Back to Journals » Research Reports in Clinical Cardiology » Volume 9
Comparison of fluoroscopy time in different catheter-engagement approaches to graft vessels in post-coronary artery-bypass graft angiography
Authors Ahmadi M, Khameneh Bagheri R, Keihanian F, Saeidinia A
Received 11 January 2018
Accepted for publication 15 August 2018
Published 25 September 2018 Volume 2018:9 Pages 27—31
DOI https://doi.org/10.2147/RRCC.S162216
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Kones
Mostafa Ahmadi,1 Ramin Khameneh Bagheri,1 Faeze Keihanian,2,3 Amin Saeidinia4
1Atherosclerosis Prevention Research Center, Imam Reza Hospital, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran; 2Pharmaceutical Research Division, Booali Research Center, Mashhad University of Medical Sciences, Mashhad, Iran; 3Cardiology Department, Imam Reza and Qaem Hospitals, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran; 4Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
Background: Although there is ongoing progress in coronary artery-bypass graft (CABG) surgery and percutaneous coronary intervention techniques and supplies, the risk of cardiac complications remains high compared with the normal population.
Aim: In this study, our aim was to compare fluoroscopy times in engagement of three different catheters in saphenous vein grafts (SVGs) in post-CABG patients undergoing angiography.
Methods: This was a single-center, cross-sectional, comparative study. We evaluated patients with previous CABG referred for invasive coronary diagnostic angiography. Patients having had SVG–obtuse marginal artery, SVG–diagonal, and SVG–posterior descending artery CABG were included. All patients underwent diagnostic angiography by each of a right diagnostic Judkins catheter, right modified Amplatz catheter, and right guiding Judkins catheter. Demographics and clinical history of patients and fluoroscopy time in different groups were evaluated.
Results: A total of 61 patients were evaluated. The distribution of baseline characteristics in the three groups of our study was normal. Mean fluoroscopy time in SVG–obtuse marginal artery was 25.70±6.70 seconds in group A, 22.23±6.51 seconds in group B, and 17.35±7.82 seconds in group C. Mean total fluoroscopy time was 86.35±16.28 seconds in group A, 73.80±10.00 seconds in group B, and 51.90±10.22 seconds in group C, which was significant (P<0.001).
Conclusion: Our data suggest that when we use the guiding Judkins catheter, fluoroscopy time decreases. However, more evaluations are needed with larger-scale studies and identification of other variables.
Keywords: cardiac catheter type, coronary artery-bypass graft surgery, percutaneous coronary intervention, fluoroscopy time
Methods
Study population
This was a single-center, cross-sectional, comparative study based on data collected from medical records and information obtained and recorded. We evaluated all patients with a history of CABG referred for invasive coronary diagnostic or therapeutic procedures between 2015 and 2017 at the Interventional Cardiology Department of Qaem Hospital, Mashhad University of Medical Sciences by one operator. Patients having had all three of SVG–obtuse marginal (OM) artery, SVG–diagonal, and SVG–posterior descending artery (PDA) CABG were included. We excluded patients with existence of tortuosity >45° in the right femoral access site. All patients underwent diagnostic angiography by femoral access with each of a right diagnostic Judkins catheter, right modified Amplatz catheter, and right guiding Judkins catheter.
Procedures
Subcutaneous infiltration with 15–20 mL 2% lidocaine was done. Then, the femoral artery was punctured under the inguinal ligament with an 18 G needle (using the modified Seldinger method) with insertion of a 6F or 7F sheath. After that, 2,500 IU UFH was prescribed. Hemostasis was achieved with manual hand compression for 2 hours, or in cases of activated clotting <180 seconds. After fluoroscopy-time calculation in diagnostic angiography, percutaneous coronary intervention was performed in patients who needed it.
