Back to Journals » ClinicoEconomics and Outcomes Research » Volume 11
Comparison Of Methods To Estimate Disease-Related Cost And Healthcare Resource Utilization For Autoimmune Diseases In Administrative Claims Databases
Authors Schroeder KM, Gelwicks S, Naegeli AN, Heaton PC
Received 19 February 2019
Accepted for publication 1 October 2019
Published 26 November 2019 Volume 2019:11 Pages 713—727
DOI https://doi.org/10.2147/CEOR.S205597
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Krista M Schroeder,1 Steve Gelwicks,1 April N Naegeli,1 Pamela C Heaton2
1Global Patient Outcomes – Real World Evidence, Eli Lilly and Company, Indianapolis, IN, USA; 2James L. Winkle College of Pharmacy, University of Cincinnati Academic Health Center, Cincinnati, OH, USA
Correspondence: Krista M Schroeder
Eli Lilly and Company, Indianapolis, IN 46285, USA
Tel +1 317 276 3039
Email [email protected]
Background: Establishing disease-related cost and/or healthcare resource utilization (HCRU) is an important aspect of health outcomes research, particularly when considering the cost offset of novel treatments. However, few studies have compared methodologies used to assess disease-related cost/HCRU.
Methods: Data from the United States IBM® MarketScan® Research Databases were used to compare four different methods of calculating disease-related cost and HCRU in patients with rheumatoid arthritis (RA). The analysis was repeated, in part, for patients with ulcerative colitis (UC) to explore the generalizability of findings to a second autoimmune disease. Four methods of disease-related cost/HCRU attribution were selected following a literature search for potential methods: Method 1, claim-wide cost/HCRU attribution based on claim-listed diagnosis codes and a predetermined disease-related medication list (pharmacy claims only); Method 2, line-item cost/HCRU attribution based on procedures/medications more likely to occur in disease cases than in matched controls at two likelihood ratio cutoffs (1.5× and 3.5×); Method 3, disease-related cost/HCRU calculated as the difference in total average cost/HCRU between cases and matched controls; Method 4, line-item cost/HCRU attribution based on clinician manual determination of procedures/medications related to the disease.
Results and conclusion: Overall, 24,373 patients with RA and 9665 with UC were included. Average total cost during 2015 was $US28,750 per patient with RA and $US20,480 per patient with UC. Disease-related cost and HCRU for RA calculated using Method 4 were most closely approximated by Methods 1 and 2 (3.5×), with Method 2 (3.5×) the closest approximation. However, in certain research scenarios, the simplest method compared in this analysis, Method 1, may provide an adequate approximation of disease-related cost and HCRU. Although Method 4 was not executed in the UC analysis because of its labor-intensive nature, similar patterns of disease-related cost and HCRU were observed for Methods 1–3 in patients with UC and RA.
Keywords: health outcomes research, methods, disease-related cost, administrative claims databases, autoimmune diseases
Plain Language Summary
When conducting research with data from administrative insurance claims, it can be difficult to connect single line-item costs with specific diagnoses. A single outpatient insurance claim often includes multiple diagnosis codes but does not match individual costs with specific diagnoses.
We descriptively compared four different methods of calculating disease-related cost and healthcare resource utilization (HCRU) with data from administrative insurance claims using a sample of patients with rheumatoid arthritis, including patient subgroups most likely to have higher or lower disease-related cost/HCRU. We repeated part of the descriptive comparison using a sample of patients with ulcerative colitis from the same databases to explore whether our initial results could be applied to a second autoimmune disease.
We found that when disease-related cost/HCRU was attributed based on procedures and/or medications people with the disease were more likely to undergo and/or receive than similar people without the disease, the results most closely resembled those from our internal standard method (manual attribution of individual disease-related cost/HCRU based on clinician experience). The simplest method we examined was claim-wide attribution of cost/HCRU based on claim-reported diagnosis codes and a predetermined medication list. We found that this method may also adequately approximate disease-related cost/HCRU in some research scenarios.
