Back to Journals » Drug Design, Development and Therapy » Volume 11
Comparison of neurotoxic potency between a novel chinbotulinumtoxinA with onabotulinumtoxinA, incobotulinumtoxinA and lanbotulinumtoxinA in rats
Authors Feng Y, Liu WC, Pan LZ , Jiang C, Zhang CX, Lu YX, Nie ZY, Jin LJ
Received 31 March 2017
Accepted for publication 31 May 2017
Published 28 June 2017 Volume 2017:11 Pages 1927—1939
DOI https://doi.org/10.2147/DDDT.S138489
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Ya Feng, Wuchao Liu, Lizhen Pan, Cong Jiang, Chengxi Zhang, Yuxuan Lu, Zhiyu Nie, Lingjing Jin
Department of Neurology, Shanghai Tongji Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China
Abstract: Four botulinumtoxin type A (BoNT/A) products, onabotulinumtoxinA (A/Ona), incobotulinumtoxinA (A/Inco), lanbotulinumtoxinA (A/Lan) and chinbotulinumtoxinA (A/Chin), are applied in the present study, among which A/Chin is newly produced. We aimed to compare the neurotoxic potency of these toxins by the gauge of muscle strength reduction. Furthermore, potential molecular and cellular mechanisms were also explored. According to our data, muscle strengths in the four toxin groups were all significantly decreased after injection for 1 week. A/Chin achieved the most obvious reduction in muscle strength as compared to the other three products at the dose of 0.5 U. However, there was no difference between the four toxins when increased to 2 U. As the toxins wore off, muscle strength recovered to basal level 12 weeks postinjection. We further measured the expression levels of key factors involved in neuromuscular junction stabilization and muscle genesis. Our results showed that nicotinic acetylcholine receptor, myogenic regulatory factors and muscle-specific receptor tyrosine kinase were all significantly upregulated upon BoNT/A treatment. Consistent with the result of muscle strength, A/Chin had the most obvious induction of gene expression. Moreover, we also found local inflammation response following BoNT/A injection. Owing to lack of complexing proteins, both A/Inco and A/Chin stimulated relatively lighter inflammation compared to that of A/Ona and A/Lan groups. In conclusion, our study provided evidence for the efficacy of the novel A/Chin and its similar functional mode to that of A/Ona, A/Inco and A/Lan. In addition, A/Chin has superiority in inducing muscle paralysis and inflammation stimulation, which may indicate faster onset and longer duration of this novel A/Chin.
Keywords: A/Chin, A/Lan, A/Ona, A/Inco, neurotoxic potency
Plain language summary
Several kinds of botulinumtoxin type A (BoNT/A) are currently available for clinical use with an increasing number of newly produced toxins. The current study was conducted to compare the neurotoxic potency of three commonly used toxins with a novel Chinese chinbotulinumtoxinA (A/Chin) that contains no complexing protein. We found that A/Chin has superiority in decreasing muscle strength and upregulating key factors involving neuromuscular junction (NMJ) stabilization. Furthermore, the role of A/Chin in stimulating inflammatory response was weaker than that of onabotulinumtoxinA (A/Ona) and lanbotulinumtoxinA (A/Lan). These results indicated the effectiveness of the novel A/Chin and the similar functional mode to the other three toxins. In addition, A/Chin has superiority in inducing muscle paralysis and inflammation stimulation. This provides evidence for the wide application of this newly produced A/Chin for clinical use in the near future.
