Back to Journals » Clinical Ophthalmology » Volume 16
Comparison of Efficacy and Safety of Two Commercial Artificial Tears Between 0.18% and 0.3% Sodium Hyaluronate for Corneal Epithelial Healing in Pterygium Excision with Conjunctival Autograft Transplantation: A Study Protocol for a Randomized Controlled Trial
Authors Chaidaroon W, Pantarote S, Upaphong P , Choovuthayakorn J
Received 31 August 2022
Accepted for publication 18 November 2022
Published 30 November 2022 Volume 2022:16 Pages 3935—3944
DOI https://doi.org/10.2147/OPTH.S388276
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Winai Chaidaroon, Suphitcha Pantarote, Phit Upaphong, Janejit Choovuthayakorn
Department of Ophthalmology, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
Correspondence: Winai Chaidaroon, Department of Ophthalmology, Faculty of Medicine, Chiang Mai University, 110 Intawaroros Road T.Suthep A.Muang, Chiang Mai, 50200, Thailand, Tel +66-53-935512 ; +66-891415512, Fax +66-53-936121, Email [email protected]
Purpose: To compare the efficacy, safety, and rate of healing on the corneal epithelial defect after pterygium surgery through the application of either 0.18% or 0.3% sodium hyaluronate (SH).
Methods: A randomized, double-blind clinical trial was performed on patients who had pterygium surgery. Eighty-six patients were randomized to 2 groups that would receive either 0.18% SH or 0.3% SH. Measurements of area of the corneal epithelial defect using ImageJ freeware were performed. All corneal epithelial defects were measured immediately after the operation (Day 0) and for the next 3 days.
Results: The mean and SD of the area of corneal epithelial defect measured on postoperative Day 0, 1, and 2 were 9.13 ± 3.09 mm2, 5.61 ± 3.26 mm2, and 3.39 ± 2.70 mm2 for 0.18% SH group, and 8.96 ± 3.17 mm2, 4.03 ± 1.99 mm2, and 1.55 ± 1.23 mm2 for 0.3% SH group. There was no statistically significant difference of the initial area of the corneal epithelial defect on Day 0 between 0.18% and 0.3% SH group (p = 0.802). The area of the defects in the 0.3% SH group was significantly smaller than that of the 0.18% SH group on both Day 1 and Day 2 (p = 0.007, p < 0.001), respectively. Similarly, the 0.3% SH group exhibited a statistically significant higher (p < 0.001) rate of healing of the corneal epithelial defect over days 0 and 1 (4.94 ± 2.16 mm²/day) when compared to that of the 0.18% SH group (3.53 ± 1.66 mm²/day).
Conclusion: With two commercial artificial tears, the corneal epithelial wound healing after pterygium surgery was faster in the 0.3% SH group than that of the 0.18% SH group. Superiority of 0.3% SH may be supported by the presence of epsilon-aminocaproic acid in this drug preparation. No significant adverse effects were exhibited during the short-term follow-up.
Keywords: 0.18% hyaluronic acid, 0.3% hyaluronic acid, hyaluronic acid, corneal epithelial defect, pterygium surgery
Introduction
Although superior conjunctival autograft transplantation for pterygium treatment was a gold standard for pterygium treatment,1 the corneal epithelial defect always existed after the surgery. Rapid corneal epithelial wound healing could decrease sight-threatening complications, such as infectious keratitis, corneal perforation, or corneal scar,2,3 and lessen comorbidities reported in these patients, including pain, eye discomfort, and photophobia.4 Various pharmacological interventions had been described to expedite corneal epithelial healing such as growth factors,5 corticosteroids,6 and sodium hyaluronate (SH).7
SH is a linear, non-sulfated glycosaminoglycan composed of b-1,3-N-acetyl-D-glucosamine and b-1,4-glucuronic acid repeating disaccharide units with viscoelastic rheology.8–10 With its extraordinary biocompatibility, excellent bioadhesion, and receptor recognition characteristics, SH had been widely used in ophthalmology, including dry eye treatment, drug delivery vehicle, ophthalmic viscoelastic device, and corneal wound repairing.10 Several studies revealed SH eye drops promoted corneal epithelialization.11–13 SH eye drops facilitated corneal epithelial healing and the rate of healing associated with the concentration of SH in the animal model study.13
The purpose of this study is to compare the efficacy between 0.18% and 0.3% SH in terms of corneal epithelial wound healing after pterygium excision with conjunctival autograft transplantation because SH has unique characteristics for corneal wound healing and the higher concentration (0.3% SH) has recently been introduced. To our best knowledge, this is the first study comparing the rate of human corneal epithelial wound healing using 0.18% and 0.3% SH after pterygium surgery.
