Back to Journals » Clinical Ophthalmology » Volume 14
Comparison of Iodide-125 and Ruthenium-106 Brachytherapy in the Treatment of Choroidal Melanomas
Authors Ghassemi F, Sheibani S, Arjmand M , Poorbaygi H, Kouhestani E , Sabour S , Samiei F, Beiki-Ardakani A , Jabarvand M, Sadeghi Tari A
Received 19 October 2019
Accepted for publication 31 December 2019
Published 4 February 2020 Volume 2020:14 Pages 339—346
DOI https://doi.org/10.2147/OPTH.S235265
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Fariba Ghassemi, 1–3 Shahab Sheibani, 4 Mojtaba Arjmand, 3 Hosein Poorbaygi, 4 Emad Kouhestani, 1 Siamak Sabour, 5 Farhad Samiei, 6 Akbar Beiki-Ardakani, 7 Mahmood Jabarvand, 1 Ali Sadeghi Tari 1
1Eye Research Center, Farabi Eye Hospital, Tehran University of Medical Sciences, Tehran, IR, Iran; 2Retina & Vitreous Service, Farabi Eye Hospital, Tehran University of Medical Sciences, Tehran, IR, Iran; 3Ocular Oncology Service, Farabi Eye Hospital, Tehran University of Medical Sciences, Tehran, IR, Iran; 4Radiation Application Research School, Nuclear Science and Technology Research Institute, Tehran, Iran; 5Safety Promotion and Injury Prevention Research Centre, Department of Clinical Epidemiology, School of Health,Shahid Beheshti University of Medical Sciences, Tehran, IR, Iran; 6Radiation Oncology Department, Cancer Institute, Imam Hospital and Medical Complex, Tehran University of Medical Sciences, Tehran, Iran; 7Radiation Physics Department, Princess Margaret Hospital, Toronto, ON, Canada
Correspondence: Fariba Ghassemi
Eye Research Center, Farabi Eye Hospital, Qazvin Square, Tehran 1336616351, Iran
Tel +98-21-55421006
Fax +98-21-55416134
Email [email protected]
Background: To compare iodine-125 ( 125I) with ruthenium-106 ( 106Ru) episcleral plaque radiation therapy in terms of the effectiveness and non-inferiority for choroidal melanoma treatment.
Objective: To report the non-inferiority of new made iodine-125 ( 125I) compared with ruthenium-106 ( 106Ru) episcleral plaque radiation.
Patients and Methods: A retrospective, non-randomized comparative case series. In this series the patients treated with 125I and 106Ru episcleral plaques for choroidal melanoma between September 2013 and August 2017 at Farabi Hospital are compared. Local control of choroidal melanomas after 125I and 106Ru plaques implantation and vision changes are the main outcome measures.
Results: A total of 35 patients were identified ( 125I = 15, 106Ru = 20). No significant difference between two groups in visual acuity, diameter and thickness changes were observed after treatment. Multivariate linear regression (MLR) analysis showed that final diameter was only, independently and significantly, correlated with the pre-treatment diameter of the tumor (β = 0.59, 95% confidence interval [CI]: 0.29, 1.34, P = 0.003). The same MLR analysis for the final thickness and visual acuity, after adjusting for age and sex showed no significant difference between two groups. A single patient treated with 106Ru had local tumor recurrence with no one in the 125I group. No statistical difference in the rate of ocular complications was observed.
Conclusion: The treatment with our 125I plaques is as effective as 106Ru plaques in controlling choroidal melanoma tumor and preserving the vision during the two and half year of follow-up. The complication rates are alike. It means that the effectiveness of 125I is not only comparable to 106Ru but also superior when the outcome of the interest is the thickness of the tumors.