Introduction
Botulinum toxins (BoNTs) are the most toxic exotoxins produced from the Gram-positive anaerobic bacterium Clostridium botulinum. Despite the hypertoxicity, they have been now extensively used to address not only cosmetic concerns but also medical specialties, including dystonia, spasm after stroke or spinal trauma, Raynaud’s syndrome and depression, with a satisfactory therapeutic efficacy.1,2 Seven different serotypes of BoNTs have been identified, but only serotypes A and B are commercially available for clinical use.3 Botulinumtoxin type A (BoNT/A) are di-chain toxins, comprising an N-terminal catalytic domain (LC) and a C-terminal heavy chain (HC), which includes a translocation domain (HN) and a receptor-binding domain (HC).4 Physiological activities of BoNTs comprise a consecutive process of binding to membrane receptors, translocation and LC-mediated proteolytic degradation of SNARE proteins, which results in local paresis and atrophy of targeted muscles.5
Attention should be paid to the fact that BoNT/As produced by different countries retain distinct potencies and various durations of effect after intramuscular administration. So far, three widely used BoNT/A formulations have been approved by both Health Canada and the US Food and Drug Administration (FDA) for many clinical applications.6 These preparations are onabotulinumtoxinA (A/Ona; Botox and Vistabel; both from Allergan Inc., Irvine, CA, USA), incobotulinumtoxinA (A/Inco; Xeomin and Bocouture; both from Merz Pharma, Frankfurt, Germany) and abobotulinumtoxinA (A/Abo; Dysport, Azzalure; both from Galderma Laboratories, Fort Worth, TX, USA).7 Besides, lanbotulinumtoxinA (A/Lan) produced by Lanzhou Institute of Biological Products of China has also become widely used in clinics. Regarding chinbotulinumtoxinA (A/Chin), it is a newly produced BoNT without complexing protein (CP). Auxiliary “non-toxic” CP surrounding the native protein might be a vital difference between toxins in addition to the different precise dose of native toxin each product contains.8 These BoNT/As are produced by different methods with unique characteristics, resulting in various effect strengths and possibly in effect duration.6,9 In addition, compensation discrepancy after injection of toxins may also exist among these different products.
Generally, clinical effects due to toxin application last for 3–6 months with gradual recovery of muscle function.10,11 Previous neuromuscular junction (NMJ) morphometry studies have demonstrated destabilization of NMJ following BoNT/A injection or muscle denervation.12 However, NMJs also retain a high degree of plasticity under conditions of nerve or muscle damage.13 The nicotinic acetylcholine receptor (nAChR) is an important component of NMJ. BoNT/A treatment has been shown to cause an increase in the expression level and spatial distributions of different subunits of nAChR.14 Muscle-specific receptor tyrosine kinase (MuSK) is a key protein that orchestrates nAChR clustering and differentiation of the NMJ.15 Myogenic regulatory factors (MRFs), including MyoD, MRF4 and myogenin, likely play an important role in the regulation of nAChR subunit expression and myogenesis.16,17 Shen et al18 identified that BoNT/A injection could significantly upregulate key molecules involved in NMJ stabilization and muscle functional recovery following BoNT/A administration. Consistent with previous studies, our previous studies also confirmed the induction of MuSK upon BoNT/A injection, which was involved in nerve sprouting and motor axon growth.19,20 As with any therapeutic protein, BoNT/As have the potential to induce immune response and local inflammation.21–23 Studies have demonstrated the anti-neurogenic inflammation role of BoNT/A in an arthritic rat model.24,25 However, the role of BoNT/A in inflammation after injection into normal animals remains to be unknown.
To better compare the toxic potency of each BoNT/A preparation, we applied a survey system (CN102599921A) comprising a fixing device, sensing means and a data handling equipment to evaluate nerve and muscle functions. Moreover, molecular and cellular mechanisms responsible for NMJ regeneration and skeletal muscle functional recovery were also explored.
Materials and methods
Animals
This study was approved by the Animal Care and Use Committee of Tongji University (approval number SYXK(hu) 2014-0026). Male Sprague Dawley rats (weighing 220–240 g, 3 months old) from B&K Universal Group Limited (Shanghai, China) were used. The animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No 85-23, revised 1996) and the Policy of Animal Care and Use Committee of Tongji University. Animals were fed with chow and water at the Animal Center of Tongji Hospital affiliated to Tongji University under controlled temperature (20°C–22°C) and a 12 h light/dark cycle.
Injections
Rats were randomly divided into five separate groups, ie, control (n=21), A/Ona (n=42), A/Inco (n=42), A/Lan (n=42) and A/Chin (n=42). Each BoNT/A preparation was reconstituted in saline (NS) for final concentrations of 0.5 U/100 μL and 2 U/100 μL. A volume of 100 μL BoNT/A was injected unilaterally into the right gastrocnemius muscle under anesthesia. The control group received an equivalent volume of NS injection.
