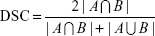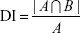Back to Journals » OncoTargets and Therapy » Volume 9
Comparison of planning target volumes based on three-dimensional and four-dimensional CT imaging of thoracic esophageal cancer
Authors Wang W , Li J, Zhang Y, Shao Q, Xu M, Fan T, Wang J
Received 15 January 2016
Accepted for publication 20 May 2016
Published 2 August 2016 Volume 2016:9 Pages 4785—4791
DOI https://doi.org/10.2147/OTT.S104315
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Wei Wang, Jianbin Li, Yingjie Zhang, Qian Shao, Min Xu, Tingyong Fan, Jinzhi Wang
Department of Radiation Oncology, Shandong Cancer Hospital Affiliated to Shandong University, Shandong Academy of Medical Sciences, Shandong, People’s Republic of China
Background and purpose: To investigate the definition of planning target volumes (PTVs) based on four-dimensional computed tomography (4DCT) compared with conventional PTV definition and PTV definition using asymmetrical margins for thoracic primary esophageal cancer.
Materials and methods: Forty-three patients with esophageal cancer underwent 3DCT and 4DCT simulation scans during free breathing. The motions of primary tumors located in the proximal (group A), middle (group B), and distal (group C) thoracic esophagus were obtained from the 4DCT scans. PTV3D was defined on 3DCT using the tumor motion measured based on 4DCT, PTV conventional (PTVconv) was defined on 3DCT by adding a 1.0 cm margin to the clinical target volume, and PTV4D was defined as the union of the target volumes contoured on the ten phases of the 4DCT images. The centroid positions, volumetric differences, and dice similarity coefficients were evaluated for all PTVs.
Results: The median centroid shifts between PTV3D and PTV4D and between PTVconv and PTV4D in all three dimensions were <0.3 cm for the three groups. The median size ratios of PTV4D to PTV3D were 0.80, 0.88, and 0.71, and PTV4D to PTVconv were 0.67, 0.73, and 0.76 (χ2=–3.18, –2.98, and –3.06; P=0.001, 0.003, and 0.002) for groups A, B, and C, respectively. The dice similarity coefficients were 0.87, 0.90, and 0.81 between PTV4D and PTV3D and 0.80, 0.84, and 0.83 between PTV4D and PTVconv (χ2=–3.18, –2.98, and –3.06; P=0.001, 0.003, and 0.002) for groups A, B, and C, respectively. The difference between the degree of inclusion of PTV4D in PTV3D and that of PTV4D in PTVconv was <2% for all groups. Compared with PTVconv, the amount of irradiated normal tissue for PTV3D was decreased by 11.81% and 11.86% in groups A and B, respectively, but was increased by 2.93% in group C.
Conclusion: For proximal and middle esophageal cancer, 3DCT-based PTV using asymmetrical margins provides good coverage of PTV4D; however, for distal esophageal cancer, 3DCT-based PTV using conventional margins provides ideal conformity with PTV4D.
Keywords: planning target volume, 4DCT, 3DCT, esophageal carcinoma
Introduction
The incidence rate of esophageal carcinoma varies considerably among different geographic regions throughout the world and is particularly high in the People’s Republic of China, where it exceeds 1 per 1,000 individuals.1,2 Historically, chemoradiotherapy has played an important role in the management of localized esophageal cancer because it provides better palliation than does radiotherapy alone and improves the likelihood of long-term, progression-free survival.3 New technological advances in radiation techniques such as intensity-modulated radiotherapy, respiratory-gated radiotherapy, image-guided radiotherapy, and positron emission tomography (PET)- or PET/computed tomography (CT)-based radiotherapy have allowed for a selective increase in the dose delivered to the target for esophageal cancer without any significant increase in the dose delivered to the organs at risk.4,5
The goal of modern radiotherapy approaches based on recent technological advances is to minimize the risk of damage to healthy tissues by improving the gross tumor volume (GTV) definition (PET or PET/CT) and reducing intrafraction motion (respiratory-gated radiotherapy; four-dimensional CT [4DCT]) and interfraction motion (image-guided radiotherapy; cone beam CT).6 Obviously, the larger the tumor and extent of involvement are, the higher the toxicity to the organs at risk will be.7,8 Therefore, this study focused on the definition of the planning target volume (PTV).