Introduction
Determining disease-related cost is often an important aspect of health outcomes research, particularly when attempting to estimate the cost offset of a new therapy. Although total cost analyses have been used for this purpose, factors such as comorbidities can have a significant effect on cost and may complicate the accurate assessment of the economic burden of a disease.1,2 Given that administrative insurance claims are generated at every interaction an individual has with the healthcare system, large closed administrative insurance claims databases, such as IBM® MarketScan® Research Databases, provide a good source of information for the investigation of scenarios, including healthcare delivery, benefits, harms and cost, for large populations.3,4 However, although claims data are frequently used for research, such usage was not their original intended purpose:5 The purpose of administrative claims is billing and reimbursement of medical care. Therefore, complexities exist when using diagnosis codes to attribute cost to a disease. This is particularly true when attempting to attribute cost for complex diseases with multiple treatment options, some of which may be indicated for more than one disease, and an array of comorbidities. Notably, autoimmune diseases are inherently complex, partly because of their heterogeneity in terms of pathophysiology and response to therapy, and because of the common pathogenic mechanisms, cytokine pathways, and systemic inflammatory cascades shared by apparently disparate conditions.6
At present, disease-related cost analyses based on administrative claims databases usually rely on the diagnosis codes included on the claims, a simple but flawed approach that can complicate the process of disease-cost attribution.7 When multiple diagnoses are included on a single claim, this approach generally results in all costs on the claim being attributed to the disease of interest regardless of whether or not individual line-item costs are related to that diagnosis code. Primary diagnosis codes can be helpful in this process, but the allocation of a primary diagnosis field or diagnosis-related group (DRG) code to the claim varies according to where the claim originated (eg, inpatient versus outpatient). For claims associated with inpatient treatment, administrative claims database allocation of a primary diagnosis field or DRG code to the patient can result in the clear attribution of claim cost to a disease. However, outpatient claims can list many diagnoses for a single procedure without identifying a primary diagnosis to which costs should be attributed. Furthermore, some healthcare professionals believe that inpatient diagnosis codes may be more reliable than diagnosis codes used in the outpatient setting.8 When multiple diagnosis codes but no primary diagnosis is listed on a single claim, line-item costs may be allocated incorrectly to a single diagnosis code. Therefore, with whole-claim cost attribution, cost on an outpatient claim may be attributed to a disease of interest if a diagnosis code for that disease exists on the claim regardless of whether the procedure cost was actually related to that disease. This can result in the potential for overestimation of the total cost attributed to a single disease of interest. For example, using this whole-claim cost attribution method based on claim-listed diagnosis codes (tested in this analysis as Method 1), a patient with chronic psoriasis who presents for an outpatient appointment associated with a broken arm would have the cost of the appointment attributed to psoriasis if the treating doctor notes the psoriasis diagnosis on the claim. Moreover, cost attribution is further complicated for pharmacy claims, which have no associated diagnoses at all, and where a single drug may have multiple indications, requiring researchers to a priori use medical judgement to compile a list of medications for which the cost should be attributed to a disease.
Several studies have attempted to examine disease-related costs. For example, Birnbaum and colleagues9 and Kappelman and colleagues10 determined the percentage of cost attributed to a disease by matching a cohort of patients with a disease with a cohort of healthy controls and subtracting the cost attributed to the control cohort from the cost of the disease cohort (an approach tested in this analysis as Method 3). Moreover, Kowal and colleagues11 utilized clinician expert opinion on disease-related procedures and medications to generate procedure and medication lists to more accurately determine disease-related cost (our internal standard approach tested in this analysis as Method 4). In addition, Kan and colleagues12 examined healthcare resource utilization (HCRU) and cost attributable to the autoimmune disease systemic lupus erythematosus (SLE) in a Medicaid population in the United States (US) using multivariate regressions to estimate incremental HCRU and cost. This study compared patients with SLE with adult patients in the database without a diagnosis of SLE and concluded that HCRU and cost were significantly higher in patients with SLE, with the highest cost observed for patients who experienced severe flares.12 However, few studies have attempted to validate the methodology underlying the assessment of disease-related cost in administrative claims databases, and no direct comparison of methods has yet been published.
The primary objective of this analysis was to use administrative claims data from the MarketScan Databases for patients with the autoimmune disease rheumatoid arthritis (RA) to descriptively compare four different methods of calculating disease-related cost and disease-related HCRU (including an internal standard clinician review method). We then sought to explore the general applicability of these findings in RA to another autoimmune disease by applying three of these methods (excluding the labor-intensive line-item cost attribution based on clinician manual determination of procedures/medications related to the disease method) to a sample of patients with ulcerative colitis (UC) derived from the same databases.
