Back to Journals » Journal of Pain Research » Volume 10
Comparison of patient-controlled intravenous analgesia with sufentanil versus tramadol in post–cesarean section pain management and lactation after general anesthesia – a prospective, randomized, double-blind, controlled study
Authors Chi X, Li M, Mei W , Liao M
Received 23 March 2017
Accepted for publication 9 June 2017
Published 3 July 2017 Volume 2017:10 Pages 1521—1527
DOI https://doi.org/10.2147/JPR.S137799
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Michael Schatman
Xiaohui Chi, Man Li, Wei Mei, Mingfeng Liao
Department of Anesthesiology, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China
Introduction: Acute pain is a common complication following cesarean section under general anesthesia. Post–cesarean section pain management is important for both the mother and the newborn. This study compared the effects of patient-controlled intravenous analgesia (PCIA) using sufentanil or tramadol on postoperative pain control and initiation time of lactation in patients who underwent cesarean section under general anesthesia.
Methods: Primiparas (n=146) scheduled for cesarean section under general anesthesia were randomized to receive PCIA with sufentanil or tramadol. Movement-evoked and rest-pain intensity were assessed by the Numerical Rating Scale (NRS) postoperatively. The number of PCIA attempts, amount of drug consumed, initiation time of lactation, and Quality of Recovery Score 40 (QoR-40) were recorded at 4, 8, 12, and 24 h postoperatively. Pre- and postoperative serum prolactin levels were recorded.
Results: No between-group difference existed in the NRS at rest at any time point postoperatively. Patients on sufentanil had more movement-evoked pain and a higher sedation score at 4, 8, and 12 h postoperatively, as compared with the tramadol group. At 24 h, the QoR-40 was higher in the tramadol group compared with the sufentanil group. No significant between-group differences were present in patient satisfaction and nausea/vomiting scores. Postpartum prolactin levels were significantly higher in the tramadol group versus the sufentanil group, corresponding with a significant delay in initiation of lactation in the latter.
Conclusion: PCIA with tramadol may be preferred due to lower movement-evoked pain, higher quality of recovery, and earlier lactation in patients following cesarean section under general anesthesia.
Keywords: sufentanil, tramadol, cesarean section, patient-controlled analgesia, prolactin
Introduction
Acute pain is a common complication after cesarean section. Post–cesarean section pain management is important for both the mother and the newborn. When postoperative pain is well managed, the mother is better able to care for her newborn and bond with her child as early as possible.1 In contrast, uncontrolled pain may delay bonding with the child and limit mobilization, further increasing the risk for thromboembolism.2,3 Therefore, appropriate analgesia is important in the short term following a cesarean section under anesthesia. Analgesia administered to post-cesarean women must be effective and reliable, while remaining safe for both the mother and her newborn.
A single-dose of neuraxial opioids provides good post-cesarean analgesia after neuraxial anesthesia.4 However, it is not suitable for pain management after general anesthesia when there is a relative contraindication for spinal puncture. Intravenous or oral administration is the preferred option. The idea of placing the patient in control of the analgesic drug dose they receive has resulted in the development of patient-controlled analgesia (PCA).5,6 Patient-controlled intravenous analgesia (PCIA) is an effective method to control postoperative pain in clinics.7 It is reported to provide better pain control with lower drug consumption, has a higher level of patient satisfaction, shorter hospital stay, and fewer adverse effects on pulmonary function.8–10
Opioids are most commonly used for PCIA, and tramadol has been shown to provide effective analgesia for moderate postoperative pain.11 Tramadol administration via either of intravenous PCA or continuous infusion modes provides efficient early postoperative analgesia in patients who undergo cesarean section. Intravenous PCA results in lower drug consumption and higher patient satisfaction.1 In addition to the satisfactory level of pain control, reduced rate of respiratory depression, sedation, and effect on intestinal motility of tramadol, this has led to its frequent use in PCA compared to strong opioids.12–14 Sufentanil is the most potent analgesic available at present. It has been demonstrated that sufentanil is associated with less respiratory suppression and better analgesia than an equal dose of fentanyl.15 PCIA with sufentanil, compared with fentanyl, provided better analgesic effects and sedation with a lower incidence of nausea and vomiting when used in postoperative patients following thoracotomy.16
Limited studies exist in the literature that compare sufentanil and tramadol as post–cesarean section analgesic agents. The aim of this study was to evaluate and compare postoperative analgesia efficacy, drug consumption, quality of recovery, and initiation time of lactation with PCIA sufentanil and tramadol in patients undergoing cesarean section under general anesthesia.
