, majority of pertinent systematic reviews addressed social preferences within healthcare as a whole.8–13 Since there are very few studies reviewing social preferences in the context of rare diseases compared with common diseases, this study undertakes a comprehensive review of the associated studies, synthesizing their similarities and differences. The goal is to elucidate societal inclinations toward rare and common diseases within the context of establishing healthcare priorities. Ultimately, the research aims to provide evidence-based guidance for determining healthcare priorities and adjusting disease thresholds.
Data and Methods
Data Sources
This study followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2009), and it involved searching, evaluating, and combing through articles written up to August 31, 2022 that were relevant to three aspects: rare diseases, social preferences, and value frameworks. Four databases—Ovid Medline, Embase, Web of Science, and Econlit (via EBSCOhost)—were searched by using medical subject headings (MeSH) and keywords, including social behavior, social cognition, social integration, social interaction, social identification, group identification, health equity, social justice, health services accessibility, gender equity, rare disease, delayed diagnosis, neglected disease, orphan drug production, and undiagnosed disease.
Inclusion and Exclusion Criteria
The following criteria for the inclusion and exclusion of studies were clarified through focus group discussions.
Inclusion criteria: (1) Studies were high-quality peer-reviewed English-language articles; (2) the sources of the studies were primary sources that had been empirically surveyed; (3) the main contents of the studies were related to rare diseases, healthcare priorities, and social preferences; (4) the subjects of the studies were the general public; (5) the methods used in the studies were quantitative trade-off analysis methods (eg, selection-based methods, personnel trade-off methods, ranking or rating tests, etc.); (6) the studies explicitly stated the inspirations for preferences and the results of their analyses.
Exclusion criteria: (1) Non-English-language studies that were not peer-reviewed (eg, identified as case reports, reviews, editorials, and other types of non-peer-reviewed articles); (2) Studies derived from non-empirical surveys or non-primary sources (eg, theoretical studies, literature reviews); (3) Studies with content beyond the rare-disease-related healthcare priorities (eg, global healthcare priorities, priorities within healthcare organizations); (4) Studies targeting subgroups of the general public (eg, patients, medical professionals, health policy decision makers); (5) studies that used qualitative research methods (eg, focus groups, structured interviews); (6) studies that did not contain explicit statements of preferences or analyses of the findings.
Screening and Information Extraction
Duplicates were excluded by using the Endnote software, and the other articles were assigned to two reviewers for independent review. Based on this, the two reviewers read the full text to determine whether an article was “included”, “excluded”, or “uncertain” according to the inclusion and exclusion criteria. For the “uncertain” or “inconsistent” articles, a focus group discussion was held to identify the articles to be finally included.
To extract information from the included studies, the two reviewers were first trained on the subject, and two randomly selected articles were pre-analyzed to ensure high quality and consistency. Second, the two reviewers extracted and categorized information according to a pre-made information extraction form (including background information, study information, information on the results, etc.) and resolved inconsistencies in information extraction through a focus group discussion.
Data Analysis and Collation
The included articles were systematically analyzed to extract information on the outcomes related to the public such as attribute preferences and preference options in the context of the comparison of rare diseases with common diseases, and the final attributes and preferences included in this study were organized and analyzed according to a focus group discussion.
Results
Search Results
In this study, 474 relevant articles were retrieved, among which 131 were retrieved from Web of Science, 115 from Ovid Medline, and 192 from Embase; no relevant articles were retrieved from Econlit. To further ensure the comprehensiveness of the retrieved articles, 36 relevant supplementary articles were manually searched, and after eliminating 166 duplicate articles, 308 articles were finally obtained. The literature screening process and its results are shown in Figure 1.
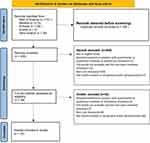 |
Figure 1 Flow diagram of the literature screening. |
Description of the Literature’s Characteristics
For the 14 articles that were included, the publication dates were concentrated after 2010; two articles were published before 2012, four were published between 2012 and 2016, and most (eight) were published in the last five years (2017–2022). In terms of the regional distribution, the data mostly originated from developed Western countries such as Norway, Canada, and the UK. In terms of respondents, the age was basically above 18 years old, and the sample size in most studies (nine articles) was over 1000 people. There were three main methods involved: Person trade-off (PTO), the Discrete choice experiment (DCE), and the Analytic hierarchy process (AHP); the details of these are shown in Table 1.