Methods
Sample Selection
The IBM MarketScan Commercial Database and the IBM MarketScan Medicare Supplemental Database are longitudinal insurance claims databases that provide access to patient-level data relating to healthcare utilization, expenditure, and enrollment across inpatient, outpatient, prescription drug, and disease-specific services from approximately 45 large employers, health plans, and government and public organizations in the US. To assess 1-year costs and negate the possibility of seasonal bias, we included all patients within the databases with continuous enrollment in both medical and prescription benefits from 1 January 2015 to 31 December 2015 (the most recent year for which complete data were available at the time of this analysis).
Eligibility Criteria
Eligible patients were aged ≥18 years on 1 January 2015, with at least two diagnosis codes for the disease of interest (RA or UC). At least one diagnosis code pre-2015 and one diagnosis code during 2015 were required to ensure prevalent RA or UC on 1 January 2015. Details of relevant diagnosis codes are provided in the online supplemental appendix. Patients were excluded from the study if they had specific cancer or autoimmune disease diagnosis codes (as detailed in the online supplemental appendix).
Controls met the same criteria as the disease cases but without a diagnosis of the respective disease of interest. Controls were exact matched 3:1 to disease cases according to age, sex, region, and insurance type (Medicare-supplemental or commercial), with individuals excluded if any one of these data points was missing.
Disease-Related Procedure/Medication List Generation For Method 2
In preparation for the Method 2 cost and HCRU analyses, which required procedure/medication lists for cost attribution, two 33% random samples of patients with each autoimmune disease, and their matched controls, were selected. Details of all procedures and medications received in 2015 by the cases and their matched controls were compiled. These procedure and medication lists were truncated at 1% of cases. For each autoimmune disease, using the generated procedure and medication list from the first 33% sample, likelihood ratios were generated to compare the proportion of cases receiving procedures and medications compared with their exact matched controls. Procedures and medications that were 1.5× or 3.5× more frequent in the cases than in the controls were compiled for subsequent analyses, with two lists generated, one for each of the two different cutoffs.
Subsequently, the cases and matched controls from the second 33% samples for each disease were used to replicate the generation of likelihood ratios for procedures and medications to test the replicability of the final procedure/medication lists in a different sample of patients with a diagnosis of the same autoimmune disease. No notable differences were observed. As specified in the study protocol, the lists generated from the first 33% RA and UC samples were used for subsequent Method 2 cost and HCRU analyses.
Disease-Related Procedure/Medication List Generation For Method 4
An RA specialist clinician reviewed the procedure and medication list compiled for the first 33% sample of patients with RA (described above; cutoff defined as procedures undergone and medications received by >1% of the sample). Procedures and medications likely to be related to RA care were manually flagged for subsequent analysis in Method 4.
Disease-Related Cost And Healthcare Resource Utilization Calculations
Using the final 33% sample of patients with RA, four methods of calculating the disease-related cost and HCRU for the calendar year 1 January to 31 December 2015 were descriptively compared to determine the percentage of total cost and HCRU attributable to RA using each method. Once these methods had been applied in RA, they were then applied to the final 33% sample of patients with UC to explore the generalizability of the findings from RA to another autoimmune disease. Given the time-consuming nature of the internal standard method, this was tested only in patients with RA. The four methods used were as follows:
Method 1 (Claim-Wide Cost And HCRU Attribution Based On Claim-Listed Diagnosis Codes And A Predetermined Disease-Related Medication List [Pharmacy Claims Only])
- Cost: Disease-related inpatient cost was attributed using the primary diagnosis field (eg, if RA was the primary diagnosis on an inpatient claim, all cost for that claim was attributed to RA). Outpatient cost was included if a diagnosis code for the disease of interest was listed on the claim (no primary diagnosis on outpatient claims). Pharmacy cost was included using a predetermined drug list (clinician-generated from clinical knowledge without access to raw patient data/drug lists; no diagnosis on pharmacy claims).
- HCRU: Disease-related inpatient visits were attributed using the primary diagnosis field. Outpatient and emergency room (ER) visits were included if the disease of interest was on the claim. Pharmacy claims were included using a predetermined drug list.