Tumor displacement and deformation affect the definition of PTV during the course of treatment. Creating a PTV for a moving target is an important but complicated clinical problem for various anatomical regions, such as the esophagus. There are many sources of fractional variations in internal structures that can occur (eg, physiological movements of the tumor and organs, mostly originating from respiratory or cardiac cycles and setup error).9 To account for these intrafractional and interfractional variations, large population-based margins are used. The incorporation of these uncertainties further increases the excessive irradiation of normal tissues. Because of the technical limitations of conventional PTV definition, new PTV definition methods have been investigated to decrease the size of the target volume and reduce normal tissue toxicity.
During free breathing, tumor and organ motions always influence the accuracy and quality of 3DCT imaging of thoracic malignancies, including esophageal carcinoma. The detailed motion of the tumor, the various spatial positions of the tumor, and anatomical information averaged over one breathing cycle should be carefully provided when using 4DCT.10–13 Breathing characteristics vary greatly among individual patients, and respiratory-induced target motion and interfraction target motion are unsymmetrical.10–15 Therefore, esophageal tumor motion must be separately assessed in each individual patient because doing so may allow the tumor margins to be decreased and consequently allow for reduction in the PTV size and the radiation exposure of normal tissue. Based on this concept, we performed patient-specific PTV definition using images acquired on 4DCT, and these PTVs were compared with the conventional PTVs formed by adding clinical experience margins and individual margins to the free-breathing planning 3DCT scan. This work was a pilot study to investigate the feasibility of using 4DCT when contouring individualized PTVs for esophageal carcinoma and to assess the differences between patient-specific and population-based target volumes as well as their inherent advantages and disadvantages.
Materials and methods
Ethics statement
All patients provided signed informed consent to participate in the study and before undergoing further imaging during radiotherapy. The study design was approved by the ethics committee of Shandong Cancer Hospital and Institute (approval ID: SDTHEC20110130).
Patient population
Forty-three patients with esophageal carcinoma who underwent 4DCT scanning were included in this study. The mean patient age was 66 years (range: 41–83 years), and 37 of the patients were men. Nine patients had pathologically confirmed adenocarcinoma of the thoracic esophagus, and 34 patients had squamous cell carcinoma of the thoracic esophagus. Thirteen patients had primary tumors in the proximal third of the esophagus (group A), whereas 18 and 12 patients had primary tumors in the middle third and distal third of the esophagus (groups B and C), respectively. All patients exhibited regular breathing patterns.
Image acquisition
Patients were immobilized in the supine position with their arms above the head using a vacuum bag. Every patient underwent 3DCT and, immediately afterward, respiration-correlated 4DCT on a 16-slice CT scanner (Philips Medical System, Cleveland, OH, USA). For 3DCT, each scan (360° rotation) took 1 second to acquire followed by a 1.8 seconds dead time and had a 2.4 cm coverage. The slice thickness in the 3DCT scan was 3 mm. During 4DCT scanning, the Varian Real-time Positioning Management system (Varian Medical Systems, Palo Alto, CA, USA) was used to monitor the patients’ breathing. The Real-time Positioning Management system uses infrared beams to track the trajectory of infrared-reflecting markers placed on the epigastric region of the patient’s abdomen. The signal was sent to the scanner to label each CT image with a time tag. GE Advantage 4D software (GE Healthcare, Waukesha, WI, USA) was used to sort the reconstructed 4DCT images into ten respiratory phases, labeled as 0%–90% based on these tags, with 0% corresponding to the end of inhalation and 50% corresponding to the end of exhalation. The slice thickness was 3 mm, and the 4DCT data set was transferred to an Eclipse treatment planning system (Eclipse 8.6; Varian Medical Systems).
Measurements of tumor motion
The same clinician contoured the GTV on both the standard free-breathing 3DCT scan and each of the ten respiratory phase volumes for each patient. Each volume was outlined using the same window and level settings. The motion amplitudes of the primary tumor for each patient throughout one respiratory cycle were measured at the center of each GTV, and the 95% upper bound of the cumulative distribution represented an extreme of motion attained by at least a portion of the GTV.
Target volume generation
The clinical target volume (CTV) was created by manually contouring the esophagus at 3 cm superior and inferior to the GTV and then adding 0.5 cm circumferentially to the created volume to account for microscopic spread. The union of all ten CTVs from the 4DCT data was used to generate the internal target volume (ITV4D). ITV3D was constructed by adding asymmetric margins to CTV3D in each spatial direction, depending on the amount of motion measured on the 4DCT phases and the expansion necessary to cover an ITV of 95% of the tumor in all three dimensions. PTVs were generated by applying 0.5 cm expansions to ITV4D and ITV3D, resulting in PTV4D and PTV3D, respectively. For PTV conventional (PTVconv), a 1.0 cm margin in all directions was added to CTV3D.