Methods
Study Material
1. 0.18% SH (Vislube® preservative-free monodose, Holopack Verpackungstechnik GmbH, Abtsgmünd-Untergröningen, Germany), a monodose unit contains 0.3 mL hypotonic (150 mOsm/L) solution. The molecular weight (MW) of SH is 1.2 × 106 Da. It consists of sodium hyaluronate, sodium chloride, potassium chloride, disodium-hydrogen phosphate, sodium citrate, magnesium chloride, calcium chloride, and water for injection.
2. 0.3% SH (Hialid® Mini 0.3 preservative-free monodose, Santen Pharmaceutical Co., Ltd, Ishikawa, Japan), a monodose unit contains 0.4 mL isotonic (286 mOsm/L) solution with 1.0 × 106 Da of MW. Each monodose contains sodium chloride, potassium chloride, epsilon aminocaproic acid, disodium edetate hydrate, sodium hydroxide, dilute hydrochloric acid, and purified water.
Because the two SH dosage levels had different packaging, open-label drug administration was required. The physician (S.P.) who administered the SH treatment was excluded from discussions about the protocol and all post-operative treatment and data collection to maintain a strict double-blind research protocol despite the open label.
SH played an important role to promote corneal wound healing and accelerating re-epithelization by activating the cluster of differentiation 44 (CD 44) receptors which were presented on corneal epithelial cells.6
Study Design
A prospective, randomized, parallel-group, open-label interventional study was conducted among patients diagnosed with primary pterygium at the Ophthalmology Clinic, Chiang Mai University Hospital between May 17, 2022 and August 15, 2022. The efficacy, in terms of corneal epithelial wound healing rate, of 0.18% SH (Vislube® preservative-free monodose, Holopack Verpackungstechnik GmbH, Germany) was compared to 0.3% SH (Hialid® Mini 0.3 preservative-free monodose, Santen Pharmaceutical Co., Ltd, Ishikawa, Japan). The study protocol was performed under Good Clinical Practice and in accordance with the tenets of the Declaration of Helsinki and approved by the Research Ethics Committee of Faculty of Medicine, Chiang Mai University (Study code: OPT-2564-08049), and Thai Clinical Trial Registry Committee (Study ID: TCTR 20210804003, https://www.thaiclinicaltrials.org/). All participants provided their written informed consent before starting the treatment. Consolidated Standards of Reporting Trials (CONSORT) guidelines were followed.
Study Population and Treatment
We recruited patients aged between 20 years and 70 years old with a diagnosis of unilateral primary pterygium. All patients enrolled in the study have comprehensive eye examinations. The following diseases and/or conditions were excluded: recurrent pterygium, corneal abnormalities, ocular surface disease,14 corneal decompensation, ocular malignancy, ocular infections, glaucoma, concurrent ophthalmic drug use, history of previous ocular surgery or trauma,15,16 and systemic diseases that interfere with corneal wound healing.17
Eligible 86 patients were divided into either 0.18% SH group or 0.3% SH group. As age was a confounding factor that influenced corneal wound healing, the patients were divided into ages <50 and ≥50 years old. Then, each age group was randomized by a permuted block of 4 into 0.18% SH group and 0.3% SH group.