Muscle strength determination
To quantify BoNT/A-induced focal muscle paralysis, a survey system (CN102599921A) composed of a fixing device, sensing means and a data handling equipment was used.19,20 Muscle strength alteration induced by as little as 0.01 U BoNT/A can be detected with this equipment. Rats were lightly anesthetized with an intraperitoneal injection of pentobarbital (30 mg/kg) and secured on a special adjustable operating table (CN202036227U) 1, 4 and 12 weeks after BoNT/A administration. Upon sciatic nerve stimulation (28 V over 0.4 ms), gastrocnemius contracted leading to plantar flexion and footboard rotation, which would then be converted to electrical signals through the muscular tension energy transducer and recorded by a specific computer.
Muscle mass determination
After administration of BoNT/A or NS for indicated time periods, body weight of each rat was measured before sacrificed. Then, the injected gastrocnemius muscle was dissected and weighed. The degree of injected muscle atrophy was expressed by the ratio of gastrocnemius to rat weight.
RNA extraction and analysis by quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA from each group of rats was isolated using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. All RNA samples were treated with DNase I (Sigma-Aldrich Co., St Louis, MO, USA), quantified and reverse transcribed into cDNA using the ReverTra Ace-α First Strand cDNA Synthesis Kit (TOYOBO CO., LTD. Life Science Department, OSAKA, Japan). qRT-PCR was conducted using a realplex4 RT-PCR detection system from Eppendorf Co Ltd (Hamburg, Germany), with SYBR-Green Real-Time PCR Master Mix (TOYOBO CO., LTD. Life Science Department) used as the detection dye. A comparative threshold cycle (Ct) was used to determine relative gene expression normalized to 18s rRNA. For each sample, the Ct values of the genes were normalized using the formula ΔCt = Ct_genes − Ct_18s rRNA. To determine relative expression level, the following formula was used: ΔΔCt = ΔCt_all_groups −ΔCt_blank control_group. The values used to plot relative expression of markers were calculated using the expression 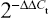 . The cDNA of each gene was amplified with primers as previously described. The main primer sequences of related genes are listed in Table 1.
. The cDNA of each gene was amplified with primers as previously described. The main primer sequences of related genes are listed in Table 1.
Immunoblotting
After specific treatment, total proteins were isolated with a mammalian cell lysis/extraction reagent (Sigma-Aldrich Co.) according to the manufacturer’s protocol. An equal amount of proteins were separated on the sodium dodecyl sulfate polyacrylamide gel electrophoresis gel and transferred to polyvinylidene fluoride membranes. After blocking, the membranes were incubated with specific primary antibodies at 4°C overnight. Then, the membranes were washed with tris-buffered saline with Tween-20 (TBST) for three times for 15 min each. After incubating with secondary antibodies for 45 min at 37°C and washing with TBST, an ECL kit (EMD Millipore, Billerica, MA, USA) was used to visualize membrane immunoreactivity. Quantification was performed using a computerized imaging program Quantity One (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Hematoxylin–eosin (HE) staining
After botulinum A or NS injection for the indicated time, rats were sacrificed by cervical dislocation to harvest the gastrocnemius for histological examination. The harvested tissues were fixed in a 4% formalin solution for 1 day and dehydrated using 20% and 30% sucrose in sequence. Then, all tissues were frozen in liquid nitrogen-cooled isopentane in optimal cutting temperature compound. The frozen sectioned muscle tissues were dyed and dipped in a Harris hematoxylin solution for 15 min and gently washed with running water for 10 min. The muscles were then dipped into eosin solution for 10 min, and the floating color was removed gently with running water. Next, gradient alcohol was used for dehydration, and the muscles were first submerged in xylene I for 15 min and then xylene II for another 15 min. Eventually, all the slides were mounted with neutral gum and observed under a microscope.
α-Bungarotoxin staining
α-Bungarotoxin is a polypeptide snake toxin that binds to nAChR with high affinity. Fluorescent conjugate of α-bungarotoxin can be used for fluorescence imaging of nAChRs at NMJ. Animals were perfusion fixed through the left ventricle with 4% paraformaldehyde. The right gastrocnemius was dissected and stored in fixative until used. Before staining, the fixed cross-section of gastrocnemius was rehydrated in PBS for 5 min at room temperature. Then, sections were blocked in immunofluorescence blocking buffer (Beyotime, Shanghai, People’s Republic of China) for 30 min at room temperature. Staining solution of 1 μg/mL α-bungarotoxin conjugated with tetramethylrhodamine (Biotium Inc., Fremont, CA, USA) in the immunofluorescence blocking buffer was used to incubate the sections in dark overnight at 4°C. After the incubation, the sections were rinsed three times in PBS for 10 min each. At last, all sections were mounted in a fluorescence anti-fade mounting medium with 4′,6-diamidino-2-phenylindole (DAPI). Fluorescent pictures were taken using Nikon instruments.