Target volume analysis
PTV3D, PTVconv, and PTV4D were compared with respect to their centroid positions, volumes, dice similarity coefficients (DSCs), and degrees of inclusion (DIs). The DSC can be used to determine the extent of spatial overlap between two regions of interest and takes values ranging from 0 (no overlap) to 1 (perfect overlap).16 The DSC is defined by the following formula:
|
|
The DI of volume A included in volume B [DI (A in B)] is given by the following equation:17
|
|
From these data, assuming that B is the reference for the standard target volume, for treatment planning based on A, 1 − DI (A in B) of A will be unnecessarily irradiated and 1 − DI (B in A) of B will be lacking irradiation.
Statistical analyses
The Friedman Z test was performed to detect the differences among the GTV centroid displacements in all three dimensions. The Wilcoxon signed-rank test was performed to determine significant differences in variability in the centroid positions and volumes of the PTVs. Additionally, comparison of the PTV volume ratios and DSCs at different locations was performed based on the Kruskal–Wallis H test. A P-value <0.05 was considered to be significant.
Results
Measurement of primary tumor motion
The average three-dimensional centroid motion amplitude of the GTVs caused by respiration was highest in the superoinferior (SI) direction for each group. The mean motions in the anteroposterior (AP) and lateral directions for tumors located in the distal third of the esophagus were larger than those for upper and middle esophageal tumors ( , P=0.008;
, P=0.008;  , P=0.011). In the SI direction, the median motion was 0.44 cm for distal tumors compared with 0.31 cm and 0.27 cm for upper and middle esophageal tumors, respectively, although this difference was not statistically significant (χ2=3.33, P=0.189). The 95th percentile values from the cumulative distribution were used to define minimum margins to account for GTV motion during target volume generation (Table 1).
, P=0.011). In the SI direction, the median motion was 0.44 cm for distal tumors compared with 0.31 cm and 0.27 cm for upper and middle esophageal tumors, respectively, although this difference was not statistically significant (χ2=3.33, P=0.189). The 95th percentile values from the cumulative distribution were used to define minimum margins to account for GTV motion during target volume generation (Table 1).
PTV conformity
The differences in the PTV centroid positions between PTV3D and PTV4D and between PTVconv and PTV4D were <0.2 cm in the lateral and AP directions and <0.3 cm in the SI direction. The median tumor volume ratios of PTV4D to PTV3D (PTV4D/PTV3D) in the upper, middle, and distal thirds of the esophagus were 0.80, 0.88, and 0.71, respectively. However, the volume ratios of PTV4D to PTVconv (PTV4D/PTVconv) had median values of 0.67, 0.73, and 0.76, respectively. Significant differences were observed in the ratios of the target volumes at different locations, particularly for the upper esophageal tumors (χ2=−3.18, −2.98, and −3.06, respectively; P=0.001, 0.003, and 0.002, respectively).
The median DSCs between PTV3D and PTV4D were 0.87 (mean: 0.87; range: 0.79–0.91), 0.90 (mean: 0.89; range: 0.79–0.91), and 0.81 (mean: 0.81; range: 0.76–0.88) for the upper, middle, and distal esophageal tumors, respectively. The median DSCs between PTVconv and PTV4D were 0.80 (mean: 0.79; range: 0.72–0.84) for group A, 0.84 (mean: 0.83; range: 0.72–0.89) for group B, and 0.83 (mean: 0.83; range: 0.78–0.90) for group C. The Kruskal–Wallis H test indicated that the DSC between PTV3D and PTV4D was significantly larger than that between PTVconv and PTV4D for group A (χ2=−3.18; P=0.001) and group B (χ2=−2.98; P=0.003) but smaller for group C (χ2=−3.06; P=0.002).
The DI of PTV3D in PTV4D exhibited median values of 0.98, 0.98, and 0.99, whereas the DI of PTVconv in PTV4D exhibited median values of 1.00, 1.00, and 0.99 for the upper, middle, and distal esophageal tumors, respectively (Table 2). The median disparities in the DI were only −0.98% (−0.96%±1.11%), −1.90% (−1.88%±1.26%), and 0.13% (0.26%±0.62%) for groups A, B, and C, respectively. In the treatment planning based on PTV3D compared with that based on PTVconv, the amount of normal tissue that unnecessarily irradiated was decreased by nearly 11.81% and 11.86% for upper and middle esophageal tumors, respectively. However, for the distal esophageal tumor patients, the average percentage of normal tissue that was unnecessarily irradiated was increased by nearly 2.93%.