Method 2 (Line-Item Cost And HCRU Attribution Based On Procedures/Medications More Likely To Occur In Cases Than In Matched Controls At Two Likelihood Ratio Cutoffs [1.5× and 3.5×])
- Cost: Disease-related inpatient cost was attributed using the primary diagnosis field as in Method 1. Using the likelihood ratios generated as described above, outpatient/pharmacy cost was included for procedures/medications occurring in a higher proportion (1.5× and 3.5×) of cases versus matched controls.
- HCRU: Disease-related inpatient visits were attributed using the primary diagnosis field as in Method 1. Using the likelihood ratios generated as described above, we included outpatient and ER visits containing one or more procedure and pharmacy claims for medications occurring in a higher proportion (1.5× and 3.5×) of cases versus matched controls.
Method 3 (Disease-Related Cost And HCRU Calculated As The Difference In Total Average Cost Between Cases And Their Matched Controls)
- Cost: Total average cost of cases – total average cost of matched controls.
- HCRU: Total average HCRU by cases – total average HCRU by matched controls.
Method 4 (Line-Item Cost And HCRU Attribution Based On Clinician Manual Determination Of Procedures/Medications Related To The Disease; RA Only)
- This method served as an internal standard and utilized a separate standalone clinician review of procedure and medication codes received by cases with RA as described above.
- Cost: As in Methods 1 and 2, disease-related inpatient cost was attributed using the primary diagnosis field. Cost attributed to outpatient and pharmacy claims flagged as related to RA in clinician review was also included.
- HCRU: As in Methods 1 and 2, disease-related inpatient visits were attributed using the primary diagnosis field. Outpatient and ER visits containing one or more procedure code and pharmacy claims determined to be related to RA in clinician review, respectively, were also included.
Exploratory Subgroup Analyses
Disease-related cost and HCRU subgroup analyses were performed to ensure the findings from the methods tested were generalizable across different types of patients, including groups most likely to be associated with high or low cost. The final 33% samples of patients with RA and UC used for the cost and HCRU analyses described above were stratified based on patients hypothesized to incur a higher or lower level of disease-related cost or HCRU.
Subgroups examined included patients who were receiving biologic/advanced therapy (yes/no) and patients who had visited the ER (yes/no) between 1 January and 31 December 2015.
Ethical Conduct
This investigation was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki, together with applicable laws and regulations of the country in which the analysis was conducted. This secondary database analysis was conducted using the IBM MarketScan Research Databases. The MarketScan Databases are fully compliant with the Health Insurance Portability and Accountability Act and meet the criteria for a limited-use dataset. Since the patient and provider data included in this analysis were fully de-identified, this study was exempt from Ethical Review Board review.
Results
Patient Samples
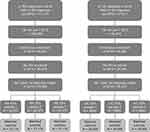
A total of 73,364 patients with RA and 29,072 patients with UC were eligible for the study. Therefore, 24,373 patients with RA and 9665 patients with UC were included in each of the 33% RA and UC samples, respectively (Figure 1). Exact-matched control samples (3:1) contained a total of 73,119 and 28,995 individuals for the 33% RA and UC samples, respectively. Overall, 245 patients with RA and 77 patients with UC could not be exact matched to controls; these patients were excluded from the study. Case samples from patients with RA or UC and control samples for both diseases were well matched (Table 1A and B). Within all three 33% RA samples and their matched controls, the mean age was around 58 years, and over three-quarters of individuals included were female. For the three 33% UC samples and their matched controls, patients had a mean age of around 50 years, and males and females were more equally represented.
 |
Table 1 Patient Demographics And Baseline Characteristics In The Three (A) Rheumatoid Arthritis Samples And (B) Ulcerative Colitis Samples And Their Matched Controls |
Assessment Of Disease-Related Cost
As described in the methods section, the first two 33% RA samples and the first two 33% UC samples were used to generate and replicate procedure/medication lists for use in Methods 2 and 4. Within RA, the top 10 medications in terms of likelihood ratios were all disease-modifying antirheumatic drugs (DMARDs). The medication used with the highest frequency was methotrexate sodium (39.4% of patients with RA, with a likelihood ratio of 319.7×). The procedure codes with the highest likelihood ratios were specialty drugs, chemotherapy injections, immunology tests, and performance tracking. Within UC, the top 10 medications in terms of likelihood ratios included several 5-aminosalicylic acid (5-ASA) medications, with 56.8% of UC cases recording a claim for mesalamine (likelihood ratio for mesalamine = 433.5×). Procedure codes with the highest likelihood ratios included specialty drugs, chemotherapy injections, other minor digestive procedures, medical supplies and devices, and chemistry/immunology/microbiology tests.