The surgery involved pterygium excision with superior conjunctival autografting associated with transferring a free conjunctival graft of superior bulbar conjunctiva to cover the bare sclera using an operating microscope.18,19 All cases were operated by one surgeon (W.C.) without retrobulbar or eyelid anesthesia. The operating time was approximately 20 minutes in each case.
Surgical Procedure
- The surgical fields were applied with 10% povidone-iodine. One drop of 2% lidocaine jelly (AstraZeneca, Xylocaine Jelly 2%®, Södertälje, Sweden) was administered to the conjunctiva and cornea for 10 minutes prior to the operation. An eyelid speculum was applied to the eyelids.
- After rinsing with 0.9% normal saline solution, 0.5 mL of 1% lidocaine/1:100,000 adrenaline mixture (AstraZeneca Xylocaine 1%®, Södertälje, Sweden) was injected into the subtenon space beneath the pterygium via a 27-gauge needle.
- Head of the pterygium and fibrovascular tissue were superficially excised by a surgical blade number 15. Pterygium was excised using Westcott scissors. The remaining subtenon tissue was extensively removed with minimal cauterization.
- 0.5 mL of one percent plain lidocaine (AstraZeneca Xylocaine 1%®, Södertälje, Sweden) was injected with a 27-gauge needle subconjunctivally between the conjunctiva and tenon at the superior border of the planned excised conjunctival graft in the 12 o’clock position when the patient looked down.
- A free conjunctival graft from superior conjunctiva was tenderly excised by Westcott scissors. After placing the conjunctival graft on the bare scleral area, the graft was sutured with 9 interrupted style stitches of 8–0 polyglactin. The eye was patched.
After postoperative recovery, the eye patch was removed and the corneal epithelial defects caused by pterygium excision were stained by a fluorescein paper strip (Chona Surgical Co., Fluorescein Sodium-Test Strip®, Delhi, India) and photographed using the digital camera equipped with SL-D701 slit-lamp (Topcon Corporation, Topcon®, Tokyo, Japan) with the cobalt blue exciter filter to record the area of the epithelial defect. This postoperative exam of all patients was done by 1 investigator (P.U.). This was followed by the application of either 0.18% SH or 0.3% SH 4 times daily by a separate physician (S.P.). Then, the eye was unpatched.
On postoperative Day 0, in both groups, a topical 0.5% levofloxacin (Santen, Cravit®, Osaka, Japan) eye drop and 1% prednisolone acetate (Allergan, Pred-Forte®, Westport, Ireland) eye drop were prescribed 4 times daily for 4 weeks.
The Measurement of Corneal Epithelial Defect
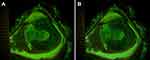
The follow-up comprised the daily ophthalmic examination and photographs at a fixed time for 4 days (Day 0 (after surgery), 1, 2, and 3). The fluorescein-stained corneal epithelial defect photos were saved as uncompressed Joint Photographic Experts Group files (6208 × 2294 pixels) RGB files using the IMAGEnet®6, version 3.0.1. Each photo was evaluated by one investigator (P.U.). The area of fluorescein staining was computed by the ImageJ freeware version 15.3k (National Institutes of Health, Rockville, MD; http://imagej.net/ImageJ) after the three times of manual delineation (Figure 1)20 and then was averaged. The fluorescein-stained area was calculated by the ImageJ freeware.
Statistical Analysis
Results from the feasibility assessment of 6 eyes were used to calculate the sample size. With the proportion for complete corneal epithelial wound healing of 67% (0.3% SH group) versus 33% (0.18% SH group) at 24 hours and assuming a drop out of 10%, a total sample size needed was 86 eyes. All data had been analyzed with the SPSS 21.0 statistical software (SPSS Inc., Chicago, IL, USA). Mean (standard deviation (SD)), median (range), t-test, and Chi-square test were conducted on the demographic and clinical characteristics of participants at baseline. The t-test was utilized to compare the area and healing rate of corneal epithelial defects between the two groups. Statistical significance was considered as a p-value less than 0.05.