 |
Table 1 Summary of Study Characteristics and Results of Included Literature |
Attributes and Preferences
Preference Setting for Rare Compared to Common Diseases Based on the Results of PTO
First, the attribute that distinguishes rare diseases from common diseases is their rarity, and studies such as those of Desser, Linley, and Chim14–16 noted that with all else being equal, the majority of the public remained neutral about treatment priorities for rare and common diseases, with no social preference for rarity. The results of two studies by Wiss and Bae17,18 indicated that a public preference for rarity existed when specific factors contributed. These specific factors included: (1) The public’s consideration of a proportional advantage for rare diseases, which refers to a preference for maximizing relative numbers at the expense of absolute numbers,17 such that individuals typically prefer to help a larger proportion (eg, 100 out of 100) rather than a smaller proportion (eg, 100 out of 10,000). The role of the proportional advantage could be important when prioritizing patients with rare diseases, as the relative proportion of patients with rare diseases that are possible to treat is far higher than that of patients with common diseases. (2) Young people showed a stronger preference for rarity.18
Second, after incorporating the cost of treatment in scenarios with a choice between rare and common diseases, the results of Desser’s19 study indicated that when the cost of rare diseases was four, eight, and twenty-five times higher than that of common diseases, the percentages given by the public for the average allocation of funds were 48.4%, 45.1%, and 38.9%, respectively, suggesting that when the opportunity cost of treating rare diseases increased, the share of funds allocated to rare diseases was affected, but to a lesser extent. Despite the high opportunity costs of rare diseases, most respondents allocated resources relatively equally between patients with rare and common diseases, and no price-responsive social preferences were found in either priority setting. The results of the study by Bae,18 however, showed that when resources were allocated between rare and common diseases, the public would provide more resources for lower-cost diseases, and this was also confirmed in Richardson’s20 study, where it was shown that the public supported the provision of services for rare diseases with lower per capita or total costs, reflecting a general public preference for low treatment costs.
Further, Desser et al19 found that members of the public who were not neutral in their choice of priority between rare and common diseases were more inclined to give priority to common diseases. Dragojlovic and Bae18,21 noted that a larger proportion of respondents preferred to fund patients with common diseases. Ramalle-Gómara,22 who used a five-point Likert scale, also noted that 72.6% of people believed that most of the budget should be spent on treating common diseases. Studies such as those of Linley and Chim15,16 incorporated the attribute of gains in health, and they showed that there was a general public preference for more health resources for common diseases with high health gains and a general preference for good health gains.
In addition, Richardson20 showed that when resources are limited, resource allocation between rare and common diseases should be reallocated from low- to high-severity conditions, indicating a priority for the treatment of severe diseases. In addition, the results of this study showed public support for effective but low-cost services for rare diseases, thus further showing a general public preference for good health benefits and low costs while focusing on effective and low-cost treatments. This is consistent with the findings of Bae’s study.18
Ranking of Attribute Preferences in the Context of Rare versus Common Diseases Based on the Results of the AHP
The two studies by Rizzardo and Yamoah23,24 used the AHP to conduct a trade-off analysis of the public’s decision-making regarding drug coverage for rare versus common diseases; they examined the relative importance of value attributes other than price in the decision and ranked the importance according to a weight. Rizzardo23 studied Canadians, and the results showed that disease rarity had a low weight (0.021) and ranked second to last out of the 13 attributes involved. In addition to rarity, the other attributes and weights were as follows: safety of the drug (0.147), the ability of the drug to act (0.140), the potential impact of treatment with the drug on quality of life (0.137), the severity of the disease (0.127), the potential for the treatment to prolong life (0.100), the lifestyle choices of the patient (0.066), drug treatment (0.063), the availability of support from the patient’s family (0.058), the patient’s age (0.046), the patient’s socioeconomic status (0.038), unmet needs (eg, no alternative medications available) (0.037), and patient compliance (0.021). Yamoah24 conducted a study on New Zealanders to explore the public’s preference for attributes of drug funding for rare diseases, and they also found that rarity was ranked second lowest in weight, with the other attributes being ranked in descending order of weight as follows: potential impact of treatment with the drug on quality of life, the ability of the drug to act, the safety of the drug, the severity of the disease, the potential of the treatment to prolong life, the equity of treatment with the drug, the age of the patient, support available from the patient’s family, the lifestyle choices of the patient, unmet needs, the socioeconomic status of the patient, and patient compliance.
