Back to Journals » Clinical Ophthalmology » Volume 13
Comparison of clinical effects of two latanoprost 0.005% solutions (Xalatan® and Arulatan®) in primary open-angle glaucoma or ocular hypertensive patients: a randomized clinical trial
Authors Brant Fernandes RA , Silva LMP , Dias DT , Pereira RH , Belfort Jr R, Prata TS
Received 14 December 2018
Accepted for publication 28 February 2019
Published 24 April 2019 Volume 2019:13 Pages 679—684
DOI https://doi.org/10.2147/OPTH.S198229
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Rodrigo A Brant Fernandes,1 Luci Meire P Silva,1 Diego Torres Dias,1 Rafael Henrique Pereira,2 Rubens Belfort Jr,1,2 Tiago Santos Prata1–3
1Department of Ophthalmology and Visual Sciences, Federal University of São Paulo, São Paulo, SP, Brazil; 2IPEPO/Vision Institute – SP, São Paulo, SP, Brazil; 3Department of Ophthalmology, Mayo Clinic, Jacksonville, FL, USA
Purpose: To evaluate the therapeutic non-inferiority between two ophthalmic latanoprost 0.005% solutions (Arulatan®, [ALT] versus the reference drug Xalatan®, [XLT]) in patients with primary open-angle glaucoma (POAG) or ocular hypertension (OH).
Patients and methods: This was a 12-week Phase IV, experimental, randomized, parallel-group, double-masked clinical trial. Consecutive patients with POAG or OH from the Glaucoma Service of Instituto Paulista de Estudos e Pesquisas em Oftalmologia (São Paulo, Brazil) were enrolled between July and December 2017. The primary outcome of the study was an analysis of therapeutic non-inferiority between ALT versus XLT at 12 weeks, while secondary outcomes were mean intraocular pressure (IOP) change from baseline at 2, 6 and 12 weeks, mean IOP at 2, 6 and 12 weeks, and topical and systemic side effects. Statistical significance was set at P<0.05. Computerized analysis was performed using the R software, version 3.4.4.
Results: A total of 45 patients were randomized to the two treatment groups: ALT (22) and XLT (23). A statistically significant reduction in IOP from baseline was observed in both treatment groups at all timepoints, while no statistically significant difference between groups was detected. By week 12, observed IOP reduction was −7.95 and −7.89 mmHg in the ALT and in the XLT groups, respectively (P=0.60). Treatment difference between the ALT and the XLT groups was −0.06 mm Hg (95% CI: −0.97, 0.85) and fell within the interval set for therapeutic non-inferiority. There was no statistically significant difference between the two groups in terms of safety profiles. The most commonly reported side effect was mild conjunctival/palpebral hyperemia.
Conclusion: ALT was considered non-inferior to XLT in achieving a statistically significant reduction in IOP at 12 weeks in POAG and OH patients. No significant difference in the occurrence of side effects was found between both groups.
Keywords: glaucoma, latanoprost, IOP reduction
Introduction
Glaucoma is a progressive optic neuropathy that results from degeneration of retinal ganglion cells and presents with a characteristic pattern of structural damage and visual field (VF) loss.1 It is the main cause of irreversible blindness worldwide,2 and it is estimated that approximately 64.3 million people between 40 and 80 years old are currently affected by the disease, and this number may rise up to 76 million, in 2020, and 111.8 million, in 2040.3
The main risk factor for glaucoma development and progression, and, so far, the only modifiable one is IOP.1 In this context, glaucoma treatment is based on reducing IOP to values capable of preventing progression of glaucomatous optic neuropathy, with minimum side effects and impact on the patient’s visual function, general and ocular health. With that in mind, prescription of topical hypotensive eyedrops is the most widely accepted form of initial treatment for glaucomatous patients.4 In this context, it is well known that prostaglandin analogs (PA) are the class of ocular hypotensive drugs most widely used as monotherapy for initial glaucoma treatment because of their good profile on hypotensive efficacy, posology (single daily dose) and tolerability.5–7
All these informations considered, latanoprost was the first PA8,9 to become commercially available and is frequently employed as initial glaucoma monotherapy. The drug is an analog of the prostaglandin F2α and studies of clinical efficacy have shown the drug to have an IOP-lowering effect superior to timolol maleate 0.5%10,11 and similar to other PAs.12 In 2011, it lost patent protection and new generic preparations are available worldwide.13,14
A new formulation of latanoprost 0.005% ophthalmic solution (Arulatan® - ALT, Bausch & Lomb, Berlin, Germany) has become commercially available since 2015. Since there is a concern whether generic medications have the same efficacy and safety profile as the reference ones,15–17 we developed this clinical trial evaluating the therapeutic non-inferiority of ALT versus the reference drug (Xalatan® - XLT, Pfizer, New York, USA) in patients with primary open-angle glaucoma (POAG) or ocular hypertension (OH).