Results
Figure 2 illustrates the CONSORT flow chart of the study. Ninety-two patients were recruited for this study. Six patients were excluded because two patients declined to participate in the protocol, two patients had previous glaucoma surgery, and two had diabetes mellitus. Therefore, 86 patients were randomized into one of the two groups, resulting in the 0.18% SH group of 43 patients in total, and 43 patients in the 0.3% SH group.
 |
Figure 2 Flow chart of the study design for a randomized controlled trial of the 0.18% SH group versus the 0.3% SH group. |
Baseline Characteristics
At the baseline, the demographic data including patient numbers, sex, age, morphologic grading of pterygium,21 and area of initial corneal epithelial defects had no significant differences (Table 1).
 |
Table 1 Demographics and Baseline Clinical Characteristics of Pterygium Patients |
Corneal Epithelial Healing
Figure 3 illustrates the corneal epithelial defects following instillation of either 0.18% SH group or 0.3% SH group. The mean ± SD of corneal epithelial defects on Day 1 and Day 2 were statistically lesser in the 0.3% SH group compared to the 0.18% group (5.61 ± 3.26 mm2 versus 4.03 ± 1.99 mm2, p = 0.007; 3.39 ± 2.70 mm2 versus 1.55 ± 1.23 mm2, p<0.001, respectively). On Day 3, all cases had complete corneal epithelial healing except one case in the 0.3% SH group and two cases in the 0.18% SH group still had small epithelial defects. All patients had complete re-epithelialization by Day 4.
 |
Figure 3 The comparison of the box plot of corneal epithelial defect on Day 0, 1, 2, and 3 between the 0.18% SH group versus the 0.3% SH group. |
The rate of corneal epithelial healing is shown in Figure 4. The 0.3% SH group exhibited a statistically significant higher (p < 0.001) rate of healing of the corneal epithelial defect over days 0 and 1 (4.94 ± 2.16 mm²/day) when compared to that of the 0.18% SH group (3.53 ± 1.66 mm²/day), whereas the healing rate of corneal epithelial defect from Day 1 to Day 2 showed no significant difference between the two groups (p < 0.363).
 |
Figure 4 Rate of corneal epithelial wound healing at postoperative period between the 0.18% SH group versus the 0.3% SH group. Notes: ● = 0.18% SH group, □ = 0.3% SH group. |
Safety Profiles
With daily slit-lamp biomicroscopy examination records and patient reports, patients in both groups had no significant adverse events that needed to discontinue the investigated medications. Only two cases in the 0.3% SH group had grittiness after 0.3% SH instillation but no adverse effect was observable in the 0.18% SH group.
