Back to Journals » OncoTargets and Therapy » Volume 12
Comparison of neoadjuvant therapy and upfront surgery in resectable pancreatic cancer: a meta-analysis and systematic review
Authors Ren X, Wei X, Ding Y, Qi F , Zhang Y, Hu X, Qin C, Li X
Received 13 October 2018
Accepted for publication 21 December 2018
Published 22 January 2019 Volume 2019:12 Pages 733—744
DOI https://doi.org/10.2147/OTT.S190810
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Xiaohan Ren,1 Xiyi Wei,1,* Yichao Ding,1,* Feng Qi,2,* Yundi Zhang,1 Xin Hu,1 Chao Qin,2 Xiao Li3
1Department of First Clinical Medical College of Nanjing Medical University, Nanjing, Jiangsu 210009, China; 2Department of Urology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu 210009, China; 3Department of Urology, Jiangsu Institute of Cancer Research, Affiliated Cancer Hospital of Nanjing Medical University, Nanjing, Jiangsu 210009, China
*These authors contributed equally to this work
Objective: The role of neoadjuvant therapy (NAT) in resectable pancreatic cancer (RPC) remains controversial. Therefore, this meta-analysis was performed to compare the clinical differences between NAT and upfront surgery in RPC.
Materials and methods: A systematic literature search was performed in PubMed, Embase, Web of Science, and the Cochrane Register of Controlled Trials databases. Only patients with RPC who underwent tumor resection and received adjuvant or neoadjuvant treatment were enrolled. The OR or HR and 95% CIs were calculated employing fixed-effects or random-effects models. The HR and its 95% CI were extracted from each article that provided survival curve. Publication bias was estimated using funnel plots and Egger’s regression test.
Results: In total, eleven studies were included with 9,386 patients. Of these patients, 2,508 (26.7%) received NAT. For patients with RPC, NAT resulted in an increased R0 resection rate (OR=1.89; 95% CI=1.26–2.83) and a reduced positive lymph node rate (OR=0.34; 95% CI=0.31–0.37) compared with upfront surgery. Nevertheless, patients receiving NAT did not exhibit a significantly increased overall survival (OS) time (HR=0.91; 95% CI=0.79–1.05).
Conclusion: In patients with RPC, R0 resection rate and positive lymph node rate after NAT were superior to those of patients with upfront surgery. The NAT group exhibited no significant effect on OS time when compared with the upfront surgery group. However, this conclusion requires more clinical evidence to improve its credibility.
Keywords: neoadjuvant therapy, resectable, pancreatic, neoplasm, prognosis, meta-analysis
Introduction
With an extremely poor prognosis and low resection rate, pancreatic cancer (PC) ranks the fourth most common cause of cancer-related deaths in the United States, and the fifth in Europe.1 PC starts when a cell in the pancreas gains genetic changes, allowing it to grow uncontrollably. Surgical resection is the only curative strategy for PC. For patients with localized disease, radical surgery may offer long-term benefits.2 However, even in patients who undergo resection, the 5-year survival rate remains only 7%–24%, and the cumulative rate of recurrence remains up to 85%, indicating that surgery alone is typically inadequate.3,4
For PC, the current standard of treatment is surgical resection with adjuvant chemotherapy.5,6 Some landmark experiments suggested the positive significance of adjuvant therapy.7,8 However, 20%–30% of patients failed to receive designated treatment due to postoperative complications, delayed recovery after surgery, patient rejection, comorbidities, or early disease recurrence, which is a major drawback of adjuvant therapy for PC.5,7,9 For borderline resectable PC, direct surgery may result in positive margins: R1 (microscopic margin) or R2 (positive margin). In recent years, neoadjuvant therapy (NAT) strategies have been increasingly employed for borderline resectable and resectable tumors.10–12 Previous studies demonstrated that NAT might improve the R0 (negative margin) resection rate and improve prognosis in borderline resectable PC (RPC).13,14
However, for RPC, no consensus has been achieved at present concerning whether it may improve the prognosis through NAT. Accordingly, we performed this study to compare the differences in overall survival (OS) time, R0 resection rate, and positive lymph nodes rate between patients who received NAT and those with upfront surgery.