Attribute Preferences in the Context of Rare versus Common Diseases Based on the Results of the DCE
Mentzakis, Bourke, and Toumi25–27 analyzed the public’s social preferences for the funding of medicines for rare diseases by using the DCE method. Mentzakis25 fund that with all else being equal, the public was not in favor of more funding for medicines for rare diseases or for increasing the lifetimes of patients with rare diseases. There was no significant difference in the weighting of attributes for rare and common diseases. In addition, in decisions about drug coverage for rare and common diseases, there was a general public preference for more serious diseases with good treatment benefits. Bourke26 used the British population as a study population, and the results showed that the public did not support more funding for rare diseases (a weight of –0.52 for rare disease treatments). In addition, the magnitudes of public preference weightings for attributes other than disease prevalence were as follows: benefits of treatment (0.86), improved quality of life (a return to normal life) (0.66), improved quality of life (somewhat improved) (0.39), the disease poses a threat to life (0.18), availability of other drug treatments (−0.075), and cost of treatment (−0.022).
Toumi,27 who used the French population, showed that there was some public preference for rarity, but it was not a significant non-priority, and no statistically significant difference was found in the public’s choice of treatment priorities for two diseases with 500 and 2000 patients, respectively, while statistically significant differences were found in the comparison of the treatment priorities for 500 or 10,000 patients (weight of 0.293) and for 500 or 20,000 patients (weight of 0.233). Statistically significant differences were found between the priorities for 500 and 10,000 patients (weight of 0.293) and for 500 and 20,000 patients (weight of 0.233). The attributes other than the number of people with the disease and the magnitude of the weights were as follows: The largest weight estimate was for the effect of a drug treatment on disease mortality, with the public preferring drugs that increased life expectancy by 30 years to drugs that extended patients’ life expectancies by 10 years (–0.964), 2 years (–0.919), or had no effect on life expectancy (–1.112). The second most important attribute according to the public was the certainty of a drug’s therapeutic effect, with the public preferring drugs with a definite therapeutic effect compared to drugs with fair (weighted at –0.560) or insufficient certainty (weighted at –0. 838) of a therapeutic effect. The public was sensitive to the availability of drug substitution therapy and drug safety. The public was more likely to choose a drug with no available alternatives (weight of 0.178) or a drug with alternatives with limited effectiveness (weight of 0. 291) than a drug with effective alternatives. Drugs with serious adverse effects were less likely to be chosen than drugs that did not cause adverse effects (weight of –0.462). The cost of treatment per capita also had an impact on the public’s choices, with drugs that had a lower cost per capita being favored, but only to a limited extent; this was only between 500,000 francs per capita (approximately USD 508,100) and 10,000 francs per capita (approximately USD 10,200), and the public’s preference for 10,000 francs was found to be statistically significant (with a weight of –0.235). Although it was generally agreed that there was a tendency for the public to prefer diseases with higher rates of causing disability, a statistically significant preference on the part of the public in terms of disease characteristics (disability and mortality due to disease) has not yet been found.
Summary of Social Preferences Related to Rare Diseases versus Common Diseases: A Combined Outcome Analysis
When the results of the PTO studies were combined, it could be seen that most of the public maintained a neutral attitude towards rare diseases in comparison with common diseases in terms of their setting of priorities, ie, health resources were equally distributed between the two types of diseases, with no social preference for rarity, but it was preferable to set a priority for rare diseases when the proportional advantage of rare diseases was considered or when the respondents were young. In contrast, among the public with a non-neutral attitude towards setting priorities for rare and common diseases, the majority of the respondents preferred to set priorities for common diseases. In terms of specific attributes of these preferences (other than rarity), the public generally tended to set priorities for diseases (treatment options/patients) with attributes such as a low cost, good health benefits, and severity when setting priorities for rare rather than common diseases.
The combined results of the AHP studies showed that the public placed a low importance on rarity, with rarity having the second lowest weighting out of the 13 attributes covered. In contrast, there was a clear preference for treatment-related factors, especially the safety of treatment, good health benefits after treatment, the prolongation of life, the improvement of quality of life, the certainty of treatment outcomes, the equity of treatment, and the severity of the disease, while no clear preferences were found for patient-related factors (eg, socioeconomic status, adherence) or rarity of the disease.