Keywords: brachytherapy, choroidal melanoma, complication, local tumor control, 125I, radiation, 106Ru, tumor size, vision preservation
Introduction
Episcleral plaque radiation therapy (brachytherapy) is the treatment of choice for small and medium-sized choroidal melanomas, while enucleation is the most commonly used therapy for large-sized choroidal melanoma tumors.1 Survival rates between patients managed by 125I brachytherapy compared to those managed by enucleation were not different in the COMS Group (Collaborative Ocular Melanoma Study Group) trial for medium-sized tumors, and a conservative approach is more appropriate for these cases.2
Brachytherapy is an accepted treatment for the management of choroidal melanomas to preserve the globe, vision, quality of life and cosmetic outcome.1–3 This treatment is a highly specialized therapy that needs experienced high workload centers and an accurate coordination between particularly trained personnel as team members.5
Since the first reports of using radon seeds radiation to treat choroidal melanoma back to 1930, physicians have used several isotopes as cobalt-60 (60Co), iodine-125 (125I), ruthenium-106 (106Ru), iridium-192 (192Ir), palladium-103 (103Pd) and strontium-90 (90Sr) to treat these tumors.4–15
In this article, we report our single institutional, early clinical outcomes of episcleral plaque brachytherapy using 125I plaques brachytherapy comparing with standard106Ru plaque brachytherapy. This study reports the achievement of local control of choroidal melanoma tumor during a short time follow-up period in both brachytherapy with 125I and 106Ru.
Methods
This study is a retrospective, non-randomized case series. We performed a chart review of patients treated with 125I and 106Ru for choroidal melanoma from September 2013 through August 2017 at Farabi Hospital, Tehran University of Medical Sciences. We thoroughly informed the patients about the procedures and we obtained a written informed consent. We followed the tenets of the Helsinki Declaration. This study was approved by the institutional ethics committee of Farabi Multi-Specialty Eye Hospital, Tehran, IR Iran.
We extracted the treatment type, age, gender, affected eye, tumor location, tumor height and maximum basal diameter, presence of subretinal fluid, visual acuity, the enucleation, the development of metastatic disease and patients’ mortality.
A single-ocular oncologist (FG) examined all patients before and after radiation therapy and we performed diagnostic ultrasonography during each examination. The main outcome measure was local tumor control, defined as decreasing or stabilizing the size of the tumor. Treatment endpoints were tumor regrowth, enucleation, and patient death. The follow-up time was the time of the first visit to the last visit, in months. We excluded a single patient in each group receiving transpupillary thermotherapy before the treatment.
A radiation oncologist (FS) and a radio physicist (MA) designated the dosimetry plan, upon the tumor features provided by the ocular oncologist (FG). All patients received 80–90 Gy radiation dose at the tumor apex. We included the scleral thickness in the measurement of tumor thickness by ultrasound. We included all the patients who had undergone 125I plaque therapy for their choroidal melanoma. At the same time period, we treated small and medium-sized choroidal melanomas with 06Ru plaques (Bebig, Eckert and Ziegler Corp., Berlin, Germany). The selection of the type of plaques was based on the tumor size, cost, market availability and rarely patient’s preference after giving enough information in this nonrandomized study. We already described the fabrication of 125I plaques.5,16 125I plaques were made by collaboration of Farabi eye hospital and Radiation Application Research School (NSTRI).
The plaques adopted for COMS plaques are designed and 125I seed sources (each by 4.7 mm length and 0.8 mm in diameter) are made (the activity of 4–6 mCi). The 125I plaques are 14, 16, 18, 20, 22 and 24 mm diameter round or notched and 1 mm thick gold shells. We used silastic carrier for 125I seeds inside the gold shells. The gold plaque had a lip or edge shield that encircles the plaque and extends on the sclera. Patients with peripapillary tumors by an incident angle over 290°, measured from the center of the optic disc, were ineligible for the operation.
We modeled each brachytherapy case at the moment with a plaque simulator to calculate doses and dose rates to ocular tissues (Plaque simulator: BEBIG; 2008; version 6.4.4). We performed the plaques implantation and removal as the standard protocol.5,17
Our hospital keeps all these brachytherapy patients hospitalized during the irradiation. The plaque is typically removed in the operating room under local or general anesthesia. The seeds on the shell have been counted in the operating room. The tumor and critical normal structures final doses have been calculated and recorded. During the study period, however, we did not use diode laser thermotherapy as routine adjuvant therapy for uveal melanoma.