Subsequently, total cost and the percentage disease-related cost by attribution method were assessed in the final 33% RA sample, with generalizability of the findings to another autoimmune disease assessed using the final 33% UC sample.
The average total cost during the 2015 calendar year was found to be $US28,750 per patient with RA and $US20,480 per patient with UC. When costs related to either RA or UC were considered as a percentage of total cost, differences were observed across the analysis methods, with Method 3 (difference in total average cost [case – control]) potentially overestimating disease-related cost for both diseases (Figure 2).
Of note, the rank order of percentage disease-related cost from highest to lowest using Methods 1, 2 (1.5×), 2 (3.5×), and 3 was similar when comparing the RA and UC samples. Method 3, which calculated disease-related cost as the difference in total average cost between patients with the disease and matched healthy controls, resulted in the highest percentage disease-related cost. The next highest percentage disease-related cost resulted from line-item cost attribution based on procedures/medications 1.5× more likely to occur in patients with RA than in matched controls (Method 2 [1.5×]) and from claim-wide cost attribution based on claim-listed diagnosis codes and a predetermined disease-related medication list (Method 1). The lowest percentage disease-related cost was found with line-item cost attribution based on procedures/medications 3.5× more likely to occur in a patient with RA than in a matched control patient (Method 2 [3.5×]).
Across analysis methods, both overall disease-related cost and disease-related cost component attribution results (ie, for ER, inpatient, outpatient-Healthcare Common Procedure Coding System [HCPCS], outpatient-other [ie, anything not HCPCS], pharmacy) differed. For example, Method 3 resulted in a much higher disease-related inpatient cost than did the other methods in both the RA and the UC samples (Figure 3A and B). Furthermore, in both the RA and the UC samples, Methods 1 and 2 (3.5×) resulted in a very low disease-related ER cost compared with Methods 2 (1.5×) and 3.
Disease-related cost for RA calculated using Method 4 (our internal standard) was found to be most closely approximated, in terms of overall disease-related cost and cost component attribution, by Methods 1 and 2 (3.5×), with Method 2 (3.5×) resulting in the closest approximation.
Assessment Of Disease-Related Healthcare Resource Utilization
As described in the methods section, disease-related HCRU was also assessed in the final 33% RA sample, with generalizability of the findings to another autoimmune disease assessed in the final 33% UC sample. Differences in disease-related HCRU were observed across the analysis methods. As with the disease-related cost analysis above, the rank order of disease-related HCRU components (inpatient, outpatient, and ER visits, and pharmacy claims) from highest to lowest mean number per patient using Methods 1, 2 (1.5×), and 2 (3.5×) was similar across the RA and UC disease samples (Figure 4A and B). Pharmacy claims represented the greatest area of disease-related HCRU for patients with both RA and UC regardless of the method used. Outpatient visits represented the second greatest area of disease-related HCRU when measured using Methods 1, 2 (1.5×) and 2 (3.5×) in both disease samples, although ER visits represented the second greatest area of HCRU when using Method 3. The lowest area of disease-related HCRU for patients with either RA or UC was represented by inpatient visits, regardless of the method applied.
As with the assessment of cost, differences in components of disease-related HCRU were observed across the methods employed and reflected in both disease samples (Figure 4A and B). Method 2 (1.5×) resulted in higher mean numbers of pharmacy claims and outpatient visits per patient than all other methods across both the RA and the UC samples. Furthermore, Method 1 showed the lowest mean number of pharmacy claims per patient compared with all other methods across both disease samples. Method 3 was associated with a higher mean number of ER visits per patient and a markedly lower mean number of outpatient visits per patient than all other methods across both the RA and the UC samples.