Materials and methods
This meta-analysis followed the PRISMA guidelines.15
Literature search
The literature was reviewed systematically by searching PubMed, Embase, Web of Science, and the Cochrane Register of Controlled Trials databases from inception until September 2018. The search strategy in PubMed included the following domains of Medical Subject Heading terms: “pancreatic neoplasm”, “neoadjuvant”, and “resectable”. These terms were combined with “AND” or “OR”, which were provided in Table S2 (supporting information). Searched documents were not subject to publication time limitations. Embase, Web of Science, and Cochrane Library searches were completed with the authors’ own terminology.
Inclusion and exclusion criteria
Studies included patients with RPC either treated by upfront surgery or NAT. Two authors independently assessed the included observational studies. Studies exclusion criteria were as follows: 1) patients without surgery were included in the study; 2) the study did not contain a control group; 3) for outcome indicators, the number of patients was unclear; and 4) the study included patients with marginally resectable or locally advanced PC.
Data extraction and quality assessment
Two authors independently evaluated the title and abstract of the primary selection and then conducted a full-text screening. Disagreements were resolved via discussion. R0 resection rate, positive lymph node rate, and survival data were extracted in each study. The HR and its 95% CI were extracted from the survival curve provided in the article with Engauge Digitizer 4.1 software (Markmitch, Boston, MA, USA).16
Quality assessment of each article was performed using the Newcastle–Ottawa scale (NOS).17 The NOS allows for the evaluation of methods of patient selection, comparability of study groups, and reporting of important outcomes. Details are available in Table S1 (supporting information).
Statistical analyses
Analyses were performed using STATA version 12.0 (Stata Corporation, College Station, TX, USA) software. All P-values were two sided, and P<0.05 was regarded as significant. The results of individual studies were summarized. The estimates were calculated using fixed-effects or random-effects models according to the heterogeneity, which was reported using the Cochrane’s Q-test18 and the inconsistency index value (I²).19 Publication bias was judged using funnel plot and Egger’s test.
Results
Characteristics of included studies
A total of eleven studies were included in the meta-analysis.20–30 The characteristics of the patients who received NAT and upfront surgery are presented in Tables 1 and 2, respectively. The upfront surgery group was regarded as the control group. Figure 1 presents the flowchart of the literature search and selection process. Age was the only available risk factor among the patients involved. However, no significant difference in age was observed between the NAT group and the upfront surgery group. The results of NOS analysis indicated that the studies we have included were of high quality.
  | Table 2 Characteristics of the preoperative group included in the meta-analysis |
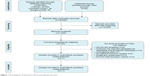  | Figure 1 Flow diagram of literature search and selection process. |
Meta-analysis results
The main results of our meta-analysis are presented in Table 3. Eleven studies involving 9,388 patients were assessed in the comparison of the R0 resection rate. As indicated in Figure 2, patients receiving NAT before surgery had a better R0 resection rate compared with patients receiving upfront surgery (nine trials; 9,388 patients; OR=1.89; 95% CI=1.26–2.83), especially in the gemcitabine (Gem)-based + RT group (four trials; 757 patients; OR=3.59; 95% CI=2.08–6.21). In the 5-fluorouracil (5-Fu)-based + RT group, the NAT group did not exhibit an obvious advantage (three trials; 487 patients; OR=1.36; 95% CI=0.67–2.75). In the neoadjuvant chemotherapy (NCT) group, patients receiving chemotherapy had a higher R0 resection rate (two trials; 8,144 patients; OR=1.50; 95% CI=1.32–1.71).