We followed the patient up at 1 and 4 months, and 4 months apart thereafter. Follow-up evaluation included best-corrected visual acuity, ophthalmoscopic and ultrasonographic assessment of local tumor control and brachytherapy-related complications. Radiation retinopathy has been described as a slowly progressing microangiopathy with at least 3 findings as microaneurysms, retinal hemorrhages, exudates, and cotton wool. For its diagnosis fluorescein angiography (FA) and optical coherence tomography (OCT) were used.
According to the COMS, local failure was defined as growth >15% increase in tumor size on ultrasound; >250-mm increase in tumor border, extra-scleral extension, or orbital recurrence.2
We did an analysis of data, using SPSS version of 20.0 (SPSS Inc., Chicago, IL, USA).
Visual outcomes, diameter and thickness changes were compared between two treatments using the t-test. For qualitative variables, chi-square test and fisher exact test were applied. Multivariate analysis was performed using linear regression test. The relation between visual acuity, the greater basal diameter and the thickness of the tumor and type of the treatment (125I versus 106Ru) adjusted for age, sex and pre-treatment vision, greater basal diameter and thickness, primary differences of these parameters and the distance of the tumor margin from fovea and optic disc and dose rate to the apex and the received dose of fovea and optic disc were assessed.
Results
We studied 35 patients with unilateral choroidal melanoma. The mean age at the time of treatment was 47.3 years (range, 21–77 years; standard deviation [SD], 13.8 years). There were 14 females and 21 males. The tumor affected the right eye choroid in 18 patients. The mean maximal basal diameter of the tumor was 13.4 mm (range, 5–20.6 mm; SD, 3.6 mm). The mean tumor height was 7.6 mm (range, 3–14.3 mm; SD, 2.9 mm). The mean pre-treatment vision was 0.98 LOGMAR (range, 0–2.6; SD, 0.83 mm). All but one choroidal melanoma was pigmented. Subretinal fluid was present over and around the tumors in 33 patients. The maximum tumor height treated in the series was 14.3 mm in a 32-year-old drug addicted man. He refused enucleation at all, despite our insistence. He had a 12 x 8 x 14.3 mm choroidal, mushroom-shaped, melanoma near inferior arcade. He felt a casual shade on the vision (zero LOGMAR) at the time of diagnosis. By 2.5 years of follow-up, at the completion of data collection in this study, the tumor remnant was 9.4 x 8.0 x 4.9 mm with a visual acuity of 1.0 LOGMAR. The scleral received dose was 431 Gy and the apex received dose was 82 Gy by 125I plaque.
No patients had clinical evidence of extraocular extension and lymph nodal involvement at the time of diagnosis. The mean follow-up of these patients was 29.7 months (range, 15–54 months; SD, 9.0 months). Sub-retinal fluid disappearance was present in 25 (71.4%) of these tumors. Ciliary body was affected at the edge of 7 (20%) tumors (5 in 106Ru group). No patient had iris melanoma in our series. Chest and abdominal imaging and liver function test were performed for all patients. No metastatic disease and patient mortality was recorded during this follow-up period. The main complications and new systemic findings during follow-up period are presented in Table 3. There was no statistical difference in overall complications in both groups (P=0.477).
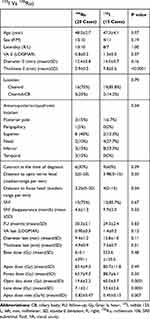 |
Table 1 Clinical Characteristics and Outcomes of Patients with Melanoma Treated with Two Types of Radioactive Plaques (125I Vs 106Ru) |
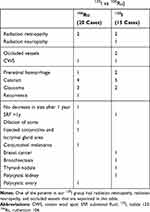 |
Table 3 Complications and Other Systemic Findings During the Follow-Up Period in Patients with Choroidal Melanoma Treated with Two Types of Radioactive Plaques (125I vs 106Ru) |
There were 15 patients treated with 125I and 20 patients with 106Ru episcleral plaque radiation therapy. Table 1 summarizes the clinical features of the patients, tumors and received doses. We enucleated a single eye because of re-growing of the tumor in 106Ru group, confirmed in consecutive two follow-ups. Pathology report affirmed the diagnosis. The pretreatment diameter was 16.5x15.0 mm with 7 mm of thickness. The absence of regrowth in 125I episcleral plaque radiation and small sample size, in this follows up period, impeded reliable comparison between two groups.