When the results of the DCE studies were combined, it was clear that rarity was not an attribute that was prioritized by the public, while attributes such as good health benefits from treatments, the ability of a treatment to extend a patient’s lifetime, the ability of a treatment to improve quality of life, the safety of the treatment, certainty, unmet needs, and the severity of the disease (eg, the disease is life-threatening) were prioritized by the public. By analyzing the included studies, this study identified the following general public preferences in setting priorities for rare and common diseases: good health benefits from a treatment, a treatment that prolongs a patient’s lifetime, a treatment that improves the quality of life, high safety of treatment options, clear treatment effects, the severity of the disease, unmet needs (ie, no alternative treatment options are available), and equity in healthcare delivery. In addition, there was a clear preference for rarity when the public considered the proportional advantage of rare diseases or when the respondents were young. Figure 2 uses a fishbone diagram to distill and summarize these specific preferences; the second construct of rarity (ie, the horizontal line) indicates that the public had a preference for rarity under these conditions.
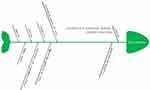 |
Figure 2 Fishbone diagram of the preference factors. |
Willingness to Pay for Rare Diseases in Society
In studies such as those of Desser, Ramalle-Gómara, and Richardson,14,20,22 the willingness to pay for rare diseases in society was measured. This method involved the researchers presenting several hypotheses in advance and asking respondents to choose to rate them on a five-point Likert scale, with a score of 1–5 that indicated full disagreement, relative disagreement, no preference, relative agreement, and full agreement. In the studies by Desser and Ramalle-Gómara,19,22 the public was invited to rate three hypothetical scenarios, the first being that “patients with rare diseases have equal access to healthcare to that of those with common diseases, regardless of cost”, the second being that “even if the cost is high, patients with rare diseases should have the same rights to treatment as other patients”, and the third being that “treatment of rare diseases at high prices is unacceptable and health budgets should be invested in patients with the greatest health benefits from treatment”. With the first hypothesis, 72.7% of people in the study by Desser14 were in full agreement (5 out of 5), and the average score of 4.6 in the study by Ramalle-Gómara22 suggested that there should be equal access to healthcare for all, regardless of the cost, and the cost of treatment for expensive and rare diseases was acceptable to society. For the second hypothesis, 67.4% of population in the study by Desser14 gave a score of 5, and the study by Ramalle-Gómara22 yielded an average score of 4.6, showing that patients with rare diseases should have the same right to treatment as others and that even if treatment is expensive, it would still be feasible if health insurance funds could afford it. The frequency of scoring in the third hypothetical situation was more dispersed, with 46.3% of the respondents in the study by Desser14 giving scores between 3 and 4 and only 38.9% giving a score of 5. The average score of 3.3 obtained in the study by Ramalle-Gómara22 suggested that the high cost of treating rare diseases was that of spending on diseases to benefit more people, and most people did not agree with this. In Richardson’s20 study, three scenarios were also developed, and the public was invited to rate them. The three scenarios were “reducing services for the majority of patients with common diseases to pay for the high cost of treatment for the minority of patients with rare diseases”, “providing basic, low-cost care for patients with rare diseases because of health insurance budget constraints”, and “setting treatment priorities for rare diseases when they are very severe rather than considering their treatment costs first”. The percentages of those who “relatively agreed” and “strongly agreed” with these three hypotheses were 43%, 25%, and 51%, respectively, suggesting that nearly half of the public would accept the high cost of rare diseases, especially when they were severe, and they would consider providing quality services to patients with rare diseases.
Discussion
This review and analysis of the literature revealed that the degree of public preference for the rarity of diseases when allocating limited health resources has received particular attention from researchers. Given this situation, this would be an important reason to give expensive drugs for rare diseases (ie, orphan drugs) a “special status” in Health technology assessments (HTAs). However, the many international studies on social preferences did not produce any evidence showing that high-cost treatments for rare diseases could only be financed on the basis of rarity. These findings also showed that public support for the treatment of rare diseases in Western countries does not differ significantly from support for the treatment of common diseases and that most of the public is less aware of rare diseases and orphan drugs and is less engaged in research. Therefore, it is difficult for policymakers to use existing measures of social value for orphan drugs when formulating orphan drug policies. Based on this, this study systematically reviewed studies on social preferences for rare diseases and discussed the following relevant attributes and preference factors mentioned in the articles.