Methods
This study was a prospective, randomized, double-blind, controlled clinical trial of tramadol PCIA or sufentanil PCIA for post–cesarean section pain control. The study was approved by the research and ethics committee of Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology and registered with Clinical Trials (ChiCTR-IOR-14005438). After obtaining written informed consent from individual patients, 146 primiparas (aged 20–40 years), belonging to the American Society of Anesthesiologists (ASA) physical status classification system risk classes I and II, scheduled to undergo elective cesarean section under general anesthesia (regional anesthesia was refused or contraindicated) were randomized into one of two groups. The study was conducted between November 2014 and February 2016 at Tongji Hospital, a general university teaching hospital with 3000 beds in Wuhan, People’s Republic of China.
Exclusion criteria included cardiovascular, respiratory, or neurologic disease; chronic antidepressants, anxiolytics, and analgesic use; a known allergy to the drugs being used; history of substance abuse; inability to understand how to use the PCIA device; and participation in another research study. Simple randomization was performed by handing out 146 opaque sealed envelopes – 73 for each group – indicating group assignment and describing the PCIA protocol. Patients were randomized immediately prior to entering the operating room. The anesthesiologist, patients, and assessor were blinded to the patients’ group.
All patients received cimetidine 200 mg preoperatively and fasted from solid food after midnight. A16-gauge catheter and standard monitoring were used. The patient was positioned supine with left uterine displacement. The patient received 100% oxygen for 3 min before the induction of anesthesia. A rapid sequence induction with cricoid pressure was performed with 0.3 mg/kg of etomidate and 1.5 mg/kg of succinylcholine, followed by orotracheal intubation. Anesthesia maintenance was achieved with 50%:50% oxygen and nitrous oxide with sevoflurane (1%). Additionally, vecuronium (0.02 mg/kg) was administered as needed for muscle relaxation, and sufentanil 0.5 μg/kg, following delivery.
Immediately after the peritoneum was closed, patients received either sufentanil or tramadol through a PCA device. The sufentanil group received PCIA sufentanil prepared as a 1.5 µg/mL solution in normal saline through a PCA device at a rate of 1.5 µg/h continuously, a 3 µg bolus injection, and PCA with a 25-min lockout interval and a limit of 10.5 µg/h through the PCA device. The tramadol group received PCIA tramadol prepared as a 10 mg/mL solution in normal saline through a PCA device at a rate of 10 mg/h continuously, a 20 mg bolus injection, and PCA with a 15-min lockout interval and a limit of 70 mg/h through the PCA device. All patients received standard care intraoperatively and in the recovery room, after which they were transferred to the postpartum wards.
All patients were interviewed in the recovery rooms and at 4, 8, 12, and 24 h postoperatively by a blinded assessor, and rated their pain on a numerical rating scale (NRS; 0=no pain, 1–3=mild pain, 3–5=moderate pain, 5–7=severe pain, 7–9=sharp pain, and 10 score=extreme pain) during both movement and at rest. During follow-up, if the NRS score of the patient was >5, the physician in charge administered a 2 mL bolus solution without changing the bolus dose or the lockout interval time of the PCIA set. The total amount of tramadol or sufentanil used during the first 24 h and complications such as nausea/vomiting (verbal descriptive scale for nausea, where 0=none, 1=mild, 2=moderate, and 3=severe nausea) were recorded for all patients. Moreover, sedation level was evaluated, on a five-grade scale using the Observer’s Assessment of Alertness/Sedation Scale (0=awake, 1=half asleep, 2=asleep, aroused by oral command, 3=asleep, aroused by shaking, and 4=unarousable). Patient satisfaction was evaluated 24 h postoperatively according to the following five-grade scale: 1=very satisfied, 2=satisfied, 3=neither satisfied nor dissatisfied, 4=dissatisfied, and 5=strongly dissatisfied. Postoperative quality of recovery was determined using the Quality of Recovery Score 40 [QoR-40, which contains five general domains: physical comfort (twelve items), emotional state (nine items), physical independence (five items), psychological support (seven items), and pain (seven items); minimum score 40; maximum score is 200] 24 h after surgery.17,18 Blood samples were taken before surgery and 24 h after surgery, and prolactin levels were determined using a chemiluminescence assay. The time at which lactation was initiated was assessed by maternal perception (breast fullness, engorgement, and leaking).