Discussion
The compromised corneal epithelial defect appeared as part of elective procedures, primarily the ablations created for pterygium excision,22 photorefractive keratectomy,23 and intraoperative epithelial debridement for good visualization during retinal surgery.24 However, the corneal epithelial defect can result in significant morbidities, including blurred vision, considerable pain, photophobia, microbial keratitis, persistent corneal epithelial defect, corneal perforation, and ultimately visual loss.2,3,25 Corneal epithelial wound healing was a significant clinical problem. Therefore, speedy epithelial wound healing may decrease these complications. Several treatment strategies have been proposed to promote re-epithelialization including discontinuation of preservatives, corticosteroids, patching or contact lenses, biological factors (eg growth factors, cytokines, fibronectin, SH), and biological substances containing SH (eg amniotic membrane, autologous serum).26
SH has a crucial role in corneal epithelial wound healing.27 The healing of corneal epithelial wound involves a number of harmonic combination events including cell migration, upregulating repair response, and inhibiting inflammatory responses.28 SH has accelerated wound healing by adhering to CD 44 receptors. CD 44 the receptors, distributed in corneal epithelium, are the receptor in the repairing wound process.19,29 Zhong et al studied the efficiency of SH in promoting cell migration and their modulation of repair factors of human corneal epithelial cell model and demonstrated that the expression levels of repair factors such as CD 44 and fibronectin increased but the levels of inflammatory processes, including cytokines, interleukin 1-beta, and matrix metalloproteinase 9 (MMP-9) decreased.28
SH has long been recognized for its ability to treat epithelial defects in many clinical and experimental situations.19,30–32 In this current study, the MW of the two investigated medications was different approximately 2.0 × 105 Da (0.18% SH = 1.2 × 106 Da, 0.3% = 1.0 × 106 Da). The MW of SH affected the binding affinity between SH and CD 44, was related to the healing process.12 Although an MW of 0.18% SH that was slightly higher than an MW of 0.3% SH, our results showed that the corneal healing process was faster in the 0.3% SH group. Benzalkonium chloride, a common preservative, had been used for a long period in multidose eye drop composition. The toxicity was manifested by cell apoptosis and decreased density of conjunctival and corneal epithelia.33 Both 0.18% and 0.3% SH used in the current study had no preservatives; therefore, the corneal epithelial wound healing would not be disturbed. We investigated two commercially available artificial tear eye drops, which had different concentrations for efficacy, in terms of the rate of corneal epithelial healing after pterygium excision with conjunctival autograft transplantation. The area of the defects in the 0.3% SH group was significantly smaller on both Day 1 and Day 2 (p=0.007, p<0.001), respectively, when compared to that of the 0.18% SH group. Moreover, the rate of healing of the corneal epithelial defect over days 0 and 1 in 0.3% SH group was significantly faster than the 0.18% SH group. Our results with clinical application in real-life practice were in good agreement with the study of Camillieri et al,13 which reported that the reparative effect of SH on corneal epithelial healing was concentration dependent in the rabbit model. Nakamura et al also found that the effects of SH on corneal epithelial wound healing relied on SH concentrations in both diabetic and non-diabetic rats which were 0.1% and 0.3%, respectively. They concluded that SH promoted the healing rate in a dose-dependent manner.34 The current results also supported that the two commercial SH had different corneal epithelialization rates. One of the components of the commercial 0.3% SH preparation used in this study was epsilon-aminocaproic acid (EACA) which may have acted as a re-epithelialization promoter contributing to the faster re-epithelialization seen in the 0.3% SH group. As an antiplasmin agent, EACA is known to have the ability to inhibit the plasminogen activator-plasmin system. As such, fibronectin would not be metabolized and promote proper adhesion of epithelium.35 A previous study showed that EACA accelerated corneal epithelial healing in the rabbit and rat models by encouraging the anchoring of the regenerating epithelium to the underlying stroma.36,37 Consequently, EACA may have played an additional role in supporting a faster rate of corneal epithelial wound healing in the 0.3% SH group, beyond effects related to the higher concentration of SH.
The physical properties of SH are also important for the management of pterygium. Based on the rheological analysis, 0.18% SH which had a higher viscosity than 0.3% SH could prolong the ocular residence time and may facilitate corneal epithelial healing.38 However, the current study showed 0.3% SH had a more rapid wound healing effect than 0.18% SH. The pH of 0.3% SH in this study was 6.0–7.0 which was a weak acidic property, whereas pH of 0.18% SH was a weak alkaline property. This difference in pH of the two SH did not affect corneal epithelial healing in terms of increased ocular residence time. The ocular discomfort may be caused by the weak acidic property of 0.3% SH.38,39
In considering adverse event issues, the 0.3% SH group had moderate discomfort. This symptom may occur after a higher concentration of SH was instilled.40 However, they were well tolerated. Three eyes in this study needed 4 days for complete re-epithelialization. There were no complications, such as loose stitches, recurrence, persistent epithelial defect, or pyogenic granuloma, seen in either groups at the 1-month follow-up.