Analysis of Results Based on the PTO Method
No Specific Preference Between Common or Rare Diseases
The Person trade-off (PTO) method is a group-decision-making-based method of measurement that requires respondents to take on the role of the decision maker and choose between two competing alternatives or remain neutral; then, the specific preferences of the respondent are judged based on the analysis of the final options of the simulated scenario.15,16 First, the public’s setting of priorities between rare and common diseases was predominantly neutral, or they chose to distribute resources equally between the two types of diseases. This indicates that a found a social preference for rarity has not yet been found.14 On the other hand, a possible explanation lies in the fact that the public is less familiar with rare diseases and has not formed a stable and ordered set of preferences. The construction of individual preferences during the interview process was susceptible to peripheral factors in the decision-making environment, and the PTO method involved the use of a horizontal slider format for respondents to allocate resources between rare and common diseases. Thus, their responses were vulnerable to a visual tendency toward the center, and the likelihood of a central tendency bias in the presence of unstable preferences was increased.14,19,21 In addition, the public rejected the “zero-sum” framework and showed an “aversion” to “dilemma” decisions, actively taking “no decision” measures in such situations, thus increasing the likelihood of choosing “neutrality” and showing a tendency to divide resources evenly between the two diseases.21
Preference for Rarity
The factors that enhanced the public’s preference for rarity included proportional advantage and age. Proportional advantage explains the conditional basis for the existence of a public preference for rarity. It was found that the public was more willing to help a larger proportion of people (eg, 100 out of 100) than a smaller proportion (eg, 100 out of 10,000), suggesting that the public prioritized solving the entirety of an incident rather than eliminating only a part of it. Therefore, the public generally tended to deal with larger problems before dealing with smaller ones, thereby somewhat ignoring the issue of efficiency.17 Meanwhile, young people are generally more educated than older people, thus increasing their awareness of rare diseases; this increased awareness can stabilize the preference for rarity.18,19
Preference for Costs
A rational explanation for the public’s neutrality—in addition to the reasons mentioned above—is that the public is insensitive to large differences in opportunity costs, does not show a clear preference based on price responses, or has a more general concern for fairness. However,19,24 the public’s preference for low costs shown in related studies, such as that of Bae,18 differed from the findings of the above analysis, which may mainly have been due to the fact that there were differences in public preferences for attributes such as rarity and fairness, which could influence the public’s judgment and value orientations towards price. In addition, the fact that the respondents were citizens of different countries, the different rates of reimbursement for rare disease treatments and orphan drugs among different countries, the different values of the public, and the different study designs in different project21 may have led to different sensitivities to price.
Preference for Health Gains
The public’s preference for treating patients with common diseases was due to the generally high individual cost of treating rare diseases and the limited availability of healthcare resources, leading to a greater tendency to use resources to maximize health benefits and provide health services to more patients.14 At the same time, Desser19 showed that the public engaged in a pre-selection “focus” exercise (ie, clarifying the principles of resource allocation before selection) and that a general preference for efficiency led to an increase in the share of resources allocated to the group with common diseases. In addition, the identification of the principles of resource allocation was more conducive to the establishment of psychological distance in moral dilemmas, and the greater psychological distance led to a tendency to allocate resources by using decontextualized information, such as the core content and objective efficiency, which increased the allocation of resources to common diseases.19
Preference for Disease Severity
The public considered severe diseases a priority, especially those with treatments that provided good health benefits. Richardson20 also noted that not all rare diseases were costly to treat and that the total cost of treatment was relatively low for rare diseases with a small number of patients, in which case the priority of rare diseases could be considered. It was also recommended that access to funding for rare diseases be broadened and the per capita cost of payment reduced to increase the availability of quality and effective services for rare diseases.
Analysis of Results Based on the AHP
The Analytic hierarchy process (AHP) is a simple, flexible, and practical multi-criteria decision-making method for the quantitative analysis of qualitative problems, and it allows for the ranking of the strength of preference for each attribute.23,24 By using the AHP, Rizzardo23 and Yamoah24 found that the public valued treatment- and disease-related factors more highly than the prevalence (rarity) of the disease, and they supported the traditional principles of drug review that emphasized the efficacy, safety, and certainty of drugs.23 However, the low value placed by the public on attributes such as unmet needs and rarity is at odds with the value preferences of policymakers who invoke the “rescue rule”, ie, the obligation or moral duty to help those in immediate life-threatening situations, those with “unmet needs”, and those with rare diseases. Rare diseases are likely to be serious life-threatening diseases, and their treatment could significantly improve the quality of life of patients while adhering to the principle of equity, ie, “equal access to healthcare for all”; therefore, rarity can be taken into account when funding medicines.23 However, given the limited funds available and the need to maximize health benefits, an ethical dilemma has emerged in the public’s consideration of funding for medicines for rare diseases. It is recommended that policymakers pay attention to the public’s attribute preferences, which can be incorporated into the medical funding and reimbursement framework after a comprehensive weighing and scientific judgment.