Statistical analysis
All data were expressed as the mean value ± standard deviation from three independent experiments. Statistical significance was determined by Student’s t-test. All data analyses were performed using SPSS version 11.5 (SPSS Inc., Chicago, IL, USA).
Results
Effect of BoNT/A on inducing muscle paresis
In the present study, we applied a survey system detecting muscle strength to evaluate the potency of four BoNT/A preparations (Figure 1). We found that muscle strength of all four toxin groups decreased significantly comparing to rats injected with NS 1 week postinjection, which indicated the presence of clinical muscle paresis. It further decreased at the dose of 2 U than 0.5 U, suggesting a stronger effect with a larger dose. Compared with the other three toxins, muscle strength of the A/Chin group was much lower at 0.5 U. Yet, no significant difference was observed among the four products at 2 U, implying that 2 U might be the saturation dosage. When prolonged to 4 weeks, muscle strength displayed a similar trend as that of 1 week. This difference of muscle strength gradually diminished 12 weeks after injection when muscle strength recovered to its baseline level.
By contrast, muscle strength in the NS group increased due to the gradual growth of rat. This implies that neither injection procedure itself nor the intramuscularly injected fluid affects muscle strength.
Induction of muscle atrophy after BoNT/A injection
In addition to the effect of BoNT/A on muscle strength, we further explored its role on muscle morphology. As shown in Figure 2A, we found the ratio of targeted muscle mass to body weight decreased significantly upon injection of toxins for 1 week compared to that of limbs injected with NS. This decrease was much more obvious 4 weeks postinjection than that of 1 week. It is worth noting that the ratio of A/Inco group presented a similar reduction degree to A/Chin. This was consistent with previous studies that A/Inco was also a kind of BoNT without CP,26,27 which might imply a similar toxic potency. The other two toxins A/Ona and A/Lan exerted a relatively weaker effect on muscle mass. With wear-off effect of BoNT/A and a gradual increase in body weight, there was no difference in the ratio between 0.5 U groups and NS group 12 weeks postinjection, but difference still existed between 2 U groups and the control group.
Accordingly, morphology of gastrocnemius muscle shown in Figure 2B confirmed the data mentioned above that BoNT/A injection could induce muscle atrophy much more obvious 4 weeks postinjection. Muscles injected with A/Chin and A/Inco displayed a similar extent atrophy as compared to the other two kinds of toxins. Moreover, HE staining confirmed the result that nucleus of atrophied muscle fibers was clearly stained in A/Chin and A/Inco groups. No obvious difference was observed between A/Ona and A/Lan groups (Figure 2C).
AChR expression profile following BoNT/A injection
AChR plays an important role in NMJ formation and regeneration.28 In this study, we measured the expression of AChR-α after BoNT/A injection. All four BoNT/As significantly upregulated AChR-α expression, reaching almost 50-fold or >1 week after injection. This increase in the AChR-α mRNA level was further enhanced at 2 U groups than 0.5 U groups. Notably, A/Chin induced a considerable increase in AChR-α expression at both dosages compared to A/Ona, A/Inco and A/Lan. There was no significant difference between the other three toxins. This increase in the AChR-α mRNA level reduced to 10–20-folds 4 weeks postinjection with less difference between the four toxins than that of 1 week postinjection. No significant difference was observed 12 weeks after injection (Figure 3A).
We further applied α-bungarotoxin staining that could bind to AChR with high specificity to further confirm the distinct induction profiles between different toxins. As shown in Figure 3B, red fluorescence indicated the presence of AChR. We found that the number and volume of AChR increased significantly after BoNT/A injection at 2 U for 1 week.