Outcomes and definitions
The efficacy of the methods studied was assessed by the success rate of the procedure, determined as completion of a coronary angiography and left ventriculography with adequate coronary and graft opacification, or in therapeutic interventions, taking a residual lesion <20%, without the need to alter the access port. The length of the process and fluoroscopy time were calculated from the start of the arterial puncture to the removal of the last catheter. However, we defined fluoroscopy time as time from the exit of 0.035-gauge wire or end of Right coronary artery angiography till the establishment of a catheter in the aorta root. Procedural safety was assessed by the occurrence of vascular adverse events contributing to the puncture site, such as hematoma >5 cm, severe bleeding, pseudoaneurysm, arteriovenous fistula, arterial occlusion, or need to repair vascular surgery.
Statistical analysis
All data were entered in SPSS version 19.0 and analyzed. Qualitative variables are listed as frequencies and percentages. Quantitative data are indicated as means ± SD. Comparisons between groups were done by c2 or Fisher’s exact test for qualitative variables and Student’s t-test or Mann–Whitney U test for quantitative variables. P<0.05 was considered statistically significant.
Ethics
Written informed consent from all patients was obtained for participation in the study. This study was done according to Mashhad University of Medical Sciences ethical committee guidelines and approved by the committee.
Results
A total of 61 patients were evaluated. The mean age of patients was 56.96±11.34 (32–80) years. Most were female (31, 50.8%). Table 1 shows the demographic data of patients.
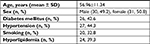  | Table 1 Demographic data of patients in the study |
The distribution of baseline characteristics in the three groups of our study can be seen in Table 2.
  | Table 2 Frequency of baseline characteristics Notes: Group A, using right diagnostic Judkins catheter; group B, using right modified Amplatz catheter; group C, using right guiding Judkins catheter. |
Mean fluoroscopy time in SVG–OM artery was 25.70±6.70 seconds in group A, 22.23±6.51 seconds in group B, and 17.35±7.82 seconds in group C. Other fluoroscopy times can be seen in Table 3 and Figure 1.
Mean total fluoroscopy time was 86.35±16.28 seconds in group A, 73.80±10.00 seconds in group B, and 51.90±10.22 seconds in group C, which was significant (P<0.001). There was no significant relationship between demographic data and fluoroscopy time (P>0.05).
Discussion
Procedures in cardiological intervention involve high-dose radiation to patients because of the extended use of fluoroscopy, various cine runs, and the difficulty of the procedures.13,14 Innovative catheter designs have been developed to allow diagnostic coronary angiography with a single catheter for both coronary arteries with the aim of reducing vasospasm, radiation dosage, and procedure time. Alternatively, conventional femoral approach catheters are also frequently used for transradial access, eg, Judkins left (JL) for the left coronary artery and Judkins right or Amplatz right I for the right coronary artery.15 Despite the lower estimation of risk of radiation exposure for interventionists, there is growing concern about this issue in cardiac catheterization.16
Long-term, low-dose exposure to radiation in the cardiac catheterization laboratory is related to a limited but not negligibly higher risk of cancers.12 Although there is no definite proof of a link between radiation exposure in the cardiac catheterization lab and higher risk of cancer, there are risk-prediction models in which the risk of cancer is deemed to be increased in lab personnel.12 In the past two decades, radiation-dose exposure for primary operators in cardiac catheterization labs not changed.17 However, advances in recent years in lowering scatter-emitted radiation by fluoroscopy/cine-angiography tools raise expectations of reduced radiation exposure for operators. This can be offset by the increased complexity of different cases that occur in modern cardiac catheterization labs. This problem and an inability to affect radiation dose for operators emphasizes the necessity for new shielding methods for lowering radiation exposure. It has been shown that radiation scatter reduction markedly reduces radiation exposure in both patients and operators during interventional fluoroscopic procedures.18