Visual acuity, diameter and thickness changes during the follow-up period in both treatment groups were compared using independent t-test. No significant difference between two groups was observed after treatment.
Pre-treatment visual acuity was 20/200 or better in 35 patients (60%). In 106Ru group, 14 cases (70%) had pretreatment visual acuity of 20/200 or better and for the 125I group it was 7 cases (46.7%) (P=0.04) (Table 1). Post-radiation last visual acuity was 20/200 or better in 12 cases (60%) in 106Ru patients and 5 cases (33%) in 125I group (P=0.34). The visual acuity change in 106Ru was 0.30 (range, −2.2–2.3; SD, 1.1) LOGMAR and in 125I was 0.16 LOGMAR (range, −1.7–2.1; SD, 1.0) (P=0.71).
Multivariate linear regression (MLR) analysis showed a significant correlation between the last diameter and the pre-treatment diameter after adjusting to age, gender, tumor thickness and dose rate (β = 0.59, 95% confidence interval [CI]: 0.29, 1.34, P = 0.003). This correlation was independent to the type of treatment.
The same MLR analysis for the final thickness showed no significant difference between two groups, after adjusting for age, sex, tumor first thickness and dose rate.
The final vision by MLR showed no significant difference between two treatments after adjusting with covariates like age, sex, first largest diameter and thickness of the lesion, dose rate and fovea and optic disc received dose (P>0.05).
After covariates adjustment (age and gender), MLR showed a significant correlation between the final vision and the distance of the tumor margin from fovea (β = -1.25, 95% CI: 0.02, 0.26, P = 0.02) and optic disc (β = 1.10, 95% CI: −0.02, 0.04, P = 0.01). This correlation was independent to the type of treatment.
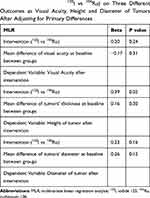
Considering the fact that height and diameter of the tumors at the baseline of the study were different in two intervention groups, we applied MLR analysis to control an important methodological issue named “confounding by indication.”
Table 2 shows that by applying MLR analysis to assess the effect of 125I compared to 106Ru on three different outcomes (visual acuity, thickness and diameter of ophthalmic tumors) adjusting for baseline differences, there is no priority between two interventions. This comparison was significant in favor of 125I in terms of tumor thickness (B=0.39, P=0.02). To make it simple, it means that the effectiveness of 125I is not only comparable to 106Ru but also superior when the outcome of the interest is the thickness of the tumors.
Discussion
The study showed that treatment with our 125I plaques was as effective as 106Ru brachytherapy to control choroidal melanoma basal diameter and vision preserving during a 30-month follow-up period. With regard to the decrease in lesion thickness, 125I plaques are more active than 106Ru plaques.
One of the reasons for the increase of brachytherapy application may be its assumed normal tissue sparing potential.5 In a retrospective study comparing the outcomes of patients with choroidal melanoma treated with 125I and 106Ru brachytherapy or proton beam radiation therapy (PBRT), patients treated with PBRT had a more rapid and substantial loss of vision than the ones treated with brachytherapy.18 The most commonly used radioactive sources to manage uveal melanoma with brachytherapy are beta emitters such as 106Ru or 90Sr plaques and low-energy gamma emitters such as 125I or 103Pd plaques.5–15
Preservation of useful visual acuity is principal goal of beta-ray treatment. In a long-term study, Lommatzsch and colleagues reported the predictability of a slow deterioration in visual acuity after 106Ru treatment to less than 20/200 (in 67.4%) and to no light perception (NLP; in 34.0%).12 Three years post-treatment, significant loss of visual acuity, defined as loss of six lines or more from baseline, was observed in 49% of the eyes treated with 125I (COMS), and 43% of them demonstrated the vision of 20/200 or worse.19 Other series utilizing 125I episcleral brachytherapy showed similar or superior preservation of visual acuity over a longer follow-up period.12,20–23 Radiation maculopathy, radiation-induced optic neuropathy and/or retinopathy, exudative retinal detachment, vitreous hemorrhage, cataract, neovascular glaucoma and/or enucleation are the most frequently recognized mechanisms for visual loss in such patients.12
In the current study after a 30-month follow-up, in 106Ru group, 60% (n=12) of the patients reserved 20/200 vision or more. We also achieved this outcome in 33% (n=5) of the 125I cases. According to the results, we observed a desirable trend in visual gain within 24 months post-treatment in the 125I group. Choroidal melanoma basal diameter and thickness and even pre-treatment vision were not influential predictors of the final vision in the current study in either group. It seems that regardless of the plaque, the distance between the tumor and fovea and the optic disc has an effect on the final visual acuity.