Disease-related HCRU calculated using Method 4 (our internal standard) in the RA sample was found to be most closely approximated, in terms of all components, by Methods 1 and 2 (3.5×); although the mean number of outpatient visits per patient was lower with Method 2 (3.5×), and the mean number of both outpatient visits and pharmacy claims per patient was lower with Method 1.
Exploratory Subgroup Analyses
As an exploratory analysis, subgroups of patients with RA and UC expected to have higher or lower disease-related cost and HCRU were compiled. Of all the methods examined in the sample of patients with RA, Method 2 (3.5×) resulted in the closest approximation of overall disease-related cost to Method 4 (our internal standard) across all high/low-cost subgroups in RA. Interestingly, Method 3 resulted in the highest total disease-related cost across all subgroups in both disease samples. In the UC sample, patients with an ER visit also appeared to incur higher costs across all methods than those with no ER visit; however, these results were not consistent with findings from the RA sample, where the effect of an ER visit on disease-related cost compared with entire sample results varied by method used (Figure 5A and B).
When HCRU was considered by component according to the subgroups of patients considered most likely to be associated with high or low HCRU, the findings broadly aligned with the disease-related cost subgroup analysis results. As anticipated, patients across both the RA and the UC samples who were receiving either biologic or advanced therapy or who had ER visits demonstrated a higher level of HCRU (particularly in terms of the main HCRU components, outpatient visits and pharmacy claims) than patients who were not receiving such therapy or did not have ER visits, respectively (except when the impact of ER visits was assessed using Method 1 in the RA sample). As observed in the entire RA and UC patient samples, Method 3 resulted in much higher levels of disease-related HCRU associated with ER visits in both disease samples than any other method in all subgroups. Furthermore, while Method 2 (1.5×) overestimated HCRU for every subgroup in the RA sample when compared with Method 4 (our internal standard), Method 2 (3.5×) once again resulted in the most comparable HCRU component estimates to Method 4 across all subgroups (Figure 6A and B).
Discussion
The findings from these analyses demonstrate that, when RA-related cost and HCRU were calculated with line-item attribution based on procedures/medications 3.5× more likely to occur in patients with RA than in matched controls (Method 2 [3.5×]), the results were most similar to those found using line-item cost or HCRU attribution based on clinician manual determination of procedures/medications related to the disease (our internal standard, Method 4) in terms of overall percentage of disease-related cost and breakdown of disease-related cost and HCRU components. However, it should be noted that when the method utilizing claim-wide cost and HCRU attribution based on claim-list diagnosis codes and a predetermined disease-related medication list (Method 1) was used, RA-related cost and HCRU estimates were also similar to those from Method 4. However, when disease-related cost and HCRU were calculated with line-item attribution based on procedures/medications 1.5× more likely to occur in case patients than in matched controls (Method 2 [1.5×]), both cost and HCRU were overestimated for both diseases relative to the other methods (with the exception of cost estimates made using Method 3 for RA and Methods 3 and 2 [3.5×] for UC), perhaps because this lower threshold allowed the inclusion of procedures/medications that were not actually disease specific. Moreover, examination of the differences in total average cost and HCRU between patients with the disease and healthy controls (Method 3) was also found to consistently overestimate both cost and, in most instances, HCRU for both diseases when compared with the other methods used, a finding that may be attributed to the inclusion of cost and HCRU relating to comorbidities being over-represented in the disease sample. Thus, Method 3 might provide a useful means of assessing the cost of disease and associated comorbidities in certain research scenarios.
These results were mirrored among RA patient subgroups in which disease-related cost and HCRU would be expected to be highest, with line-item cost and HCRU attribution based on procedures/medications 3.5× more likely to occur in patients with RA than in matched controls (Method 2 [3.5×]) found to be most similar to line-item cost and HCRU attribution based on clinician manual determination of procedures/medications related to the disease (Method 4, our internal standard). Although Method 4 was not executed in the UC sample because it was too labor intensive, similar patterns of disease-related cost and HCRU were observed for the other three methods assessed in patients with UC as in those with RA. This was true for both the overall UC sample and the patient subgroups in whom disease-related cost and HCRU would be expected to be highest. Costs were considerably higher in patients with RA or UC requiring biologic or advanced therapy, an observation that aligns with the assumption that such individuals may have more severe disease.