Presentation of observations
The primary outcome was movement-evoked pain intensity assessed using the NRS, 4 h after the surgery. The secondary outcomes were movement-evoked pain at 8, 12, and 24 h; rest pain at 4, 8, 12, and 24 h; number of PCIA attempts at 4, 8, 12, and 24 h; the amount of drug consumed; the QoR-40 at 24 h; prolactin levels at 24 h; and the time at which lactation was initiated after surgery. Movement-evoked pain was defined as pain while changing position.
Statistics
Sample size calculation was based on an initial pilot study where the standard deviation within each group was ~1.5. To achieve 80% power at α=0.05 level to detect a two-tailed difference of at least 1.5 NRS points, we required 64 patients in each group. A further 15% was then added to the cohort; therefore, 73 patients were enrolled in each group. For sample size estimation and power consumption, we used G*Power software, version 3.1.0.
Descriptive statistics were computed for all study variables. The Kolmogorov–Smirnov and normal-quantize plots were used to determine whether continuous variables were normally distributed. Age, weight, height, body mass index (BMI), duration of surgery, cost, and QoR-40 were analyzed using one-way analysis of variance (ANOVA). Non-normally distributed data were analyzed by the Kruskal–Wallis test between groups. Repeated measurements (NRS) were analyzed using two-way ANOVA and a Tukey’s test was used for post hoc testing. A P-value <0.05 was considered significant. An adjusted P-value was applied to post hoc pairwise comparisons. The adjusted P-value was 0.01. Numerical calculations were performed using SPSS (Version 12, SPSS, Chicago, IL, USA).
Results
During the study period, 191 patients were assessed for eligibility, of which 146 females were included (Figure 1). Data from all 146 patients were included in the final analysis. No difference was found between the two groups in terms of age, height, weight, BMI, surgery duration, hospital stay, and cost (P>0.05; Table 1).
  | Figure 1 Study patient disposition flow chart. Abbreviation: PCIA, patient-controlled intravenous analgesia. |
  | Table 1 Patients characteristics and postoperative variables Note: Data are expressed as mean ± SD. |
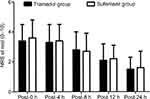
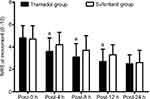
There was no difference in the NRS at rest between the two groups at any time point after surgery (P>0.05; Figure 2). There was no difference in the movement-evoked NRS between the two groups at the end of the surgery. The movement-evoked NRS in the tramadol group was lower compared with the sufentanil group at 4 h (P=0.0030), 8 h (P=0.0013), and 12 h (P=0.0033) after surgery. In addition, no significant difference was found between the two groups at 24 h postoperatively (P>0.05; Figure 3). The sufentanil group was associated with a lower number of PCIA attempts at 8 h (3.49±2.57 vs. 5.25±2.76, P=0.0001) and 12 h (4.08±3.56 vs. 8.05±4.50, P<0.0001), whereas there was no significant difference at 4 or 24 h postoperatively (P>0.05; Table 2). The amount of opioids used in the sufentanil group was lower than in the tramadol group at 8 h (14.49±4.53 mL vs 19.50±4.67 mL, P<0.0001), 12 h (18.40±7.71 mL vs 27.47±10.53 mL, P<0.0001; Table 2). During follow-up, 10 patients in the sufentanil group and eight patients in the tramadol group received a bolus of supplementary rescue analgesia; however, there was no significant difference between the two groups (13.7% vs 10.9%, P=0.8020). The sedation score was higher in the sufentanil group compared with the tramadol group at 4 h (P=0.0153), 8 h (P=0.0043), and 12 h (P=0.0091) after surgery (Figure 4).
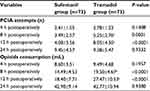  | Table 2 PCIA attempts and opioid consumption Notes: Data are expressed as mean ± SD. aP<0.01 vs. the sufentanil group. Abbreviation: PCIA, patient-controlled intravenous analgesia. |
  | Figure 4 The sedation score between the two groups after surgery. Notes: Data are expressed as mean ± SD. aP<0.05 vs. the sufentanil group. |
The QoR-40 was higher in the tramadol group compared with the sufentanil group at 24 h postoperatively (159.77±9.19 vs 152.27±10.58, P<0.0001). There was no significant difference in patient satisfaction scores and nausea/vomiting scores between the two groups. Prolactin levels in the tramadol group were significantly higher than that in the sufentanil group during the postpartum period (P=0.0039), and there was no significant differenc3 between the two groups prior to surgery (P>0.05). The start of lactation was delayed in the sufentanil group compared to the tramadol group (P=0.0422; Table 3).