  | Figure 2 Forest plots of R0 resection rate. |
Regarding the positive lymph node rate (Figure 3), the NAT group exhibited a remarkably reduced rate compared with the upfront surgery group (eleven trials; 9,386 patients; OR=0.34; 95% CI=0.31–0.37). In the subgroup analysis for positive lymph nodes, the Gem-based + RT group (five trials; 803 patients; OR=0.34; 95% CI=0.25–0.47), 5-Fu-based + RT group (four trials; 529 patients; OR=0.22; 95% CI=0.14–0.33), and the NCT group (two trials; 8,054 patients; OR=0.35; 95% CI=0.31–0.38) were associated with lower positive lymph node rates.
  | Figure 3 Forest plots of positive lymph nodes. |
In the eleven studies included above, a total of seven studies (seven trials; 1,012 patients) provided either survival curves or HR and its 95% CI. The HR sum of survival for NAT compared with the upfront surgery group with a pooled HR of 0.91 (95% CI=0.79–1.05) indicated no significant survival advantage for NAT. In the subgroup analysis for OS time (Figure 4), the Gem-based + RT group (three trials; 280 patients; HR=0.88; 95% CI=0.69–1.12) and the 5-Fu-based + RT group (four trials; 738 patients; HR=0.93; 95% CI=0.78–1.11) consistently exhibited insignificant advantages in OS time.
  | Figure 4 Forest plots of OS time. |
Test of heterogeneity
For the comparison of the R0 resection rate, moderate heterogeneity was observed. However, heterogeneity decreased when subgroup analyses were conducted based on neoadjuvant treatment methods. In addition, no significant heterogeneity was observed in overall and subgroup comparisons for positive lymph node rate analysis and OS analysis.
Sensitivity analysis
Sensitivity analysis was utilized to detect the influence of each study by repeating the meta-analysis while omitting one study each time. Figures 5A and 6A revealed that the results were reliable given that no individual study affected the pooled OR or HR significantly.
  | Figure 5 Sensitivity analysis and funnel plot of the R0 resection rate. |
  | Figure 6 Sensitivity analysis and funnel plot of OS. |
Publication bias
Figure 5B indicates that no significant publication bias for the R0 resection rate was observed, and this finding was confirmed by Egger’s test (P=0.346). Moreover, the positive lymph node rate (Figure 7) did not exhibit publication bias (Egger’s test: P=0.122). For the OS time comparison (Figure 6B), no significant publication bias was detected (Egger’s test: P=0.707).
  | Figure 7 Egger’s funnel plot for publication bias test of positive lymph nodes. |
Discussion
PC is classified as resectable, marginally resectable, or locally advanced.31,32 However, to date, the only potentially curative technique for managing PC is surgical resection with radiotherapy and/or chemotherapy regimens, which may improve disease-free and OS time.33 At present, NAT is increasingly used in various types of PCs given its benefits for vascularization improvement, early treatment of micrometastasis, and potential downgrading effect on borderline resectable PC.34 Some previous analyses of PC revealed that patients receiving NAT exhibited a longer survival time.35,36 However, most of these studies only analyzed marginal resectable or locally advanced PC, which exhibited a poor prognosis if only upfront surgery treatment was administered. NAT is considered safe for resectable PC,37 and neoadjuvant therapy with multiple drugs is usually superior to monotherapy.38 Thus, new adjuvant therapies are increasingly being applied for RPC. The transition from upfront surgical to a systemic approach in the form of NAT is being investigated for the treatment of RPC.39,40 The driving force behind this shift is the growing recognition of the systematic early origins of the disease.41 However, previous studies on NAT for RPC were limited, and the results were inconclusive. For example, one study reported no difference in OS time between the NAT group and the upfront surgery group.42 However, some studies demonstrated that the survival rate of the NAT group was significantly improved compared with the upfront surgery group.21,30
Meta-analysis can provide more reliable results compared with a single study and serves as a powerful tool to explain controversial conclusions. For this reason, we performed a meta-analysis to clarify whether NAT has significant benefits for RPC compared with upfront surgery. This meta-analysis was not the first meta-analysis that focused on the effect of NAT on RPC. However, we included newer and higher-quality studies and obtained a larger sample size to arrive at a more accurate conclusion. As a result, the comparative analysis between the NAT group and upfront surgery group revealed that NAT played a positive role on R0 resection rate and positive lymph node rate. This finding was consistent with our expectations.35,43 However, the benefits of NAT for OS time were statistically insignificant. In the studies we included, 73.6% of the patients who were judged resectable were resected after NAT. This rate was similar to published resection rates of 78%–96%.44