Discussion
For thoracic esophageal carcinoma, several factors lead to uncertainties in target displacement. The most important causes are patient positioning variability, breathing motion, and changes in the shape of the tumor. Recently, Hawkins et al18 have demonstrated that the alignment “clipbox” and selected registration method can affect the displacements obtained. Additionally, for lung tumors, the three-dimensional tumor trajectory exhibits hysteresis ranging from 1 mm to 5 mm.19 Therefore, the previously described optimal multidisciplinary approach to the measurement of tumor movement using multiple CT scanning might introduce significant artifacts and inaccuracies in the 3DCT images. The acquisition time of 4DCT is >60 seconds, allowing for the capture of CT data in separate phases of the respiratory cycle. Additionally, the coregistration of all phases provides precise information regarding the amplitude of the structure motion as well as the position and anatomic deformation information of the structure in each phase of the breathing cycle.
We initially analyzed the respiratory motion of primary esophageal cancers using 4DCT and found that the tumor motion caused by respiration was greatest in the SI direction for all three groups of tumor patients. Compared with upper and middle esophageal cancers, a large intrafractional radial margin (0.36 cm in the lateral direction and 0.47 cm in the AP direction) for distal esophageal cancer would provide tumor motion coverage for 95% of the cases in our study population. These results are comparable to those of previous studies that have investigated three-dimensional tumor motion using time-resolved 4DCT.10–13 Thus, asymmetric margins are recommended because of variations in tumor central displacement in different directions and in different regions caused by respiratory or cardiac cycles.
Delineation accuracies in 3DCT images are influenced by artifacts and partial volume effects that arise with the motion of the thoracic contents caused by respiration.20,21 PTV3D based on 3DCT CTV allows for the definition of an asymmetric margin to increase the target volume. We observed mean volumetric differences between PTV3D and PTV4D of 20%, 12%, and 29% for groups A (proximal esophagus cancer), B (middle esophagus cancer), and C (distal esophagus cancer), respectively. However, the centers of the masses differed by <0.3 cm. 4DCT imaging for target volume definition and motion has been well studied in non-small-cell lung cancer,17,22–24 but it is not quite ready for routine clinical use for esophageal cancer PTV definition for radiotherapy. In their non-small-cell lung cancer study, Li et al17 evaluated the positional and volumetric differences between PTV4D and PTVvector in 28 patients. Their results indicated a necessity to expand the internal margin isotropically in a single direction for 3DCT treatment planning. The PTVs derived from 3DCT encompassed a relatively large proportion of normal tissues. To the best of our knowledge, ours is the first study to compare DSC and DI values between PTV4D and PTV values determined by adding conventional margins and individual margins using 3DCT to delineate the esophageal cancer target volume.
For esophageal cancer, PTVs constructed by applying asymmetric margins to standard 3DCT scans (PTV3D) provide good coverage of patient-specific PTVs based on the unions of 4DCT CTVs (PTV4D). PTV3D allows for an average reduction of 2% in the unirradiated target volume compared with PTV4D. These results indicate that the use of PTV3D to permit customization of the target volumes leads to a geographic miss of only 2%. In addition, we found that mean volumes of 22%, 16%, and 31% of the surrounding healthy tissues were unnecessarily irradiated when using PTV3D compared with PTV4D for groups A, B, and C, respectively. The latter findings demonstrated that PTV3D could provide good coverage of PTV4D, but the risk of increasing the affected volume of normal tissues should be noted, particularly for upper and distal esophageal cancers, for treatment planning performed using PTV3D. 4DCT generates up to ten times more data than conventional 3DCT, and 4DCT also incurs an increased workload by requiring the contouring of multiple target volumes. Our study demonstrated that PTV definition using asymmetric margins applied to planning 3DCT scans (PTV3D) requires an amount of time that is shorter by a factor of 8.75, on average, than that required for the delineation of ten scans and the creation of a composite PTV4D.
Conventional PTV definition for esophageal tumors is often based on helical treatment-planning CT, in which the visible primary tumor volume is enlarged by individual isotropic margins for the CTV. The coverage of PTV4D by PTVconv was very high, with mean values of 99%, 99%, and 97% for groups A, B, and C, respectively. The amount of normal tissue that was unnecessarily irradiated was decreased to nearly 12% for upper and middle esophageal tumors; however, for the distal esophageal tumor patients, the average percentage of the adjacent normal tissue to be included within the irradiation field was actually slightly increased, by 2.93%. These findings suggest that PTVconv can compensate for the additional effects that the implementation of 4DCT in target volume definition can offer, but only in the case of distal esophageal cancer.