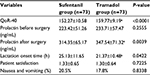  | Table 3 Postoperative variables in study groups Notes: Data are expressed as mean ± SD, or (%). aP<0.05 vs. the sufentanil group. Abbreviation: QoR-40, Quality of Recovery Score 40. |
Discussion
This prospective, randomized, double-blind, controlled study compared the analgesic effects of sufentanil PCIA with those of tramadol PCIA in patients undergoing cesarean section under general anesthesia. We found that the tramadol PCIA was significantly more effective than the sufentanil PCIA with regard to pain control (movement-evoked pain) at 4, 8, and 12 h postoperatively. Furthermore, a higher QoR-40 score was observed in the tramadol group 24 h after the surgery. Our study also found that both tramadol and sufentanil PCIA were able to achieve satisfactory pain relief during the first 24 h after surgery. The mean intensity of pain was <6 for all of the pain scores used and at all time points. There was no significant difference in rest pain between the two groups. Prolactin levels and the time at which lactation was initiated were influenced in patients who received sufentanil PCIA.
Our results are supported by the study of Vercauteren et al, who reported that rest pain did not differ between post-cesarean women receiving patient-controlled extradural analgesia with tramadol (10 mg/mL) and sufentanil (2 µg/mL).19 However, in their study, they did not observe movement-evoked pain, which is usually more severe compared to rest pain and should be measured as an outcome in every postsurgical trial.20 A lower movement-evoked pain score was associated with improved postoperative outcomes.5 Therefore, better management of movement-evoked pain may lead to improved early postoperative functional recovery. Possible explanations that account for the lower movement-evoked pain score associated with tramadol in our study may include that a greater number of PCIA attempts were used in the tramadol group and that a lower dose of sufentanil was used. A preclinical study demonstrated that the opioid μ receptor-binding affinity for sufentanil and tramadol was 0.1380 and 12.486 µM, respectively.21 One clinical trial found that tramadol is 10 times less potent than morphine,6 and morphine is 400–1000 times less potent than sufentanil.22 Thus, 1 mg of sufentanil may be considered equipotent to 4000–10,000 mg of tramadol. Therefore, we chose 10 mg/mL tramadol and 1.5 µg/mL sufentanil for the PCIA drug protocol in this study, which led to both groups receiving equivalent levels of analgesia. The number of PCIA attempts was lower in the sufentanil group at 8 and 12 h postoperatively compared to the tramadol group. The level of opioid consumption of tramadol was 270 mg (10 mg/mL * 27 mL) while that of sufentanil was 27 µg (1.5 µg/mL * 18 mL), at 12 h after surgery. However, there was no difference in the number of PCIA attempts or opioid consumption between the two groups, 24 h after surgery.