BoNT/A injection–induced MRF4 expression
MRF4 is a member of MRFs vital for the regulation of nAChR expression.16 From Figure 4, we find that the mRNA level of MRF4 increased significantly 1 week after BoNT/A injection with the A/Chin group displaying the most obvious induction both at 0.5 and 2 U. This was in accordance with the expression profile of nAChR. However, the mRNA level of MRF4 had a slight increase to ~15-fold change 12 weeks after BoNT/A injection as compared to that of 4 weeks after injection. This discrepancy might explain the late-stage effect of BoNT/As.
BoNT/A injection–induced MuSK expression
Upon BoNT/A injection for 1 week, the mRNA level of MuSK gene increased significantly, most of which reached 20–40-fold compared to the NS group. Yet, there was no difference between all toxin groups at the dose of 0.5 U. When increased to 2 U, the A/Chin group induced a higher expression than the other three groups. The mRNA level of MuSK diminished when extended to 4 weeks postinjection, and no increase in the MuSK mRNA level was observed at 12 weeks after injection of toxins (Figure 5).
Effects of BoNT/A injection on muscle inflammation
Inflammation response after BoNT/A administration might be a vital factor contributing to therapy failure. Thus, we further evaluated the expression patterns of proinflammatory factors following BoNT/A injection. As shown in Figure 6A, all four toxins led to an obvious increase in the interleukin (IL)-1β mRNA level but with different profiles. This effect had a slight increase 4 weeks after BoNT/A treatment. Yet, it decreased sharply 12 weeks postinjection, but still showed approximately fivefold increase than the NS group. Among all toxin groups, the effect of A/Chin on inflammation response was relatively smooth (Figure 6A). A/Ona and A/Inco showed no obvious difference in stimulating IL-1β expression. In addition, we found that only the A/Lan group showed a significant difference in inducing IL-1β expression when compared to the A/Chin group at 1 week postinjection. Lack of CP may account for this difference. No difference was observed between the four toxins in inducing IL-1β expression for 12 weeks after the injection.
Similarly, mRNA levels of IL-6 after BoNT/A injection presented an analogous trend of increase as that of IL-1β. What made it different from IL-1β was the smaller degree of induction of IL-6 at 12 weeks postinjection (Figure 6B).
Different from proinflammatory factors IL-1β and IL-6, the mRNA level of tumor necrosis factor (TNF)-α had a slight increase that displayed no more than 10-fold change. In addition, A/Chin had the least effect on induction of TNF-α expression comparing to A/Ona, A/Inco and A/Lan (Figure 6C).
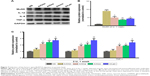
Induction of key factors upon BoNT/A injection on protein level
To further confirm different induction profiles of key factors of the four preparations, we applied immunoblotting to measure the induction of key factors following injection of toxins. From Figure 7, we can find that the protein level of MuSK was significantly increased after BoNT/A treatment for 1 week at the dose of 2 U in comparison to that of the NS group. A/Chin induced the most obvious increase in the MuSK expression. As to proinflammatory factors, IL-1β, IL-6 and TNF-α showed a similar increase trend, among which the TNF-α mRNA level had the slightest induction. For all four toxins, A/Chin had a lower potency in stimulating local inflammatory response compared to the other three types.
Discussion
In the present study, we first made a comparison of physical characteristics between A/Chin, A/Lan, A/Ona and A/Inco (Table 2). In our experiment, we found that four BoNT/A preparations (A/Chin, A/Lan, A/Ona and A/Inco) induced different degrees of muscle paresis in vivo. The neurotoxic potency of A/Chin was higher than that of the other three kind of toxins in decreasing muscle strength at the dose of 0.5 U. There was no significant difference between the four kinds of toxins at 2 U 1 and 4 weeks postinjection in inducing muscle paresis. Based on this, we further explored the possible compensation mechanisms counteracting the effects of toxins. Our data confirmed that key factors involved in NMJ formation and stabilization were apparently upregulated, which indicated reversely the toxic potency of four different preparations. Besides, proinflammatory factors such as IL-1β, IL-6 and TNF-α were all induced with different profiles after injection of toxins. To our knowledge, this was the first study to compare these four kinds of toxins in vivo.