Analysis of Results Based on the DCE Method
A discrete choice experiment (DCE) is a method of multivariate analysis that requires subjects to choose a situation that they prefer from different hypothetical scenarios (corresponding to different variables and different levels of the variables) and analyzing each attribute preference and weight size, rather than choosing directly from different levels of a single variable to reflect their preferences.26,27 The results of the DCE suggested that the severity of the disease and the effectiveness of the treatment were confirmed as important factors in judgments and that rarity was not a prioritized attribute, which was generally consistent with the results derived from the AHP. Meanwhile, the DCE results also indicated a lower sensitivity to the disabling nature of a disease due to the fact that the study reflected social preferences (ie, preferences for others) rather than personal preferences (ie, people’s preferences for themselves); these were more focused on the duration of life and less on the quality of life, rather valuations based on personal preferences. In addition, the public’s preference for unmet needs (ie, drugs for which there is no alternative) was influenced by the concept of the equitable distribution of healthcare resources even if a treatment is expensive, and the public was willing to provide healthcare to patients who needed it most.
Analysis of Results Based on Relative Willingness to Pay in Society
The findings of the study on relative willingness to pay revealed that the public’s willingness to pay for rare diseases was moderate and mainly influenced by their belief in equity - that every individual has the right to life and health. As a result, the majority of the population favored the idea that patients with rare diseases should have equal access to healthcare, even if the costs were high. Conversely, most individuals disagreed with the notion of reducing the high cost of treatment for rare diseases to expand the number of patients with access to healthcare. Additionally, the priority of treatments for rare diseases was heavily influenced by the disease’s severity, with this attribute deemed as higher-priority than the treatment’s cost. Quality healthcare services for individuals with rare diseases should be offered within the limits of affordability for health insurance funds.
Future Research Directions
Attaining health equity, wherein all individuals can fully realize their health and well-being, assumes paramount significance. Nevertheless, the escalating demands of patients coupled with the scarcity of financial resources engender a conflict between efficiency and equity. Consequently, certain groups may inadvertently face neglect in the allocation of healthcare resources, driven by the pursuit of maximum efficiency. A prime example of this phenomenon exists in the context of patients afflicted with rare diseases, as their prevalence significantly pales in comparison to common ailments, rendering them susceptible to receiving unequal and inequitable medical care. These observations, confirmed by our current study, suggest minimal societal bias towards rare diseases. Moving forward, an equitable health care paradigm demands adherence to principles of both horizontal and vertical equity, involving uniform resource distribution among individuals with equivalent needs and disparate allocation among those with varying requirement intensities, respectively. Future research should thus direct its focus onto the development of resource distribution strategies that align with societal perceptions of equity.
Limited resources available and increasing healthcare spending require payers to consider opportunity costs and societal concerns about the treatment of rare diseases when deciding whether to fund a particular treatment. The analysis here revealed a wide variation in the distribution of weights in different studies, which was probably due to the differences in the methods, contexts, countries, sample sizes, and attribute-related definitions considered. Striking a balance between the needs of individuals with rare diseases and the broader goals of public health requires careful consideration of various factors, including disease prevalence, severity, and stakeholder interests. Developing a fair and efficient approach to resource allocation that recognizes these complexities is essential for fostering equitable healthcare access and outcomes for all population groups.