Catheter choice usually is dependent on such factors as operator experience, training, orientation of ostia, and shape of the aorta. For instance, a large aorta makes it very hard to use a Judkins catheter to reach the vein-graft ostium. Similarly, Amplatz catheters have been used successfully in patients having vein grafts with superior takeoff. Cannulation of grafts on the right side may be dependent on the orientation of the right coronary ostium. Most cases with horizontal takeoff might be cannulated more easily with Judkins right catheters. Some right coronary grafts may have steep takeoff, making Judkins catheter use technically challenging, and may be better served using multipurpose catheters with shallow angulation. Therefore, we consider these factors during procedures. Our results showed that using a right guiding Judkins catheter in post-CABG patients can significantly reduce fluoroscopy time over right diagnostic Judkins and Amplatz catheters in diagnostic angiography. This can help to reduce procedure time and lessen radiation exposure for the patient and operator, lowering the risk of radiation-induced cancer. In this study, we excluded patients who underwent more than one-time try to engagement to the artery, in order to eliminate the confounding factor engagement difficulty. Our study population was limited to these three types of grafts, which was a limitation. This issue was related to the fact that most of our patients had these three types of venous grafts in the center and there were no other venous grafts to be compared. Our results also showed a priority for SVG–OM rather than SVG–diagonal or SVG–PDA grafts. This priority can be due to the anterolateral position of the SVG–OM ostium and higher level of its origin, which allows more time to maneuver and higher probability of engagement. There have been few investigations to compare catheter shape and rate of procedural success for the transfemoral approach in coronary angiography. Vorpahl et al15 demonstrated that fluoroscopy time was significantly less in a Tiger II (2.4±1.5 minutes) than a conventional catheter (3.1±2.5 minutes; P=0.01), a major reason for which was the higher use of supplemental catheters (crossover) in Tiger II. In addition, fluoroscopy times after crossover were significantly greater in the conventional catheter (5.8±0.7, P=0.0001) than the Tiger II (7.6±3.0 minutes, P=0.0001). Fluoroscopy time was very similar between the conventional catheter and the Tiger II without crossover (2.2±1.2 min vs 2.3±1.2 min). In 2006, Kim et al19 made a comparison of the Tiger II and Judkins left catheter by measuring procedure time and fluoroscopy time. They found superiority for right coronary angiographic quality with the Tiger II and a marked benefit in process and fluoroscopy time without difference for left coronary angiographic quality. Overall, fluoroscopy time in the prospective randomized trial of Kim et al was significantly lower in the Tiger II (1.55 minutes) vs conventional catheter (2.3 minutes).19 SVG markers assist the angiographer by pinpointing the ostium of the aorta vein-graft anastomosis and by demonstrating the number of vein-graft ostia that must be cannulated at catheterization, significantly decreasing fluoroscopy time. However, in this study, we routinely used the markers by angiographer and all of the processes were performed in the same way in all three groups.
By reducing fluoroscopy time in our study with the right guiding catheter, the risk of cancer in patients and operators can be lowered. Another benefit is better engagement and lowering manipulation that can lead to lower rate of emboli risk in patients. Also, by lowering fluoroscopy time, the usage of dye will decrease and thus lower the rate of contrast-induced nephropathy. However, assessment of contrast-induced nephropathy in this study was not logical, because we did not have any patient with it.
Limitations
There are several limitations to our study. The design was based on the existence of previous cases, and this made our study affected by some confounding factors. While during patient engagement, these points were followed, we did not access documented reports for all cases. We plan future studies based on this report and hope to resolve these limitations. In our setting, the three reported vein grafts were the most frequent, and we did not have other venous grafts. The findings from our study are hypothesis-generating and may need further validation by a larger prospective randomized trial.
Conclusion
This study and other similar publications highlight the importance of catheter choice and operator training as key components in successful procedures. Our data suggest that when the guiding Judkins catheter is used, fluoroscopy time will decrease and lead to the benefits mentioned. However, more evaluations are needed in the form of large-scale studies and identification of other variables, eg, contrast volume, success in engagement, and other confounding factors.
Acknowledgment
We thank all nurses of the Cardiac Catheterization Laboratory of Qaem Hospital for their cooperation in performing the study.
Disclosure
The authors report no conflicts of interest in this work.