A previous study demonstrated that the curve for local tumor control did not plateau within the first 5 years of 106Ru treatment.24 Papageorgiou and colleagues reported the control rate of 85.7% (14 recurred while 13 did not respond) in their 189 patients treated with 106Ru plaques during 33 months of follow-up.24 In a recent retrospective study on 143 eyes with uveal melanoma treated with 106Ru plaque brachytherapy, the estimated local tumor recurrence rates at 12, 24, and 48 months after irradiation were 3%, 8.4%, and 14.7%, respectively.25 A comparative study showed that five-year melanoma recurrence rates were 11% and 4% in 106Ru and 125I plaque brachytherapy, respectively.18 Lommatzsch and colleagues reported a higher recurrence rate of 37% fifteen years after using 106Ru plaque brachytherapy.12 The control rate after 125I plaque treatment of choroidal melanoma was 95% during the first 10 years after plateauing at 3 years.26 This result was favorably comparable to the COMS study, where the 5-year local control rate was reported as 89.7%.18 Similar results were found in other institutional reports.11,27–30 In the current study, the lesion recurred only in a single case (4%) of 106Ru group and that eye was enucleated and confirmed in the pathology report. The pretreatment diameter was 16.5x15.0 mm with 7 mm of height in this case.
The decrease in tumor diameter and thickness showed a comparable course during a 30-month period in the two groups in the current study. In terms of the type of treatment, only the pretreatment diameter was correlated with the final diameter. Tumor size (largest basal diameter and thickness) is one of the most important clinical prognostic features of uveal melanoma.4,31–33 Due to the short follow-up period and small sample size, the prognostic factors could not be assessed with the current study database. The current study has no sufficient power for evaluating the type of regression pattern.34,35 According to the radiation dose, the application of 106Ru plaque brachytherapy is limited by a steep dose-gradient in tissue, with the upper limit of penetration judged to be approximately 5 mm.14,36 That is to say, in 106Ru plaques (as compared with 125I or 103Pd), the dose deposition of the plaque to the basal part of the tumor can be nine times more than that of the apex dose.24 Therefore, this radioisotope is only suitable for small to medium-sized melanomas. In contrast, centers using 125I or 103Pd plaques do not restrict their treatments based on tumor thickness.5 In the present study, the cases had comparable apex and basal doses in both groups, despite a higher apex dose rate in 106Ru. The mean apex dose rate in the patients was 0.82 Gy/hour for 106Ru compared with 0.45 Gy/hour for 125I plaques (Table 1).
Complications after eye plaque are caused by radiotherapy-specific factors (dose, dose rate, dose volume) and tumor-related factors (eg, tumor size, location, and its biologically variable response to irradiation).37,38 Due to different methods of dosimetry and tumor characteristics in different studies, it is difficult to compare different studies for the rate of complication. Radiation retinopathy and cataract are the most common complications.37–40 The rate of the radiation retinopathy was reported to be up to 95%.41 Another study comparing the 125I with 106Ru plaques in the tumors less than 5 mm thickness reported the radiation retinopathy in 50% and 74%, respectively, at 5 years of follow up.40 The rate of cataract was 3% and 37%, consequently, at the same time. In our study, the rate of radiation retinopathy was 13.3% for the 125I and 10% for 1°6Ru plaques. Vascular occlusion as simultaneous arteriolar and venular occlusions were observed only in the Iodide group. Overall, in 30 months of follow-up, no statistical difference in the rate of ocular complications was observed in this study.