Generally, the onset of BoNT/A effect relates closely to the volume of toxin injected, with a faster onset upon a larger volume.29,30 Thus, the toxin will reach more motor endplates and induce a stronger paresis due to a broader intramuscular diffusion. For this reason, we standardized the injected volume of the four products (100 μL into each gastrocnemius) to enable the accuracy of neurotoxic potency. Despite the un-interchangeable characteristic of different toxins, we set two distinct doses, 0.5 and 2 U, for each kind of toxin and explored diverse toxic potencies of the four toxins. The reason was that the unit is a universally recognized standard to describe doses of different BoNTs. It is also widely used in clinics to state the amount of toxins patients use. Besides, to quantify the extent of effect toxins can exert on inducing muscle paralysis, we applied a survey system invented by our team to measure affected muscle strength following injection of toxins.
According to our results, the novel A/Chin also induced decrease in muscle strength, indicating its effectiveness in inducing muscle paralysis. Meanwhile, it had the similar function mode to the other three kinds of toxins whose effect duration was ~3 months. Consistent with previous studies, our present study also confirmed the strongest effect of toxins 1 week postinjection and the gradual recovery of muscle function to base level after injection for 12 weeks.19,31 This was in line with clinical data that effective duration of BoNT/A would range from ~3 to 6 months. Gradually, wear-off of toxins and recovery of muscle function might imply that some compensative mechanisms exist to counteract the action of toxins upon injection. Studies have confirmed that BoNT/A injection could effectively induce muscle paralysis, followed by a profile of gene transcription changes leading to muscle recovery.28,32 Two stages may be involved in this rehabilitation process: an early aneural stage and a later neural stage. The first stage refers to the time period from toxin administration to ~2–3 weeks, which is muscle intrinsic and nerve independent. In this aneural stage, induced MRF4 unregulates AChR expression, and MuSK contributes to the early formation of postsynaptic endplates. Approximately 4–6 weeks after injection of toxins, the second neural stage begins with the wear-off of BoNT/A effect. In this stage, reinnervation and myogenesis gradually occur. Factors released by motor neurons refine the initial AChR clusters and NMJ formation. This leads to NMJ stabilization and muscle functional recovery ~3–6 months after injection of toxins.28 In the current study, we also confirmed the significant induction of AChR expression after toxin treatment. Compared to other toxins, the A/Chin group showed the most obvious increase in AChR induction, implying the highest toxin potency of A/Chin. Similar conclusion could be drawn from the results of MRF and MuSK, providing evidence for the high potency of A/Chin.
Additionally, we also measured effect of BoNT/A on inflammation. We proved in vivo that BoNT/A treatment could stimulate inflammation response in all four toxin products with different extent profiles. Inflammation induction occurring in our experiment may result from long-time paralysis of affected muscles after BoNT/A injection. Previous studies have demonstrated that CP around the native neurotoxins could induce immune response and inflammation stimulation.33,34 As intended to be, A/Chin exerted the least effect on inflammatory response. Studies have demonstrated that one limitation of BoNT/A products in treating diseases is the presence of a high protein load that may increase antigenicity. Lack of CPs and the highly purified characteristic make it possible for A/Chin to confer a lower risk of immunogenicity. This may explain the phenomenon of the relatively less effect of A/Chin on inflammatory stimulation.35 Yet, induction of inflammation by BoNT/A was opposed to what was reported previously. In recent years, BoNT/A has been approved for treating arthritic pain, which might be related to its anti-neurogenic inflammation role.36,37 This discrepancy may result from the different conditions under which toxins are applied. In this study, we evaluated the effect of BoNT/A on inflammation under normal state that differs from pathological states as reported. There may exist immense difference between animals of disease models and normal animals reacting to noxious or non-noxious stimuli. This difference in rats themselves can be a reason behind their divergent responses after the same treatment, which needs more researches.
Conclusion
We established that BoNT/A produced by different manufacturers achieved different toxic potencies and a newly produced toxin A/Chin displayed a similar function mode to A/Inco, A/Ona and A/Lan. Some limitations existed in this study, including lack of a precise dose equivalence ratio and the difference between physiological and pathological states. The current study does provide experimental evidences for effectiveness and low antigenicity of the newly produced toxin A/Chin. These data can lay a solid foundation for the wide application of A/Chin in the near future.