Currently, various NAT methods have been used in clinical practice.20–30 Most studies supported the use of Gem or 5-Fu in the neoadjuvant setting. In fact, different neoadjuvant settings may lead to different clinical outcomes, suggesting that the prognostic indicators of PC can vary notably due to methodological differences. As a result, stratified analysis was performed based on neoadjuvant therapy methods. Regarding the R0 resection rate, the NCT group without radiation therapy also had a higher R0 resection rate, indicating the significant role of chemotherapy drugs in resectable PC. In fact, for resectable PC, neoadjuvant radiotherapy has often been used in conjunction with chemotherapy to improve marginal negative resection rates.45 In the subgroup analysis with radiation therapy, the Gem-based + RT group exhibited more advantages compared with the 5-Fu-based + RT group. There are several possible mechanisms to explain this result. First, Gem has potent radiosensitizing properties that are critical in a disease with a propensity for positive surgical margins and local recurrence.46 Second, compared with 5-Fu, the use of Gem as a radiosensitizer generates greater tumor cell killing. Moreover, Gem provides superior systemic treatment of micrometastases.10
After surgical resection, ~80% of patients exhibited a potential risk of extrapancreatic metastasis and required adjuvant therapy. Indeed, the surgical approach and postoperative recovery process may lead to a delayed or reduced dose of radiotherapy and chemotherapy, thus objectively delaying adjuvant therapy. Previous studies demonstrated that NAT may lead to a more favorable regression of lymph node metastasis compared with adjuvant therapy.47 At the time of surgery, patients who received NAT had smaller tumor sizes. Although the specific mechanism is unclear, the positive lymph node rate was significantly reduced in the NAT group in most studies.20,21,23 The results of our study were consistent with previous studies, which further showed that NAT played an important role in positive lymph nodes. For RPC, neoadjuvant chemoradiation may exhibit a reduced positive lymph node rate compared with neoadjuvant chemotherapy, and the addition of radiation therapy may improve the quality of NAT.48 However, further studies are needed to determine whether the difference is statistically significant. Moreover, the addition of radiotherapy may increase costs.48
Overall, survival time is one of the most prognostic indicators for survival in RPC patients. Differences in neoadjuvant settings exhibit different impacts on prognosis. Finally, the results of our meta-analysis for RPC demonstrated similar OS values for the Gem-based + RT group and 5-Fu-based + RT group compared to the upfront surgery group. These effects may be attributed to several possible mechanisms. First, compared with marginal resectable or locally advanced PC, RPC exhibits better tissue and cell characteristics, which may explain why NAT did not have significant benefits for RPC. In addition, patients exclusively treated with upfront surgery generally received additional adjuvant therapy at a later date. However, a small number of patients refused to receive adjuvant therapy or terminated it ahead of schedule for various reasons, which may make the associations unreliable.
Furthermore, despite the overall robust statistical evidence generated through this analysis, some limitations have been identified. First, among the studies we included, only three studies were randomized controlled trials with a total of 165 patients. Currently, the number of completed RCT is insufficient, which may be due to the slow recruitment of patients and patient choice. Moreover, it is difficult for multicenter experimental institutions to reach a unified opinion given the diversity of neoadjuvant treatment options. As a result, the small number of RCT studies is inevitable to some extent. Therefore, more studies are required to provide more clear conclusions. Second, in addition to age, other information about the included population, such as gender and lifestyle, was not available in most studies. Moreover, as a multifactorial disease, PC results from a combination of various complicated factors, including a variety of genetic and environmental factors. Therefore, if all patient data had been available, the results would have been more accurate.