Large population-based margins further increase the excessive irradiation of normal tissues; however, there is no benefit of a larger PTV with respect to minimizing local regional recurrence and limiting the toxicity to normal surrounding tissues or increasing overall survival. Currently, there are several lines of clinical evidence to suggest a local control and survival advantage with radiation dose escalation. However, radiation-induced lung injury has been a hindering factor in dose escalation, particularly for patients with abnormal heart and pulmonary functions.7,8,25,26 As we have demonstrated, esophageal tumors move substantially during the respiratory cycle, particularly in the SI direction of the distal thoracic esophagus.10–15 Wang et al14 have corroborated that systematic gastroesophageal junction displacements in all three dimensions are correlated, to varying degrees, with variations in tidal volume and diaphragmatic excursion during treatment. Intrapatient variability may be caused by the effect of changes in breathing patterns, and interpatient variability is most likely induced by inherent physical differences among patients, for example, tumor lengths and pulmonary functions. Therefore, normal, free-breathing conditions with regular respiratory rhythms are of fundamental importance to the improvement of PTV definition for esophageal cancer. The primary objective of performing a pretreatment CT scan is to reduce the uncertainty in the tumor location resulting from respiration, swallowing, and patient positioning variability.
Conclusion
We explored the methods of PTV delineation to minimize the damage to sensitive normal tissues within irradiated fields without sparing the primary cancer for esophageal patients. Asymmetric margins are recommended for proximal and middle thoracic esophagus cancer because of tumor displacement and deformation during the respiratory cycle. This approach reduces the time required for planning and not only provides adequate coverage of PTV4D but also encompasses a relatively large volume of surrounding healthy tissues. In addition, for distal esophageal cancer, adequate coverage of the moving target within the radiation field can be achieved without excessive irradiation of the surrounding normal tissue by applying clinical experience or the published margin guidelines reported in the literature for PTV definition. Therefore, the thoracic esophageal cancer target volume must be separately assessed because it may influence the size and spatial location of the tumors.
Acknowledgments
The abstract of this paper was presented at the ASTRO 54th/57th annual meeting and RSNA2012 in the name “Comparison of the planning target volume based on three-dimensional CT and four-dimensional CT images of thoracic esophageal cancer as a poster presentation with interim findings”. The poster’s abstract was published in “Poster Abstracts” in the Journal of Thoracic Oncology and International Journal of Radiation Oncology, Biology, Physics in the name “Comparison of the planning target volume based on three-dimensional CT and four-dimensional CT images of thoracic esophageal cancer”. The actual paper, however, has never previously been published. This manuscript was edited for English language by American Journal Experts. This work was supported by National Key Research Program of China (2016YFC0904700), the Medicine and Science Technology Development Program of Shandong Province (2015GSF118011 and 2015GSF118027), and the Medicine and Health Science Technology Development Program of Shandong Province (2015WS0160).
Disclosure
The authors report no conflicts of interest in this work.