Our study faced an important methodological issue named “confounding by indication.” The patients with more severe illness are likely to receive more intensive treatments, and when comparing the interventions, the more intensive intervention will appear to result in poorer outcomes. This is called “confounding by severity,” emphasizing that the degree of illness is the confounder.41
Confounding by indication is not conceptually different from confounding by other factors, and we controlled it by multivariate adjustment (MLR adjusted it to the primary differences of the measures).42,43 Concluding this analysis, it is shown that after adjusting for baseline differences, there was no significant difference between the two interventions considering regression in the thickness and diameter, recurrence and vision.
There are several other limitations that should be considered in this retrospective series. The most important ones are the short follow-up period for local recurrence, small sample size, study and the risk of bias arising from the retrospective collection nature and single center of the study. Because regrowth or complications after irradiation reached a peak during the first 3 years after treatment,12 the findings should be treated more carefully.
Conclusion
In summary, by an average of 30-month follow-up, 125I plaque radiotherapy resulted in 100% local control rate and vision preservation in 33% of cases by an average 2.5 years of follow-up. Patients treated with our 125I plaques experienced no new complications, which might preclude its acceptance. Our 125I plaques were comparable with 106Ru plaques brachytherapy in terms of efficacy and globe and vision saving even superior regarding thickness control.
Ethical Approval
This manuscript involves no conflict of interest or ethical adherence issues. This study was performed at Farabi Hospital (University Hospital), Tehran University of Medical Sciences, IR Iran. Ethical Approval code: 94-04-43-31148-390594
Acknowledgments
We thank all members of brachytherapy group for their kind help in this study.
Disclosure
Mr Mojtaba Arjmand reports he is Tehran University of Medical Sciences Research Deputy, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Jovanovic P, Mihajlovic M, Djordjevic-Jocic J, Vlajkovic S, Cekic S, Stefanovic V. Ocular melanoma: an overview of the current status. Int J Clin Exp Pathol. 2013;6:1230–1244.
2. Diener-West M, Earle JD, Fine SL, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma III: initial mortality findings. COMS Report No. 18. Arch Ophthalmol. 2001;119:969–982.
3. Melia M, Moy CS, Reynolds SM, et al.; Quality of Life Study Group. Quality of life after iodine 125 brachytherapy vs enucleation for choroidal melanoma: 5-year results from the Collaborative Ocular Melanoma Study: COMS QOLS Report No. 3. Arch Ophthalmol. 2006;124:226–238.
4. Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No 28. Arch Ophthalmol. 2006;124:1684–1693. doi:10.1001/archopht.124.12.1684
5. American Brachytherapy Society Ophthalmic Oncology Task Force. The American Brachytherapy Society consensus guideline for plaque brachytherapy of uveal melanoma and retinoblastoma. Brachytherapy. 2014;13:1–14. doi:10.1016/j.brachy.2013.11.008
6. Shishe Boran D, Grange JD, Patricot LM, et al. Histopathologic study of melanoma of the choroid after proton therapy. Ann Pathol. 1997;17:187–192.