Conclusion
The results of the present meta-analysis suggested that NAT can increase the R0 resection rate and decrease the positive lymph node rate. However, patients receiving NAT exhibited comparable survival as patients receiving upfront surgery. These results indicate that NAT offers insignificant advantages for postoperative survival. Although upfront surgery continues to be the standard and only curative treatment option for RPC, the results of this meta-analysis demonstrate the increasing use of NAT with some favorable outcomes. Numerous RCT studies are ongoing, for example, the NCT01372735.49 These studies focus on RPC and its prognosis after receiving NAT. We believe that when these studies are completed, we will draw more accurate conclusions after analyzing their data.
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2018;65(1):7–30. | ||
Schmidt CM, Turrini O, Parikh P, et al. Effect of hospital volume, surgeon experience, and surgeon volume on patient outcomes after pancreaticoduodenectomy: a single-institution experience. Arch Surg. 2010;145(7):634–640. | ||
National Cancer Institute Surveillance, Epidemiology and End Results Program SEER Stat Fact Sheets. Pancreas cancer. Available from: http://seer.cancer.gov/statfacts/html/pancreas.html. Accessed July 13, 2016. | ||
Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. | ||
Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310(14):1473–1481. | ||
Heestand GM, Murphy JD, Lowy AM. Approach to patients with pancreatic cancer without detectable metastases. J Clin Oncol. 2015;33(16):1770–1778. | ||
Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200–1210. | ||
Maeda A, Boku N, Fukutomi A, et al. Randomized phase III trial of adjuvant chemotherapy with gemcitabine versus S-1 in patients with resected pancreatic cancer: Japan Adjuvant Study Group of Pancreatic Cancer (JASPAC-01). Jpn J Clin Oncol. 2008;38(3):227–229. | ||
Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358(9293):1576–1585. | ||
Isaji S, Mizuno S, Windsor JA, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18(1):2–11. | ||
Jang JY, Han Y, Lee H, et al. Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: a prospective, randomized, open-label, multicenter phase 2/3 trial. Ann Surg. 2018;268(2):215–222. | ||
Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26(21):3487–3495. | ||
Sutton JM, Abbott DE. Neoadjuvant therapy for pancreas cancer: past lessons and future therapies. World J Gastroenterol. 2014;20(42):15564–15579. | ||
Heinrich S, Pestalozzi BC, Schäfer M, et al. Prospective phase II trial of neoadjuvant chemotherapy with gemcitabine and cisplatin for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26(15):2526–2531. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. | ||
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8(1):16. | ||
Wells G, Shea B, O’Connell D, et al [webpage on the Internet]. The Newcastle-Ottawa scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed March 15, 2018. | ||
Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–826. | ||
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. | ||
Fujii T, Satoi S, Yamada S, et al. Clinical benefits of neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreatic head: an observational study using inverse probability of treatment weighting. J Gastroenterol. 2017;52(1):81–93. | ||
Mokdad AA, Minter RM, Zhu H, et al. Neoadjuvant therapy followed by resection versus upfront resection for resectable pancreatic cancer: a propensity score matched analysis. J Clin Oncol. 2017;35(5):515–522. | ||
Casadei R, Di Marco M, Ricci C, et al. Neoadjuvant chemoradiotherapy and surgery versus surgery alone in resectable pancreatic cancer: a single-center prospective, randomized, controlled trial which failed to achieve Accrual targets. J Gastrointest Surg. 2015;19(10):1802–1812. | ||