Strengths of this current study were found, such as all patients in both arms had regular follow-ups. Additionally, instead of manual measurement with width and length, ImageJ freeware provided precision in measuring the area of epithelial defect patients that decreased the personal bias. Different levels of pressure on eye patching may occur and affect the corneal epithelial wound repairing. Hence, after SH was instilled, no eye patch was needed afterward. This procedure would avoid the difference in pressure-related eye patches.
Study Limitations
- The medications between arms could not be blinded due to the difference in commercials containing packages of SH.
- The results of the current study may not represent the pure pharmacologic effect of SH, according to the EACA contained in 0.3% SH.
- We could not investigate the levels of inflammatory and repairing markers such as CD44, fibronectin, cytokines, and MMP-9 that affected corneal wound healing. These data would add objective support to the results obtained here.
Conclusion
With two commercial artificial tears, this first-in-human study provides evidence of the speed of corneal epithelial wound healing of monodose 0.3% SH on faster corneal re-epithelialization rate over monodose 0.18% SH without clinically significant adverse events during short-term follow-up. Superiority of 0.3% SH may be supported by the presence of EACA in this drug preparation. However, moderate discomfort occurred after 0.3% SH instillation on corneal epithelial defect following pterygium surgery.
Data Sharing Statement
The data used to support the findings of this study are included within the article. Further data or information is available from the corresponding author upon request.
Consent for Publication
All participants provided written informed consent prior to the study.
Ethics Approval and Informed Consent
The study protocol was conducted in accordance with the tenets of the Declaration of Helsinki and approved by the Ethics committee of Faculty of Medicine, Chiang Mai University (Study code: OPT-2564-08049), and Thai Clinical Trial Registry Committee (Study ID 20210804003, https://www.thaiclinicaltrials.org/). Written informed consent was obtained from all participants.
Consent for Publication
All participants provided written informed consent prior to the study.
Acknowledgments
The authors thank Miss Kittika Kanjanarattanakorn for kind assistance with the statistical analysis and Dr. Robert P. Batzinger, PhD., for revision of the English manuscript.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding
This study was supported by the Faculty of Medicine Endowment Fund, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand (Grant Number 027/2565).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Shusko A, Schechter BA, Hovanesian JA. Pterygium surgery utilizing limbal conjunctival autograft and subconjunctival amniotic membrane graft in high-risk populations. Clin Ophthalmol. 2020;14:2087–2090. doi:10.2147/OPTH.S243584
2. Wirostko B, Rafii M, Sullivan DA, Morelli J, Ding J. Novel therapy to treat corneal epithelial defects: a hypothesis with growth hormone. Ocul Surf. 2015;13:204–212. doi:10.1016/j.jtos.2014.12.005
3. Chaidaroon W, Isipradit S, Upaphong P, Dejkriengkraikul C. A randomized controlled trial to manage postoperative ocular pain after pterygium excision with conjunctival autograft transplantation with a single application of 2% sodium hyaluronate. Pain Res Manag. 2022;2022:5144516. doi:10.1155/2022/5144516
4. Malafa MM, Coleman JE, Bowman RW, Rohrich RJ. Perioperative corneal abrasion: updated guidelines for prevention and management. Plast Reconstr Surg. 2016;137:790e–798e. doi:10.1097/PRS.0000000000002108