The impetus for conducting a systematic review on the social equity of healthcare and access to rare disease drugs acquires paramount importance, as it strives to consolidate pertinent empirical evidence with the potential to guide forthcoming research and policy-making. Through the rigorous synthesis and analysis of the existing literature, this paper aspires to elucidate the multifaceted dimensions and disparities that undergird the accessibility and affordability of healthcare services and rare diseases, further bridging the knowledge gap between various stakeholders. By doing so, this systematic review not only sheds light on critical issues underscoring social equity in healthcare domains, but also supplies an impetus for the adoption of more equitable health policies and interventions. Since 2018, China has gradually incorporated health technology assessment into the national drug reimbursement negotiation process, gradually shaping a value-based pricing mechanism for the access of innovative pharmaceuticals. A key factor in this decision-making process is the incremental cost-effectiveness ratio (ICER) threshold. With the characteristic of holding large and diverse population under its social welfare system, the measure it adopts to balance the offering to public or individual becomes more crucial. In recent years, an increasing number of studies have explored societal inclination for rare diseases, with the aim of providing scientific support for adjustments to the ICER threshold. To address the current needs and value prioritization in orphan drug negotiations, this study plans to further explore preference characteristics within the context of rare diseases. As we endeavor to explore the intricate interplay between individual, social, and systemic factors, this paper will serve as an invaluable resource for scholars and practitioners who seek to unravel the complexities of healthcare disparities and drive the development of more equitable healthcare landscapes for individuals afflicted by rare diseases, ultimately contributing to more informed decision-making and resource allocation in the healthcare sector. In addition, There is also an objective possibility of differences between population groups from different social backgrounds, so their perceptions and preferences for treatments for rare diseases and orphan drugs may have differed to some extent. The studies that were retrieved were mainly from Western countries, and it is hoped that more research on the social preferences of Asian populations will be published in the future.
Conclusion
Given the scarcity of healthcare resources, the allocation of resources between rare and common diseases has gained prominence on an international level. There has been a surge in studies examining the social preference for rare diseases. Our study, which analyzed multiple related studies, has found that there is no significant preference for rarity when evaluating equal circumstances. However, a social preference for rarity was observed when respondents perceived a proportional advantage of rare diseases or when the respondents were young. Additionally, the public considers various attributes such as health benefits, equity, and disease severity when setting healthcare priorities. Therefore, while deciding whether to fund rare or common diseases, payers should be cognizant of the opportunity costs and societal preference for rare disease treatments, balancing the interests of patients with rare diseases and the broader goals of public health. This requires a deliberate consideration of several factors, including disease prevalence, severity, and stakeholder interests.
Acknowledgments
We owe a special debt of gratitude to all the professors and editors, whose hard work benefited us a lot and academically reviewed the paper.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stafinski T, Menon D, Marshall D, Caulfield T. Societal values in the allocation of healthcare resources is it all about the health gain? Patient. 2011;4(4):207–225. doi:10.2165/11588880-000000000-00000
2. Whitty JA, Lancsar E, Rixon K, Golenko X, Ratcliffe J. A systematic review of stated preference studies reporting public preferences for healthcare priority setting. Patient. 2014;7(4):365–386. doi:10.1007/s40271-014-0063-2
3. Albertsen A. Rare diseases in healthcare priority setting: should rarity matter? J Med Ethics. 2022;48(9):624–628. doi:10.1136/medethics-2020-106978
4. Sandman L, Gustavsson E. The (Ir)relevance of group size in health care priority setting: a reply to juth. Health Care Anal. 2017;25(1):21–33. doi:10.1007/s10728-016-0333-3
5. Magalhaes M. Should rare diseases get special treatment? J Med Ethics. 2022;48(2):86–92. doi:10.1136/medethics-2021-107691
6. Juth N, Henriksson M, Gustavsson E, Sandman L. Should we accept a higher cost per health improvement for orphan drugs? A review and analysis of egalitarian arguments. Bioethics. 2021;35(4):307–314. doi:10.1111/bioe.12786
7. Juth N. For the sake of justice: should we prioritize rare diseases? Health Care Anal. 2017;25(1):1–20. doi:10.1007/s10728-014-0284-5
8. Devlin N, Parkin D. Does NICE have a cost-effectiveness threshold and what other factors influence its decisions? A binary choice analysis. Health Econ. 2004;13(5):437–452. doi:10.1002/hec.864
9. Harris AH, Hill SR, Chin G, Li JJ, Walkom E. The role of value for money in public insurance coverage decisions for drugs in Australia: a retrospective analysis 1994–2004. Med Decis Making. 2008;28(5):713–722. doi:10.1177/0272989X08315247
10. Clement FM, Harris A, Li JJ, Yong K, Lee KM, Manns BJ. Using effectiveness and cost-effectiveness to make drug coverage decisions a comparison of Britain, Australia, and Canada. JAMA. 2009;302(13):1437–1443. doi:10.1001/jama.2009.1409
11. Canadian Agency for Drugs and Technologies in Health. Guidelines for the Economic Evaluation of Health Technologies. Canada: Canadian Agency for Drugs and Technologies in Health; 2006.