Medications used in this study did not cause any major adverse side effects during the study period, although observation over a longer period is needed to confirm long-term safety. While a high dose of tramadol can be associated with serious adverse events, especially seizures, the dose of tramadol used in our study was within the safe range.23 Postoperative adverse events were common but minor in this study and included nausea and vomiting; the incidence of these side effects in the present study was similar to those in the study of Demirel et al.1 The nausea and vomiting were mainly due to the intraoperative and postoperative opioids used and the fact that antiemetic medication was not administered prophylactically. Nausea and vomiting are the main adverse effects of tramadol. Antiemetics (either pharmacological or acupressure) should be offered to reduce the incidence of nausea and vomiting.24 Respiratory depression evaluated by ventilator use was not observed in this study. In a study with healthy volunteers, Bailey et al found that sufentanil produced shorter lasting respiratory depression and longer lasting analgesia compared to fentanyl.15 The sedation score was higher in the sufentanil group compared to the tramadol group in the first 12 h after surgery in our study. This could be due to the sedative effect of sufentanil, as both epidural and intravenous sufentanil administration are known to have analgesic as well as sedative properties.25
The tramadol group reported lower movement-evoked pain and sedative scores than the sufentanil group, and also benefited from earlier start of lactation. Prolactin promotes milk synthesis during lactation, and is required for successful lactation. Prolactin levels are affected by many factors. General anesthesia did not suppress the secretion of prolactin, while effective analgesia is beneficial to prolactin secretion.26 Postoperative pain causes sympathetic nerve excitement, hypersecretion of catecholamines, and an increase in the levels of lactation-hormone-inhibitive factors from the hypothalamus, which in turn inhibit the secretion of prolactin and delay the production of colostrum, thereby delaying the time to initiation of breastfeeding. Effective analgesia can reduce sympathetic nerve excitement and secretion of catecholamines to promote the secretion of prolactin.27 In our study, patients in the tramadol group had higher prolactin levels, and this could be due to effective movement-evoked pain relief by PCIA. Meanwhile, lactation is dependent on effective suckling from the baby, which can stimulate the sympathetic nerves of the nipple and breast to promote prolactin release. Prolonged sedation leads to reduced energy levels in the mother and a delay in communication between the mother and newborn and, subsequently, in initiation of lactation, despite sedative effects being within the safe range. After anesthesia, early and continual breastfeeding was encouraged once the mother was awake and able to hold her newborn. A number of analgesics were recommend to the mother, including those with minimal sedation.28 PCIA with tramadol may be preferred to sufentanil as it had a lower sedative score, and promoted early lactation.
There are some limitations to the present study. We did not compare the effects of tramadol with morphine, which is the most commonly used analgesic in the postoperative analgesia studies. Lactation quantity and breastfeeding duration were not observed in the present study. The concentration of sufentanil and tramadol in breast milk was not measured as we did not have facilities to evaluate this. Sufentanil is lipophilic and can be potentially stored in the fatty breast tissue and slowly released into breast milk. Therefore, further investigation into maternal and neonatal safety and communication after cesarean section is required in future studies.
Conclusion
PCIA with tramadol may be preferred due to lower movement-evoked pain scores, higher quality of recovery, and earlier commencement of lactation for patients after cesarean section under general anesthesia.
Acknowledgment
This work was supported by Research Founding of Tongji Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
Demirel I, Ozer AB, Atilgan R, et al. Comparison of patient-controlled analgesia versus continuous infusion of tramadol in post-cesarean section pain management. J Obstet Gynaecol Res. 2014;40(2):392–398. | ||
Dualé C, Frey C, Bolandard F, Barrière A, Schoeffler P. Epidural versus intrathecal morphine for postoperative analgesia after Caesarean section. Br J Anaesth. 2003;91(5):690–694. | ||
Loane H, Preston R, Douglas MJ, Massey S, Papsdorf M, Tyler J. A randomized controlled trial comparing intrathecal morphine with transversus abdominis plane block for post-cesarean delivery analgesia. Int J Obstet Anesth. 2012;21(2):112–118. | ||
Carvalho B, Roland LM, Chu LF, Campitelli VA 3rd, Riley ET. Single-dose, extended-release epidural morphine (DepoDur) compared to conventional epidural morphine for post-cesarean pain. Anesth Analg. 2007;105(1):176–183. | ||