Acknowledgments
This study is supported by the National Natural Science Foundation of China (81470582 and 81500970), Shuguang Program (14SG21) and Talent Development Foundation of Putuo district (2014-A-21). The authors highly appreciate Lanzhou Institute of Biological Products of China for provision of A/Chin and A/Lan. The authors thank Professor Dressler and Professor Xueping Zhang for the revision of the manuscript, and all the members of the Department of Neurology, Shanghai Tongji Hospital and Tongji University School of Medicine for their help.
Disclosure
The authors report no conflicts of interest in this work.
References
Dressler D, Adib Saberi F. Botulinum toxin in myotonia congenita: it does not help against rigidity and pain. J Neural Transm (Vienna). 2014;121(5):531–532. | ||
Wanitphakdeedecha R, Ungaksornpairote C, Kaewkes A, Sathaworawong A, Lektrakul N, Manuskiatti W. The efficacy of 2 formulations of botulinum toxin type A for masseter reduction: a split-face comparison study. J Dermatolog Treat. 2016;6:1–4. | ||
Setler PE. Therapeutic use of botulinum toxins: background and history. Clin J Pain. 2002;18(6 suppl):S119–S124. | ||
Kroken AR, Blum FC, Zuverink M, Barbieri JT. Entry of botulinum neurotoxin subtypes A1 and A2 into neurons. Infect Immun. 2016;85(1):e795–e716. | ||
Weingart OG, Loessner MJ. Nerve cell-mimicking liposomes as biosensor for botulinum neurotoxin complete physiological activity. Toxicol Appl Pharmacol. 2016;313:16–23. | ||
Chen JJ, Dashtipour K. Abo-, inco-, ona-, and rima-botulinum toxins in clinical therapy: a primer. Pharmacotherapy. 2013;33(3):304–318. | ||
Bonaparte JP, Ellis D, Quinn JG, Rabski J, Hutton B. A comparative assessment of three formulations of botulinum toxin type A for facial rhytides: a systematic review with meta-analyses. Plast Reconstr Surg. 2016;137(4):1125–1140. | ||
Miyashita S, Sagane Y, Suzuki T, Matsumoto T, Niwa K, Watanabe T. “Non-toxic” proteins of the botulinum toxin complex exert in-vivo toxicity. Sci Rep. 2016;6:31043. | ||
Frevert J. Content of botulinum neurotoxin in botox(R)/vistabel(R), dysport(R)/azzalure(R), and xeomin(R)/bocouture(R). Drugs R D. 2010;10(2):67–73. | ||
Ma J, Elsaidi GA, Smith TL, et al. Time course of recovery of juvenile skeletal muscle after botulinum toxin A injection: an animal model study. Am J Phys Med Rehabil. 2004;83(10):774–780; quiz 781–783. | ||
Mehlan J, Brosig H, Schmitt O, Mix E, Wree A, Hawlitschka A. Intrastriatal injection of botulinum neurotoxin-A is not cytotoxic in rat brain – a histological and stereological analysis. Brain Res. 2016;1630:18–24. | ||
Alderson K, Holds JB, Anderson RL. Botulinum-induced alteration of nerve-muscle interactions in the human orbicularis oculi following treatment for blepharospasm. Neurology. 1991;41(11):1800–1805. | ||
Santos AF, Caroni P. Assembly, plasticity and selective vulnerability to disease of mouse neuromuscular junctions. J Neurocytol. 2003;32(5–8):849–862. | ||
Witzemann V, Brenner HR, Sakmann B. Neural factors regulate AChR subunit mRNAs at rat neuromuscular synapses. J Cell Biol. 1991;114(1):125–141. | ||
DeChiara TM, Bowen DC, Valenzuela DM, et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85(4):501–512. | ||
Charbonnier F, Della Gaspara B, Armand AS, et al. Specific activation of the acetylcholine receptor subunit genes by MyoD family proteins. J Biol Chem. 2003;278(35):33169–33174. | ||
Weintraub H, Davis R, Tapscott S, et al. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991;251(4995):761–766. | ||
Shen J, Ma J, Elsaidi GA, et al. Gene expression of myogenic regulatory factors following intramuscular injection of botulinum A toxin in juvenile rats. Neurosci Lett. 2005;381(3):207–210. | ||