5. Gallego-Muñoz P, Ibares-Frías L, Valsero-Blanco MC, Cantalapiedra-Rodriguez R, Merayo-Lloves J, Martínez-García MC. Effects of TGFβ1, PDGF-BB, and bFGF, on human corneal fibroblasts proliferation and differentiation during stromal repair. Cytokine. 2017;96:94–101. doi:10.1016/j.cyto.2017.03.011
6. Mobaraki M, Abbasi R, Omidian Vandchali S, Ghaffari M, Moztarzadeh F, Mozafari M. Corneal repair and regeneration: current concepts and future directions. Front Bioeng Biotechnol. 2019;7:135. doi:10.3389/fbioe.2019.00135
7. Chang WH, Liu PY, Lin MH, et al. Applications of hyaluronic acid in ophthalmology and contact lenses. Molecules. 2021;26:2485. doi:10.3390/molecules26092485
8. Bayer IS. Hyaluronic acid and controlled release: a review. Molecules. 2020;25:2649. doi:10.3390/molecules25112649
9. Zhang X, Wei D, Xu Y, Zhu Q. Hyaluronic acid in ocular drug delivery. Carbohydr Polym. 2021;264:118006. doi:10.1016/j.carbpol.2021.118006
10. Lin T, Gong L. Sodium hyaluronate eye drops treatment for superficial corneal abrasion caused by mechanical damage: a randomized clinical trial in the People’s Republic of China. Drug Des Devel Ther. 2015;9:687–694. doi:10.2147/DDDT.S77270
11. Ho WT, Chiang TH, Chang SW, Chen YH, Hu FR, Wang IJ. Enhanced corneal wound healing with hyaluronic acid and high-potassium artificial tears. Clin Exp Optom. 2013;96:536–541. doi:10.1111/cxo.12073
12. Zhong L, Liu Y, Xu L, et al. Exploring the relationship of hyaluronic acid molecular weight and active targeting efficiency for designing hyaluronic acid-modified nanoparticles. Asian J Pharm Sci. 2019;14:521–530. doi:10.1016/j.ajps.2018.11.002
13. Camillieri G, Bucolo C, Rossi S, Drago F. Hyaluronan-induced stimulation of corneal wound healing is a pure pharmacological effect. J Ocul Pharmacol Ther. 2004;20:548–553. doi:10.1089/jop.2004.20.548
14. Hilmi MR, Che Azemin MZ, Mohd Kamal K, Mohd Tamrin MI, Abdul Gaffur N, Tengku Sembok TM. Prediction of changes in visual acuity and contrast sensitivity function by tissue redness after pterygium surgery. Curr Eye Res. 2017;42:852–856. doi:10.1080/02713683.2016.1250277
15. Mohd Radzi H, Khairidzan MK, Mohd Zulfaezal CA, Azrin EA. Corneo-pterygium total area measurements utilising image analysis method. J Optom. 2019;12:272–277. doi:10.1016/j.optom.2019.04.001
16. Hilmi MR, Khairidzan MK, Azemin ZC, Azami MH, Ariffin AE. Measurement of contrast sensitivity using the M&S Smart System II compared with the standard Pelli–Robson chart in patients with primary pterygium. Makara J Heal Res. 2018;22:167–171.
17. Torkildsen G, Brujic M, Cooper MS, et al. Evaluation of a new artificial tear formulation for the management of tear film stability and visual function in patients with dry eye. Clin Ophthalmol. 2017;11:1883–1889.
18. Kenyon KR, Wagoner MD, Hettinger ME. Conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology. 1985;92:1461–1470. doi:10.1016/S0161-6420(85)33831-9
19. Chaidaroon W, Satayawut N, Tananuvat N. Effect of 2% hyaluronic acid on the rate of healing of corneal epithelial defect after pterygium surgery: a randomized controlled trial. Drug Des Devel Ther. 2021;15:4435–4443. doi:10.2147/DDDT.S336372
20. Rasband WS, Image J. U. S. National Institutes of Health, Bethesda, Maryland, USA. Available from: https://imagej.nih.gov/ij/.
21. Tan DT, Chee SP, Dear KB, Lim AS. Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol. 1997;115:1235–1240. doi:10.1001/archopht.1997.01100160405001
22. Hirst LW. Cosmesis after pterygium extended removal followed by extended conjunctival transplant as assessed by a new, web-based grading system. Ophthalmology. 2011;118:1739–1746.