References
Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996;28(3):616–626. | ||
Kim MS, Wang TY, Ou FS, et al. Association of prior coronary artery bypass graft surgery with quality of care of patients with non-ST-segment elevation myocardial infarction: a report from the National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With the Guidelines. Am Heart J. 2010;160(5):951–957. | ||
Szavits-Nossan J, Stipic´ H, Sesto I, Kapov-Svilicic´ K, Sipic´ T, Bernat R. Angiographic control and percutaneous treatment of myocardial ischemia immediately after CABG. Coll Antropol. 2012;36(4):1391–1394. | ||
Fabricius AM, Gerber W, Hanke M, Garbade J, Autschbach R, Mohr FW. Early angiographic control of perioperative ischemia after coronary artery bypass grafting. Eur J Cardiothorac Surg. 2001;19(6):853–858. | ||
Hanratty CG, Koyama Y, Ward MR. Angioplasty and stenting of the distal coronary anastomosis for graft failure immediately after coronary artery bypass grafting. Am J Cardiol. 2002;90(9):1009–1011. | ||
He PY, Yang YJ, Qiao SB, et al. A comparison of the transradial and transfemoral approaches for the angiography and intervention in patients with a history of coronary artery bypass surgery: in-hospital and 1-year follow-up results. Chin Med J. 2015;128(6):762. | ||
FriscI. Invasive compared with non-invasive treatment in unstable coronary-artery disease: FRISC II prospective randomised multicentre study. FRagmin and Fast Revascularisation during In Stability in Coronary artery disease Investigators. Lancet. 1999;354(9180):708–715. | ||
Cannon CP, Weintraub WS, Demopoulos LA, Vicari R, Frey MJ, Lakkis N. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. New England Journal of Medicine 2001;344:1879–87. | ||
Fox K, Poole-Wilson P, Henderson R, Clayton T, Chamberlain D, Shaw T. Interventional versus conservative treatment for patients with unstable angina or non-ST-elevation myocardial infarction: the British Heart Foundation RITA 3 randomised trial. The Lancet 2002;360:743–51. | ||
Asrar Ul Haq M, Rudd N, Mian M, et al. Predictors and outcomes of early coronary angiography in patients with prior coronary artery bypass surgery presenting with non-ST elevation myocardial infarction. Open Heart. 2014;1(1):e000059. | ||
Brasselet C, Blanpain T, Tassan-Mangina S, et al. Comparison of operator radiation exposure with optimized radiation protection devices during coronary angiograms and ad hoc percutaneous coronary interventions by radial and femoral routes. Eur Heart J. 2008;29(1):63–70. | ||
Venneri L, Rossi F, Botto N, et al. Cancer risk from professional exposure in staff working in cardiac catheterization laboratory: insights from the National Research Council’s Biological Effects of Ionizing Radiation VII Report. Am Heart J. 2009;157(1):118–124. | ||
Fletcher DW, Miller DL, Balter S, Taylor MA. Comparison of four techniques to estimate radiation dose to skin during angiographic and interventional radiology procedures. J Vasc Interv Radiol. 2002;13(4):391–397. | ||
Lobotessi H, Karoussou A, Neofotistou V, Louisi A, Tsapaki V. Effective dose to a patient undergoing coronary angiography. Radiat Prot Dosimetry. 2001;94(1-2):173–176. | ||
Vorpahl M, Koehler T, Foerst J, et al. Single Center Retrospective Analysis of Conventional and Radial TIG Catheters for Transradial Diagnostic Coronary Angiography. Cardiol Res Pract. 2015;2015:862156–6. | ||
Brasselet C, Blanpain T, Tassan-Mangina S, et al. Comparison of operator radiation exposure with optimized radiation protection devices during coronary angiograms and ad hoc percutaneous coronary interventions by radial and femoral routes. Eur Heart J. 2008;29(1):63–70. | ||
Kim KP, Miller DL, Balter S, et al. Occupational radiation doses to operators performing cardiac catheterization procedures. Health Phys. 2008;94(3):211–227. | ||
Dromi S, Wood BJ, Oberoi J, Neeman Z. Heavy metal pad shielding during fluoroscopic interventions. J Vasc Interv Radiol. 2006;17(7):1201–1206. | ||
Kim S-M, Kim D-K, Kim D-I, Kim D-S, Joo S-J, Lee J-W. Novel diagnostic catheter specifically designed for both coronary arteries via the right transradial approach. Int J Cardiovasc Imaging. 2006;22(3-4):295–303. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