Relatively few studies have examined the methods used to assess cost and HCRU among patients. Those that have attempted to estimate disease-related cost have followed a similar approach to this study, by comparing costs in patients with a disease with those in control patients without the disease.9,10,12 Other studies, such as that by Wimo and colleagues13 to examine the relationship between cost, HCRU, and disease severity in individuals with Alzheimer’s disease, have applied unit costs to HCRU in an attempt to generate an estimate of healthcare and social care costs.
Strengths of this study include the large sample sizes and head-to-head comparison of methods in samples of patients with the same disease. In an attempt to limit bias and confounding, this analysis was designed to draw three 33% samples for each autoimmune disease from the databases, two of which were used to generate and validate procedure/medication lists, and one of which was used for the assessment of disease-related cost and HCRU. All cost and HCRU calculation method comparisons were undertaken within the same final 33% samples from the databases for each autoimmune disease of interest. Furthermore, the assessment of two autoimmune diseases provides some verification of the applicability and utility of the methods employed and ensures that the conclusions drawn from each method are not disease specific.
Limitations of this study include the small number of diseases in which it has been practical to test the methodology thus far and the retrospective nature of the data analyzed. Moreover, it was not possible to fully ascertain disease severity from the data provided within the databases, a factor that would assist with the identification of patients most likely to incur higher disease cost and HCRU. However, the exploratory subgroup analysis of patients receiving biologic or advanced treatment would be expected to target patients with more severe disease. Furthermore, the potential for selection bias existed because of the multiple codes required for confirmation of a diagnosis of RA or UC and the exclusion of comorbid autoimmune and other specified diseases. However, such an approach was necessary to increase sample specificity for the purpose of methods testing and to limit the potential influence of cost due to related or highly costly diseases on some methods (such as Methods 1 and 3). Although line-item attribution of cost and HCRU based on procedures/medications 3.5× more likely to occur in cases than in matched controls (Method 2 [3.5×]) could be expected to provide a good estimate of cost in such patients, it is unlikely that a concomitant treatment for cancer would generate a likelihood ratio above 3.5×, with the exception of immunosuppressive therapies used in both cancer and RA/UC. The internal standard method employed in this study was based on expert review of claim codes as opposed to chart reviews, with the expert having access only to the codes for procedures and medications received by patients with RA. The expert then had to determine which procedures and medications were likely to have been due to RA treatment, which may have resulted in the incorrect attribution of some codes. Moreover, uncertainty surrounds the generalizability of our findings to other disease settings and administrative claims data sources.
Conclusion
The findings from these analyses indicate that disease-related line-item cost and HCRU attribution based on procedures/medications 3.5× more likely to occur in patients with RA or UC than in matched controls (Method 2 [3.5×]) is a good method of calculating disease-related cost and HCRU using data from administrative claims databases. This observation remains true even in patients for whom disease-related cost and HCRU would be expected to be highest. In patients with RA included in this study, using a 3.5× likelihood ratio cutoff with line-item cost and HCRU attribution based on procedures/medications more likely to occur in cases than in matched controls (Method 2 [3.5×]) produced an accurate estimation of actual cost and HCRU due to a specific disease state without the intensive labor required to undertake line-item cost attribution based on clinician manual determination of procedures/medications related to the disease (Method 4). Moreover, the generalizability of these findings to another autoimmune disease was demonstrated when the method was applied to the UC sample. However, this methodology should be further tested and validated in other disease settings/datasets to confirm the findings of the current analyses and to ensure the suitability of this methodology across a range of disease states. Moreover, we suggest that the findings from these analyses also indicate that the use of claim-wide cost attribution based on claim-listed diagnosis codes and a predetermined disease-related medication list (Method 1) to assess disease-related cost and HCRU, the simplest and least labor-intensive method included in our comparison, may be a reasonable option in some research scenarios, depending on the research question.
Abbreviations
5-ASA, 5-aminosalicylic acid; DMARD, disease-modifying anti-rheumatic drug; DRG, diagnosis-related group; ER, emergency room; HCPCS, Healthcare Common Procedure Coding System; HCRU, healthcare resource utilization; HIV, human immunodeficiency virus; ICD, International Classification of Diseases; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; UC, ulcerative colitis; US, United States.