23. Tomás-Juan J, Murueta-Goyena Larrañaga A, Hanneken L. Corneal regeneration after photorefractive keratectomy: a review. J Optom. 2015;8:149–169. doi:10.1016/j.optom.2014.09.001
24. Tosi GM, Baiocchi S, Balestrazzi A, et al. Corneal complications during and after vitrectomy for retinal detachment in photorefractive keratectomy treated eyes. Medicine. 2015;94:e2215. doi:10.1097/MD.0000000000002215
25. Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med. 2001;226:653–664. doi:10.1177/153537020222600711
26. Ziaei M, Greene C, Green CR. Wound healing in the eye: therapeutic prospects. Adv Drug Deliv Rev. 2018;126:162–176. doi:10.1016/j.addr.2018.01.006
27. Gomes JA, Amankwah R, Powell-Richards A, Dua HS. Sodium hyaluronate (hyaluronic acid) promotes migration of human corneal epithelial cells in vitro. Br J Ophthalmol. 2004;88:821–825. doi:10.1136/bjo.2003.027573
28. Zhong J, Deng Y, Tian B, et al. Hyaluronate acid-dependent protection and enhanced corneal wound healing against oxidative damage in corneal epithelial cells. J Ophthalmol. 2016;2016:6538051. doi:10.1155/2016/6538051
29. Kim DJ, Jung MY, Pak HJ, et al. Development of a novel hyaluronic acid membrane for the treatment of ocular surface diseases. Sci Rep. 2021;11:2351. doi:10.1038/s41598-021-81983-1
30. Durrie DS, Wolsey D, Thompson V, Assang C, Mann B, Wirostko B. Ability of a new crosslinked polymer ocular bandage gel to accelerate reepithelialization after photorefractive keratectomy. J Cataract Refract Surg. 2018;44:369–375. doi:10.1016/j.jcrs.2018.01.018
31. Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277:4589–4592. doi:10.1074/jbc.R100038200
32. Inoue M, Katakami C. The effect of hyaluronic acid on corneal epithelial cell proliferation. Invest Ophthalmol Vis Sci. 1993;34:2313–2315.
33. Coroi MC, Bungau S, Tit M. Preservatives from the eye drops and the ocular surface. Rom J Ophthalmol. 2015;59:2–5.
34. Nakamura M, Sato N, Chikama TI, Hasegawa Y, Nishida T. Hyaluronan facilitates corneal epithelial wound healing in diabetic rats. Exp Eye Res. 1997;64:1043–1050. doi:10.1006/exer.1997.0302
35. Regnier A, Cazalot G, Cantaloube B. Topical treatment of non-healing corneal epithelial ulcers in dogs with aminocaproic acid. Vet Rec. 2005;157:510–513. doi:10.1136/vr.157.17.510
36. Williams PB, Crouch ER
37. Crouch ER, Crouch ER Jr, Williams PB. Aminocaproic acid and tranexamic acid increase the rate of acute corneal reepithelialization in Sprague Dawley rats. J Ocul Pharmacol Ther. 1998;14:109–118. doi:10.1089/jop.1998.14.109
38. Che Arif FA, Hilmi MR, Kamal MK, Ithnin MH. Evaluation of 18 artificial tears based on viscosity and pH. Malaysian J Ophthalmol. 2020;2:96–111. doi:10.35119/myjo.v2i2.109
39. Durrani AM, Farr SJ, Kellaway IW. Influence of molecular weight and formulation pH on the precorneal clearance rate of hyaluronic acid in the rabbit eye. Int J Pharm. 1995;118:243–250. doi:10.1016/0378-5173(94)00389-M
40. Ridder WH, Lamotte JO, Ngo L, Fermin J. Short-term effects of artificial tears on visual performance in normal subjects. Optom Vis Sci. 2005;82:370–377. doi:10.1097/01.OPX.0000162646.30666.E3
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.