Gilron I, Orr E, Tu D, O’Neill JP, Zamora JE, Bell AC. A placebo-controlled randomized clinical trial of perioperative administration of gabapentin, rofecoxib and their combination for spontaneous and movement-evoked pain after abdominal hysterectomy. Pain. 2005;113(1–2):191–200. | ||
Casali R, Lepri A, Cantini Q, Landi S, Novelli GP. [Comparative study of the effects of morphine and tramadol in the treatment of postoperative pain]. Minerva Anestesiol. 2000;66(3):147–152. Italian [with English abstract]. | ||
Costa JR, Coleman R. Post-operative pain management using patient-controlled analgesia. Clin Podiatr Med Surg. 2008;25(3):465–475. | ||
Momeni M, Crucitti M, De Kock M. Patient-controlled analgesia in the management of postoperative pain. Drugs. 2006;66(18):2321–2337. | ||
Thomas V, Heath M, Rose D, Flory P. Psychological characteristics and the effectiveness of patient-controlled analgesia. Br J Anaesth. 1995;74(3):271–276. | ||
Lebovits AH, Zenetos P, O’Neill DK, et al. Satisfaction with epidural and intravenous patient-controlled analgesia. Pain Med. 2001;2(4):280–286. | ||
Schnabel A, Reichl SU, Meyer-Frießem C, Zahn PK, Pogatzki-Zahn E. Tramadol for postoperative pain treatment in children. Cochrane Database Syst Rev. 2015(3):CD009574. | ||
Cattabriga I, Pacini D, Lamazza G, et al. Intravenous paracetamol as adjunctive treatment for postoperative pain after cardiac surgery: a double blind randomized controlled trial. Eur J Cardiothorac Surg. 2007;32(3):527–531. | ||
Karanikolas M, Aretha D, Kiekkas P, Monantera G, Tsolakis I, Filos KS. Case report. Intravenous fentanyl patient-controlled analgesia for perioperative treatment of neuropathic/ischaemic pain in haemodialysis patients: a case series. J Clin Pharm Ther. 2010;35(5):603–608. | ||
Unlugenc H, Vardar MA, Tetiker S. A comparative study of the analgesic effect of patient-controlled morphine, pethidine, and tramadol for postoperative pain management after abdominal hysterectomy. Anesth Analg. 2008;106(1):309–312. | ||
Bailey PL, Streisand JB, East KA, et al. Differences in magnitude and duration of opioid-induced respiratory depression and analgesia with fentanyl and sufentanil. Anesth Analg. 1990;70(1):8–15. | ||
Lin CS, Lu G, Ruan LY, Gu MN. [Patient-controlled intravenous analgesia with sufentanil and fentanyl after thoracotomy: a comparative study]. Nan Fang Yi Ke Da Xue Xue Bao. 2006;26(2):240–241, 244. | ||
Idvall E, Berg K, Unosson M, Brudin L, Nilsson U. Assessment of recovery after day surgery using a modified version of quality of recovery-40. Acta Anaesthesiol Scand. 2009;53(5):673–677. | ||
Tanaka Y, Wakita T, Fukuhara S, et al. Validation of the Japanese version of the quality of recovery score QoR-40. J Anesth. 2011;25(4):509–515. | ||
Vercauteren MP, Mertens E, Schols G, Mol IV IV, Adriaensen HA. Patient-controlled extradural analgesia after caesarean section: a comparison between tramadol, sufentanil and a mixture of both. Eur J Pain. 1999;3(3):205–210. | ||
Srikandarajah S, Gilron I. Systematic review of movement-evoked pain versus pain at rest in postsurgical clinical trials and meta-analyses: a fundamental distinction requiring standardized measurement. Pain. 2011;152(8):1734–1739. | ||
Volpe DA, McMahon Tobin GA, Mellon RD, et al. Uniform assessment and ranking of opioid μ receptor binding constants for selected opioid drugs. Regul Toxicol Pharmacol. 2011;59(3):385–390. | ||
Cafiero T, Di Minno RM, Sivolella G, Di Iorio C. Immediate postoperative pain management in patients undergoing major abdominal surgery after remifentanil-based anesthesia: sufentanil vs tramadol. Minerva Anestesiol. 2004;70(9):661–669. | ||
Mitra S, Khandelwal P, Sehgal A. Diclofenac-tramadol vs. diclofenac-acetaminophen combinations for pain relief after caesarean section. Acta Anaesthesiol Scand. 2012;56(6):706–711. | ||
Practice Guidelines for Obstetric Anesthesia: An Updated Report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia and the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2016;124(2):270–300. | ||
Armstrong KP, Kennedy B, Watson JT, Morley-Forster PK, Yee I, Butler R. Epinephrine reduces the sedative side effects of epidural sufentanil for labour analgesia. Can J Anaesth. 2002;49(1):72–80. | ||
Kutlucan L, Seker İS, Demiraran Y, et al. Effects of different anesthesia protocols on lactation in the postpartum period. J Turk Ger Gynecol Assoc. 2014;15(4):233–238. | ||
Hirose M, Hara Y, Hosokawa T, Tanaka Y. The effect of postoperative analgesia with continuous epidural bupivacaine after cesarean section on the amount of breast feeding and infant weight gain. Anesth Analg. 1996;82(6):1166–1169. | ||
Cobb B, Liu R, Valentine E, Onuoha O. Breastfeeding after anesthesia: a Review for anesthesia providers regarding the transfer of medications into breast milk. Transl Perioper Pain Med. 2015;1(2):1–7. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.