Acknowledgments
The authors would like to acknowledge Dr Sarah Birch and Sue Williamson (Rx Communications, Mold, UK) for medical writing assistance with the preparation of this manuscript, funded by Eli Lilly and Company.
Author Contributions
Krista M Schroeder was involved in the design and interpretation of the data for the work, Steve Gelwicks was involved in the design of the work and the analysis of the data. April N Naegeli and Pam C Heaton were involved in the interpretation of the data for the work. All authors contributed sufficiently to the work and provided critical revision of the manuscript for important intellectual content. All authors give their approval of the manuscript to be submitted and published in ClinicoEconomics and Outcomes Research, and agree to be accountable for all aspects of the work.
Disclosure
Krista M Schroeder and April N Naegeli are employees of and own stock in Eli Lilly and Company. Steve Gelwicks was an employee of Eli Lilly and Company at the time the study was conducted and is a minor stockholder of Eli Lilly and Company. Pamela C Heaton reports grants from Eli Lilly and Company, during the conduct of the study and outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Chen W, Lynd LD, FitzGerald JM, et al. Canadian Respiratory Research Network. Excess medical costs in patients with asthma and the role of comorbidity. Eur Respir J. 2016;48(6):1584–1592. doi:10.1183/13993003.01141-2016
2. Cortaredona S, Ventelou B. The extra cost of comorbidity: multiple illnesses and the economic burden of non-communicable diseases. BMC Med. 2017;15(1):216. doi:10.1186/s12916-017-0978-2
3. Cadarette SM, Wong L. An introduction to health care administrative data. Can J Hosp Pharm. 2015;68(3):232–237. doi:10.4212/cjhp.v68i3.1457
4. Riley GF. Administrative and claims records as sources of health care cost data. Med Care. 2009;47(7 Suppl 1):S51–S55. doi:10.1097/MLR.0b013e31819c95aa
5. Preussler JM, Mau LW, Majhail NS, et al. Administrative claims data for economic analyses in hematopoietic cell transplantation: challenges and opportunities. Biol Blood Marrow Transplant. 2016;22(10):1738–1746. doi:10.1016/j.bbmt.2016.05.005
6. Cho JH, Feldman M. Heterogeneity of autoimmune diseases: pathophysiologic insights from genetics and implications for new therapies. Nat Med. 2015;21(7):730–738. doi:10.1038/nm.3897
7. Ford JH, Schroeder K, Nyhuis AW, Foster SA, Aurora SK. Cycling through migraine preventive treatments: implications for all-cause total direct costs and disease-specific costs. J Manag Care Spec Pharm. 2019;25(1):46–59. doi:10.18553/jmcp.2018.18058
8. Voss EA, Ma Q, Ryan PB. The impact of standardizing the definition of visits on the consistency of multi-database observational health research. BMC Med Res Methodol. 2015;15:13. doi:10.1186/s12874-015-0001-6
9. Birnbaum HG, Kessler RC, Lowe SW, et al. Costs of attention deficit-hyperactivity disorder (ADHD) in the US: excess costs of persons and their family members in 2000. Curr Med Res Opin. 2005;21(2):195–206. doi:10.1185/030079904X20303
10. Kappelman MD, Rifas-Shiman SL, Porter CQ, et al. Direct health care costs of Crohn’s disease and ulcerative colitis in US children and adults. Gastroenterology. 2008;135(6):1907–1913. doi:10.1053/j.gastro.2008.09.012
11. Kowal SL, Dall T, Chakrabarti R, Storm MV, Jain A. The current and projected economic burden of Parkinson’s disease in the United States. Mov Disord. 2013;28(3):311–318. doi:10.1002/mds.25292
12. Kan HJ, Song X, Johnson BH, Bechtel B, O’Sullivan D, Molta CT. Healthcare utilization and costs of systemic lupus erythematosus in Medicaid. Biomed Res Int. 2013;2013:808391. doi:10.1155/2013/808391
13. Wimo A, Reed CC, Dodel R, et al. The GERAS study: a prospective observational study of costs and resource use in community dwellers with Alzheimer’s disease in three European countries – study design and baseline findings. J Alzheimers Dis. 2013;36(2):385–399. doi:10.3233/JAD-122392
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.