References
Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24(14):2137–2150. | ||
Koshy M, Esiashvilli N, Landry JC, Thomas CR Jr, Matthews RH. Multiple management modalities in esophageal cancer: epidemiology, presentation and progression, work-up, and surgical approaches. Oncologist. 2004;9(2):137–146. | ||
Kleinberg L, Gibson MK, Forastiere AA. Chemoradiotherapy for localized esophageal cancer: regimen selection and molecular mechanisms of radiosensitization. Nat Clin Pract Oncol. 2007;4(5):282–294. | ||
Welsh J, Palmer MB, Ajani JA, et al. Esophageal cancer dose escalation using a simultaneous integrated boost technique. Int J Radiat Oncol Biol Phys. 2012;82(1):468–474. | ||
Fakhrian K, Oechsner M, Kampfer S, et al. Advanced techniques in neoadjuvant radiotherapy allow dose escalation without increased dose to the organs at risk: Planning study in esophageal carcinoma. Strahlenther Onkol. 2013;189(4):293–300. | ||
Wang YC, Hsieh TC, Yu CY, et al. The clinical application of 4D 18F-FDG PET/CT on gross tumor volume delineation for radiotherapy planning in esophageal squamous cell cancer. J Radiat Res. 2012;53(4):594–600. | ||
Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):70–76. | ||
Dähn D, Martell J, Vorwerk H, et al. Influence of irradiated lung volumes on perioperative morbidity and mortality in patients after neoadjuvant radiochemotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2010;77(1):44–52. | ||
Hashimoto T, Shirato H, Kato M, et al. Real-time monitoring of a digestive tract marker to reduce adverse effects of moving organs at risk (OAR) in radiotherapy for thoracic and abdominal tumors. Int J Radiat Oncol Biol Phys. 2005;61(5):1559–1564. | ||
Dieleman EM, Senan S, Vincent A, Lagerwaard FJ, Slotman BJ, van Sörnsen de Koste JR. Four-dimensional computed tomographic analysis of esophageal mobility during normal respiration. Int J Radiat Oncol Biol Phys. 2007;67(3):775–780. | ||
Yaremko BP, Guerrero TM, McAleer MF, et al. Determination of respiratory motion for distal esophagus cancer using four dimensional computed tomography. Int J Radiat Oncol Biol Phys. 2008;70(1):145–153. | ||
Patel AA, Wolfgang JA, Niemierko A, Hong TS, Yock T, Choi NC. Implications of respiratory motion as measured by four-dimensional computed tomography for radiation treatment planning of esophageal cancer. Int J Radiat Oncol Biol Phys. 2009;74(1):290–296. | ||
Yamashita H, Kida S, Sakumi A, et al. Four-dimensional measurement of the displacement of internal fiducial markers during 320-multislice computed tomography scanning of thoracic esophageal cancer. Int J Radiat Oncol Biol Phys. 2011;79(2):588–595. | ||
Wang J, Lin SH, Dong L, et al. Quantifying the interfractional displacement of the gastroesophageal junction during radiation therapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;83(2):e273–e280. | ||
Li J, Wang L, Wang X, et al. Preliminary study of the internal margin of the gross tumor volume in thoracic esophageal cancer. Cancer Radiother. 2012;16(7):595–600. | ||
Ehler ED, Tome WA. On correlated sources of uncertainty in four dimensional computed tomography data sets. Technol Cancer Res Treat. 2010;9(3):299–306. | ||
Li FX, Li JB, Zhang YJ, et al. Comparison of the planning target volume based on three-dimensional CT and four-dimensional CT images of non-small-cell lung cancer. Radiother Oncol. 2011;99(2):176–180. | ||
Hawkins MA, Aitken A, Hansen VN, McNair HA, Tait DM. Cone beam CT verification for oesophageal cancer-impact of volume selected for image registration. Acta Oncol. 2011;50(8):1183–1190. | ||
Seppenwoolde Y, Shirato H, Kitamura K, et al. Precise and real-time measurement of 3D tumor motion in lung due to breathing and heartbeat, measured during radiotherapy. Int J Radiat Oncol Biol Phys. 2002;53(4):822–834. | ||
Lorchel F, Dumas JL, Noël A, Wolf D, Bosset JF, Aletti P. Dosimetric consequences of breath-hold respiration in conformal radiotherapy of esophageal cancer. Phys Med. 2006;22(4):119–126. | ||
Chen GT, Kung JH, Beaudette KP. Artifacts in computed tomography scanning of moving objects. Semin Radiat Oncol. 2004;14(1):19–26. | ||
Rietzel E, Liu AK, Doppke KP, et al. Design of 4D treatment planning target volumes. Int J Radiat Oncol Biol Phys. 2006;66(1):287–295. | ||
Hof H, Rhein B, Haering P, Kopp-Schneider A, Debus J, Herfarth K. 4D-CT-based target volume definition in stereotactic radiotherapy of lung tumours: comparison with a conventional technique using individual margins. Radiother Oncol. 2009;93(3):419–423. | ||
Vinogradskiy Y, Castillo R, Castillo E, et al. Use of 4-dimensional computed tomography-based ventilation imaging to correlate lung dose and function with clinical outcomes. Int J Radiat Oncol Biol Phys. 2013;86(2):366–371. | ||
Minsky B, Pajak T, Ginsberg R, et al. INT0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer high-dose versus standard dose radiation therapy. J Clin Oncol. 2002;20(5):1167–1174. | ||
Button MR, Morgan CA, Croydon ES, Roberts SA, Crosby TD. Study to determine adequate margins in radiotherapy planning for esophageal carcinoma by detailing patterns of recurrence after definitive chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2009;73(3):818–823. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.