12. National Institute for Health and Clinical Excellence. Social Value Judgements: Principles for the Development of Nice Guidance. National Institute for Health and Clinical Excellence; 2008.
13. Australian Government Department of Health and Ageing, Pharmaceutical Benefits Advisory Committee. Guidelines for Preparing Submissions to the Pharmaceutical Benefits Advisory Committee (Version 4.3). Canberra: Australian Government Department of Health and Ageing, Pharmaceutical Benefits Advisory Committee; 2013.
14. Desser AS, Gyrd-Hansen D, Olsen JA, Grepperud S, Kristiansen IS. Societal views on orphan drugs: cross sectional survey of Norwegians aged 40 to 67. BMJ. 2010;341(sep22 3):c4715. doi:10.1136/bmj.c4715
15. Linley WG, Hughes DA. Societal views on nice, cancer drugs fund and value-based pricing criteria for prioritising medicines: a cross-sectional survey of 4118 adults in Great Britain. Health Econ. 2013;22(8):948–964. doi:10.1002/hec.2872
16. Chim L, Salkeld G, Kelly P, Lipworth W, Hughes DA, Stockler MR. Societal perspective on access to publicly subsidised medicines: a cross sectional survey of 3080 adults in Australia. PLoS One. 2017;12(3):e0172971. doi:10.1371/journal.pone.0172971
17. Wiss J, Levin LA, Andersson D, Tinghög G. Prioritizing rare diseases: psychological effects influencing medical decision making. Med Decis Making. 2017;37(5):567–576. doi:10.1177/0272989X17691744
18. Bae EY, Lim MK, Lee B, Bae G. Who should be given priority for public funding? Health Policy (New York). 2020;124(10):1108–1114. doi:10.1016/j.healthpol.2020.06.010
19. Desser AS. Prioritizing treatment of rare diseases: a survey of preferences of Norwegian doctors. Soc Sci Med. 2013;94:56–62. doi:10.1016/j.socscimed.2013.06.019
20. Richardson J, Iezzi A, Chen G, Maxwell A. Communal sharing and the provision of low-volume high-cost health services: results of a survey. PharmacoEconomics. 2017;1(1):13–23. doi:10.1007/s41669-016-0002-3
21. Dragojlovic N, Rizzardo S, Bansback N, Mitton C, Marra CA, Lynd LD. Challenges in measuring the societal value of orphan drugs: insights from a Canadian stated preference survey. Patient. 2015;8(1):93–101. doi:10.1007/s40271-014-0109-5
22. Ramalle-Gomara E, Ruiz E, Quinones C, Andres S, Iruzubieta J, Gil-de-Gomez J. General knowledge and opinion of future health care and non-health care professionals on rare diseases. J Eval Clin Pract. 2015;21(2):198–201. doi:10.1111/jep.12281
23. Rizzardo S, Bansback N, Dragojlovic N, et al. Evaluating Canadians’ values for drug coverage decision making. Value Health. 2019;22(3):362–369. doi:10.1016/j.jval.2018.08.008
24. Yamoah L, Dragojlovic N, Smith A, Lynd LD, Marra CA. Evaluating New Zealanders’ values for drug coverage decision making: trade-offs between treatments for rare and common conditions. Pharmacoeconomics. 2021;39(1):109–119. doi:10.1007/s40273-020-00974-8
25. Mentzakis E, Stefanowska P, Hurley J. A discrete choice experiment investigating preferences for funding drugs used to treat orphan diseases: an exploratory study. Health Econ Policy Law. 2011;6(3):405–433. doi:10.1017/S1744133110000344
26. Bourke SM, Plumpton CO, Hughes DA. Societal preferences for funding orphan drugs in the United Kingdom: an application of person trade-off and discrete choice experiment methods. Value Health. 2018;21(5):538–546. doi:10.1016/j.jval.2017.12.026
27. Toumi M, Millier A, Cristeau O, Thokagevistk-Desroziers K, Dorey J, Aballea S. Social preferences for orphan drugs: a discrete choice experiment among the French general population. Front Med. 2020;7. doi:10.3389/fmed.2020.00323
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