7. Finger PT. Radiation therapy for choroidal melanoma (therapeutic review). Surv Ophthalmol. 1997;42:215–232. doi:10.1016/S0039-6257(97)00088-X
8. Lommatzsch PK. Results after beta-irradiation (106Ru/106Rh) of choroidal melanomas: 20 years’ experience. Br J Ophthalmol. 1986;70:844–885. doi:10.1136/bjo.70.11.844
9. Potter PD, Shields CL, Shields JA, Cater JR, Brady LW. Plaque radiotherapy for juxtapapillary choroidal melanoma. Arch Ophthalmol. 1996;114:1357–1365. doi:10.1001/archopht.1996.01100140557006
10. Mameghan H, Karolis C, Fisher R, et al. Iodine-125 irradiation of choroidal melanoma. Clinical experience from the Prince of Wales and Sydney Eye Hospitals. Australas Radiol. 1992;36:249–252. doi:10.1111/j.1440-1673.1992.tb03161.x
11. Packer S, Stoller S, Lesser ML, Mandel FS, Finger PT. Long-term results of iodine 125 irradiation of uveal melanoma. Ophthalmology. 1992;99:767–773. doi:10.1016/S0161-6420(92)31899-8
12. Lommatzsch PK, Werschnik C, Schuster E. Long-term follow- up of Ru-106/Rh-106 brachytherapy for posterior uveal melanoma. Graefes Arch Clin Exp Ophthalmol. 2000;238:129–137. doi:10.1007/PL00007880
13. Finger PT, Berson A, Ng T, Szechter A. Palladium-103 (103Pd) plaque radiotherapy for choroidal melanoma: an 11-year study. Int J Radiat Oncol Biol Phys. 2002;54:1438–1445. doi:10.1016/S0360-3016(02)03751-3
14. Valcarcel F, Valverde S, Cardenes H, et al. Episcleral iridium- 192 wire therapy for choroidal melanomas. Int J Radiat Oncol Biol Phys. 1994;30:1091–1097. doi:10.1016/0360-3016(94)90314-X
15. van Gindeuren R, van Limbergen E, Spileers W. 18 years’ experience with high dose rate strontium-90 brachytherapy of small to medium sized posterior uveal melanoma. Br J Ophthalmol. 2005;89:1306–1310. doi:10.1136/bjo.2005.068460
16. Sheibani S, Poorbeighi H, Ghassemi F, et al. Production, quality control, and running animal phase of 125i radioactive ophthalmic plaques. J Ophthalmol Bina. 2016;21:208–217.
17. Boldt HC, Melia BM, Liu JC, Reynolds SM; Collaborative Ocular Melanoma Study Group. I-125 brachytherapy for choroidal melanoma photographic and angiographic abnormalities: the Collaborative Ocular Melanoma Study: COMS Report No. 30. Ophthalmology. 2009;116:106–115.e1. doi:10.1016/j.ophtha.2008.10.013
18. Wilson MW, Hungerford JL. Comparison of episcleral plaque and proton beam radiation therapy for the treatment of choroidal melanoma. Ophthalmology. 1999;106:1579–1587. doi:10.1016/S0161-6420(99)90456-6
19. Damato B. Progress in the management of patients with uveal melanoma. the 2012 ashton lecture. Eye. 2012;26:1157–1172. doi:10.1038/eye.2012.126
20. Hungerford JL. Current trends in the treatment of ocular melanoma by radiotherapy. Clin Experiment Ophthalmol. 2003;31:8–13. doi:10.1046/j.1442-9071.2003.00611.x
21. Perez BA, Mettu P, Vajzovic L, et al. Uveal melanoma treated with iodine-125 episcleral plaque: an analysis of dose on disease control and visual outcomes. Int J Radiation Oncol Biol Phys. 2014;89:177–186. doi:10.1016/j.ijrobp.2014.01.026
22. Vonk DT, Kim Y, Javid C, Gordon JD, Stea B. Prescribing to tumor apex in episcleral plaque iodine-125 brachytherapy for medium-sized choroidal melanoma: A single-institutional retrospective review. Brachytherapy. 2015;14:726–733. doi:10.1016/j.brachy.2015.05.002
23. Berry JL, Dandapani SV, Stevanovic M, et al. Outcomes of choroidal melanomas treated with eye physics: a 20-year review. JAMA Ophthalmol. 2013;131:1435–1442. doi:10.1001/jamaophthalmol.2013.4422
24. Papageoriou KI, Cohen VM, Bunce C, Kinsella M, Hungerford JL. Predicting local control of choroidal melanomas following 106Ru plaque brachytherapy. Br J Ophthalmol. 2011;95:166–170. doi:10.1136/bjo.2009.176198