Golcher H, Brunner TB, Witzigmann H, Marti L. Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: results of the first prospective randomized phase II trial. Strahlenther Onkol. 2015;191:7–16. | ||
Tzeng CW, Tran Cao HS, Lee JE, et al. Treatment sequencing for resectable pancreatic cancer: influence of early metastases and surgical complications on multimodality therapy completion and survival. J Gastrointest Surg. 2014;18(1):16–25. | ||
Motoi F, Unno M, Takahashi H, et al. Influence of preoperative anti-cancer therapy on resectability and perioperative outcomes in patients with pancreatic cancer: Project Study by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2014;21(2):148–158. | ||
Papalezova KT, Tyler DS, Blazer DG, et al. Does preoperative therapy optimize outcomes in patients with resectable pancreatic cancer? J Surg Oncol. 2012;106(1):111–118. | ||
Tajima H, Ohta T, Kitagawa H, et al. Pilot study of neoadjuvant chemotherapy with gemcitabine and oral S-1 for resectable pancreatic cancer. Exp Ther Med. 2012;3(5):787–792. | ||
Barbier L, Turrini O, Grégoire E, Viret F, Le Treut YP, Delpero JR. Pancreatic head resectable adenocarcinoma: preoperative chemoradiation improves local control but does not affect survival. HPB. 2011;13(1):64–69. | ||
Vento P, Mustonen H, Joensuu T, Kärkkäinen P, Kivilaakso E, Kiviluoto T. Impact of preoperative chemoradiotherapy on survival in patients with resectable pancreatic cancer. World J Gastroenterol. 2007;13(21):2945–2951. | ||
Moutardier V, Turrini O, Huiart L, et al. A reappraisal of preoperative chemoradiation for localized pancreatic head ductal adenocarcinoma in a 5-year single-institution experience. J Gastrointest Surg. 2004;8(4):502–510. | ||
Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer, third edition. Cancer. 2005;103(7):1457–1467. | ||
Fritz A, Percy C, Jack A. International Classification of Diseases for Oncology. 3rd ed. Geneva: World Health Organization; 2000. | ||
National Comprehensive Cancer Network, NCCN Guideline: Pancreatic Adenocarcinoma. Version 1.2019. Available from: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed September 1, 2019. | ||
Artinyan A, Anaya DA, McKenzie S, Ellenhorn JD, Kim J. Neoadjuvant therapy is associated with improved survival in resectable pancreatic adenocarcinoma. Cancer. 2011;117(10):2044–2049. | ||
Versteijne E, Vogel JA, Besselink MG, et al. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg. 2018;105(8):946–958. | ||
Sultana A, Tudur Smith C, Cunningham D, Starling N, Neoptolemos JP, Ghaneh P. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer: results of secondary end points analyses. Br J Cancer. 2008;99(1):6–13. | ||
Heinrich S, Schäfer M, Weber A, et al. Neoadjuvant chemotherapy generates a significant tumor response in resectable pancreatic cancer without increasing morbidity. Ann Surg. 2008;248(6):1014–1022. | ||
Palmer DH, Stocken DD, Hewitt H, et al. A randomized phase 2 trial of neoadjuvant chemotherapy in resectable pancreatic cancer: gemcitabine alone versus gemcitabine combined with cisplatin. Ann Surg Oncol. 2007;14(7):2088–2096. | ||
Tempero MA, Malafa MP, Behrman SW. Pancreatic adenocarcinoma, version 2.2014: featured updates to the NCCN guidelines. J Natl Compr Cancer Netw. 2014;12:1083–1093. | ||
Abrams RA, Lowy AM, O’Reilly EM, Wolff RA, Picozzi VJ, Pisters PW. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16(7):1751–1756. | ||
Sohal DP, Walsh RM, Ramanathan RK, Khorana AA. Pancreatic adenocarcinoma: treating a systemic disease with systemic therapy. J Natl Cancer Inst. 2014;106(3):dju011. | ||
Spitz FR, Abbruzzese JL, Lee JE, et al. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol. 1997;15(3):928–937. | ||
Chua TC, Saxena A. Preoperative chemoradiation followed by surgical resection for resectable pancreatic cancer: a review of current results. Surg Oncol. 2011;20(4):e161–e168. | ||
Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246(2):173–180. | ||
Badiyan SN, Molitoris JK, Chuong MD, Regine WF, Kaiser A. The role of radiation therapy for pancreatic cancer in the adjuvant and neoadjuvant settings. Surg Oncol Clin N Am. 2017;26(3):431–453. | ||
Crane CH, Wolff RA, Abbruzzese JL, et al. Combining gemcitabine with radiation in pancreatic cancer: understanding important variables influencing the therapeutic index. Semin Oncol. 2001;28(3 Suppl 10):25–33. | ||
Roland CL, Yang AD, Katz MHG, et al. Neoadjuvant therapy is associated with a reduced lymph node ratio in patients with potentially resectable pancreatic cancer. Ann Surg Oncol. 2015;22(4):1168–1175. | ||
Hoffe S, Rao N, Shridhar R. Neoadjuvant vs adjuvant therapy for resectable pancreatic cancer: the evolving role of radiation. Semin Radiat Oncol. 2014;24(2):113–125. | ||
University Hospital Heidelberg. Trial of Neoadjuvant Short Course IMRT Followed by Surgery and IORT for Resectable Pancreatic Cancer (NEOPANC). Available from: https://clinicaltrials.gov/ct2/show/NCT01372735. NLM identifier: NCT01372735. Accessed August 2011. |
Supplementary materials
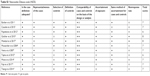  | Table S1 Newcastle–Ottawa scale (NOS) |
  | Table S2 Search strategy |
References
Barbier L, Turrini O, Grégoire E, Viret F, Le Treut YP, Delpero JR. Pancreatic head resectable adenocarcinoma: preoperative chemoradiation improves local control but does not affect survival. HPB. 2011;13(1):64–69. | ||
Casadei R, Di Marco M, Ricci C, et al. Neoadjuvant chemoradiotherapy and surgery versus surgery alone in resectable pancreatic cancer: a single-center prospective, randomized, controlled trial which failed to achieve accrual targets. J Gastrointest Surg. 2015;19(10):1802–1812. | ||
Papalezova KT, Tyler DS, Blazer DG, et al. Does preoperative therapy optimize outcomes in patients with resectable pancreatic cancer? J Surg Oncol. 2012;106(1):111–118. | ||
Golcher H, Brunner TB, Witzigmann H, Marti L. Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: results of the first prospective randomized phase II trial. Strahlenther Onkol. 2015;191:7–16. | ||
Mokdad AA, Minter RM, Zhu H, et al. Neoadjuvant therapy followed by resection versus upfront resection for resectable pancreatic cancer: a propensity score matched analysis. J Clin Oncol. 2017;35(5):515–522. | ||
Moutardier V, Turrini O, Huiart L, et al. A reappraisal of preoperative chemoradiation for localized pancreatic head ductal adenocarcinoma in a 5-year single-institution experience. J Gastrointest Surg. 2004;8(4):502–510. | ||
Vento P, Mustonen H, Joensuu T, Kärkkäinen P, Kivilaakso E, Kiviluoto T. Impact of preoperative chemoradiotherapy on survival in patients with resectable pancreatic cancer. World J Gastroenterol. 2007;13(21):2945–2951. | ||
Tajima H, Ohta T, Kitagawa H, et al. Pilot study of neoadjuvant chemotherapy with gemcitabine and oral S-1 for resectable pancreatic cancer. Exp Ther Med. 2012;3(5):787–792. | ||
Motoi F, Unno M, Takahashi H, et al. Influence of preoperative anti-cancer therapy on resectability and perioperative outcomes in patients with pancreatic cancer: Project Study by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2014;21(2):148–158. | ||
Fujii T, Satoi S, Yamada S, et al. Clinical benefits of neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreatic head: an observational study using inverse probability of treatment weighting. J Gastroenterol. 2017;52(1):81–93. | ||
Tzeng CW, Tran Cao HS, Lee JE, et al. Treatment sequencing for resectable pancreatic cancer: influence of early metastases and surgical complications on multimodality therapy completion and survival. J Gastrointest Surg. 2014;18(1):16–25. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