Guo Y, Pan L, Liu W, Pan Y, Nie Z, Jin L. Polyclonal neural cell adhesion molecule antibody prolongs the effective duration time of botulinum toxin in decreasing muscle strength. Neurol Sci. 2015;36(11):2019–2025. | ||
Jin L, Pan L, Liu W, et al. IGF-1 antibody prolongs the effective duration time of botulinum toxin in decreasing muscle strength. Int J Mol Sci. 2013;14(5):9051–9061. | ||
Dressler D. Five-year experience with incobotulinumtoxinA (Xeomin((R))): the first botulinum toxin drug free of complexing proteins. Eur J Neurol. 2012;19(3):385–389. | ||
Bryant AM, Cai S, Singh BR. Comparative immunochemical characteristics of botulinum neurotoxin type A and its associated proteins. Toxicon. 2013;72:126–132. | ||
Dressler D, Hallett M. Immunological aspects of botox, dysport and myobloc/neurobloc. Eur J Neurol. 2006;13(suppl 1):11–15. | ||
Kaneguchi A, Ozawa J, Moriyama H, Yamaoka K. Nociception contributes to the formation of myogenic contracture in the early phase of adjuvant-induced arthritis in a rat knee. J Orthop Res. 0000. Epub 2016 Sep 1. | ||
Egloff C, Hart DA, Hewitt C, Vavken P, Valderrabano V, Herzog W. Joint instability leads to long-term alterations to knee synovium and osteoarthritis in a rabbit model. Osteoarthritis Cartilage. 2016;24(6):1054–1060. | ||
Santamato A, Micello MF, Panza F, et al. Safety and efficacy of incobotulinum toxin type A (NT 201-xeomin) for the treatment of post-stroke lower limb spasticity: a prospective open-label study. Eur J Phys Rehabil Med. 2013;49(4):483–489. | ||
Lee JH, Park JH, Lee SK, et al. Efficacy and safety of incobotulinum toxin A in periocular rhytides and masseteric hypertrophy: side-by-side comparison with onabotulinum toxin A. J Dermatolog Treat. 2014;25(4):326–330. | ||
Shen J, Ma J, Lee C, et al. How muscles recover from paresis and atrophy after intramuscular injection of botulinum toxin A: study in juvenile rats. J Orthop Res. 2006;24(5):1128–1135. | ||
Kutschenko A, Manig A, Reinert MC, Monnich A, Liebetanz D. In-vivo comparison of the neurotoxic potencies of incobotulinumtoxinA, onabotulinumtoxinA, and abobotulinumtoxinA. Neurosci Lett. 2016;627:216–221. | ||
Kim HS, Hwang JH, Jeong ST, et al. Effect of muscle activity and botulinum toxin dilution volume on muscle paralysis. Dev Med Child Neurol. 2003;45(3):200–206. | ||
Patil S, Willett O, Thompkins T, et al. Botulinum toxin: pharmacology and therapeutic roles in pain states. Curr Pain Headache Rep. 2016;20(3):15. | ||
Tsai SW, Chen HL, Chang YC, Chen CM. Molecular mechanisms of treadmill therapy on neuromuscular atrophy induced via botulinum toxin A. Neural Plast. 2013;2013:593271. | ||
Lee JC, Yokota K, Arimitsu H, et al. Production of anti-neurotoxin antibody is enhanced by two subcomponents, HA1 and HA3b, of Clostridium botulinum type B 16S toxin-haemagglutinin. Microbiology. 2005;151(pt 11):3739–3747. | ||
Kukreja R, Chang TW, Cai S, et al. Immunological characterization of the subunits of type A botulinum neurotoxin and different components of its associated proteins. Toxicon. 2009;53(6):616–624. | ||
Lamb YN, Scott LJ. IncobotulinumtoxinA: a review in upper limb spasticity. Drugs. 2016;76(14):1373–1379. | ||
Li T, Wang L, Wang K, et al. Intra-articular injection of botulinum toxin A reduces neurogenic inflammation in CFA-induced arthritic rat model. Toxicon. 2016;126:70–78. | ||
Wanitphakdeedecha R, Ungaksornpairote C, Kaewkes A, Rojanavanich V, Phothong W, Manuskiatti W. The comparison between intradermal injection of abobotulinumtoxinA and normal saline for face-lifting: a split-face randomized controlled trial. J Cosmet Dermatol. 2016;15(4):452–457. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.