25. Tarmann L, Wackernagel W, Avian A, et al. Ruthenium-106 plaque brachytherapy for uveal melanoma. Br J Ophthalmol. 2015;99:1644–1649. doi:10.1136/bjophthalmol-2015-306666
26. Wagner A, Chen A, Cook T, Faber D, Winward K, Sause W. Outcomes and control rates for I-125 plaque brachytherapy for uveal melanoma: a community- based institutional experience. ISRN Ophthalmol. 2014;2014:950–975. doi:10.1155/2014/950975
27. Jampol LM, Moy CS, Murray TG, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: IV. Local treatment failure and enucleation in the first 5 years after brachytherapy. COMS report no. 19. Ophthalmology. 2002;109:2197–2206. doi:10.1016/S0161-6420(02)01277-0
28. Nag S, Wang D, Wu H, Bauer CJ, Chambers RB, Davidorf FH. Custom-made “Nag” eye plaques for 125I. Brachytherapy. Int J Radiation Oncol Biol Phys. 2003;56:1373–1380. doi:10.1016/S0360-3016(03)00324-9
29. Jensen AW, Petersen IA, Kline RW, Stafford SL, Schomberg PJ, Robertson DM. Radiation complications and tumor control after 125I plaque brachytherapy for ocular melanoma, Quivey JM, Augsburger J, Snelling L, Brady LW. Int J Radiation Oncol Biol Phys. 2005;63:101–108. doi:10.1016/j.ijrobp.2005.01.022
30. Qivey JM, Snelling L, Brady LW. 125 I plaque therapy for uveal melanoma. Analysis of the impact of time and dose factors on local control. Cancer. 1996;77:2356–2362. doi:10.1002/(SICI)1097-0142(19960601)77:11<2356::AID-CNCR26>3.0.CO;2-V
31. Kujala E, Mäkitie T, Kivelä T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44:4651–4659. doi:10.1167/iovs.03-0538
32. The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma II: initial mortality findings. COMS report no 10. Am J Ophthalmol. 1998;125:779–796. doi:10.1016/S0002-9394(98)00039-7
33. Shields CL, Furuta M, Thangappan A, et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol. 2009;127:989–998. doi:10.1001/archophthalmol.2009.208
34. Rashid M, Heikkonen J, Kivelä T. Tumor regression after brachytherapy for choroidal melanoma: reduction of thickness and cross-sectional area by shape and regression pattern. IOVS. 2015;56:2612–2623.
35. Abramson DH, Servodidio CA, McCormick B, Fass D, Zang E. Changes in height of choroidal melanomas after plaque therapy. Br J Ophthalmol. 1990;74:359–362. doi:10.1136/bjo.74.6.359
36. Summanen P, Immonen I, Kivelä T, Tommila P, Heikkonen J, Tarkkanen A. Radiation related complications after ruthenium plaque radiotherapy of uveal melanoma. Br J Ophthalmol. 1996;80:732–739. doi:10.1136/bjo.80.8.732
37. Finger PT. Tumour location affects the incidence of cataract and retinopathy after ophthalmic plaque radiation therapy. Br J Ophthalmol. 2000;84:1068–1070. doi:10.1136/bjo.84.9.1068
38. Nag S, Quinvey JM, Earle JD, Followill D, Fontanesi J, Finger PT; American Brachytherapy Society. The American Brachytherapy Society recommendations for brachytherapy of uveal melanomas. Int J Radiat Oncol Biol Phys. 2003;56:544–555. doi:10.1016/S0360-3016(03)00006-3
39. Myers CA, Abramson DH. Radiation protection. Choroidal melanoma and iodine-125 plaques. J Ophthalmic Nurs Technol. 1988;7:103–107.
40. Takiar V, Voong KR, Gombos DS, et al. A choice of radionuclide: comparative outcomes and toxicity of ruthenium-106 and iodine-125 in the definitive treatment of uveal melanoma. Pract Radiat Oncol. 2015;5:e169–e176. doi:10.1016/j.prro.2014.09.005
41. Kyriacou DN, Lewis RJ. Confounding by indication in clinical research. JAMA. 2016;316:1818–1819. doi:10.1001/jama.2016.16435
42. Greenland S, Robirts J. Confounding and misclassification. Am J Epidemiol. 1985;122:495–506. doi:10.1093/oxfordjournals.aje.a114131
43. Leon DA. Failed or misleading adjustment for confounding. Lancet. 1993;342:479–481. doi:10.1016/0140-6736(93)91599-H
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.