Material and methods
This protocol was developed according to the Good Clinical Practices of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. It was also approved by the ethics committee and the institutional review board of the Federal University of São Paulo and adhered to the tenets of the declaration of Helsinki.
Study design
This was a 12-week Phase IV, experimental, randomized, parallel-group, double-masked clinical trial, designed to evaluate the therapeutic non-inferiority of the IOP-lowering effect between a generic latanoprost 0.005% ophthalmic solution (ALT) and the reference drug (XLT).
Inclusion and exclusion criteria (Table 1) were assessed during the screening visit after obtaining patient’s written informed consent form and were confirmed at the randomization visit (28 days later). At the screening visit, parameters were evaluated as follows: demographic data, medical history and use of concomitant systemic medication were assessed; all participants had undergone a comprehensive ophthalmological evaluation, including best-corrected visual acuity (BCVA), slit-lamp biomicroscopy, Goldmann applanation tonometry, gonioscopy, dilated fundoscopy, VF testing (24–2 Swedish interactive threshold algorithm, Humphrey Field Analyzer II; Carl Zeiss Meditec, Inc., Dublin, CA), and color/red-free fundus imaging; a pregnancy test was performed in women of childbearing potential; pulse and blood pressure were measured; patients under topical hypotensive medication initiated a 28-day washout period. At the randomization visit, patients were included in the study if an unmedicated IOP from 21 to 36 mmHg was detected. All patients were submitted to a single hypotensive eyedrop regimen, which could be either ALT or XLT. Patients in need for multiple drugs to promote IOP control were not included in the study. Control follow-up visits were then scheduled at 2, 6 and 12 weeks in which medical history and use of concomitant systemic medication, pulse, blood pressure, BCVA, slit-lamp biomicroscopy, Goldmann applanation tonometry and the presence of eyedrops side effects and adverse effects were assessed. At the 12th week after randomization, the patient came back to the hospital for the end-of-study visit.
 | Table 1 Inclusion and exclusion criteria |
Participants and outcomes
Consecutive patients with POAG or OH from the Glaucoma Service of Instituto Paulista de Estudos e Pesquisas em Oftalmologia (São Paulo, Brazil) who met the inclusion criteria and any one of the exclusion criteria were enrolled between July and December 2017.
The primary outcome of the study was an analysis of therapeutic non-inferiority between ALT versus XLT at 12 weeks, while secondary outcomes were mean iIOP change from baseline at 2, 6 and 12 weeks, mean IOP at 2, 6 and 12 weeks, and topical and systemic side effects.
POAG was defined as the presence of an untreated IOP ≥21 mmHg with characteristic signs of glaucomatous optic neuropathy (GON), associated or not with characteristic VF defects, while OH was defined as the presence of an untreated IOP ≥21 mmHg without characteristic signs of GON and normal VF tests.
GON was defined as cup-to-disc ratio >0.6, asymmetry between eyes ≥0.2, the presence of localized defects of the retinal nerve fiber layer, and/or neuroretinal rim in the absence of any other anomalies that could explain such findings. Characteristic glaucomatous VF defect was defined as glaucoma hemifield test results outside normal limits and the presence of at least three contiguous test points within the same hemifield on the pattern deviation plot at p<1%, with at least one at p<0.5%, excluding points on the edge of the field or those directly above and below the blind spot.
Safety endpoints of this study were corneal pachymetry, pulse/blood pressure, dilated fundoscopy, BCVA, slit-lamp biomicroscopy, VF, eyedrops side effects and adverse events. Analysis of these endpoints consisted of average changes from baseline whenever appropriate and descriptive statistics such as frequency and percentages for adverse events and eyedrops side effects.
Randomization and statistical analysis
The randomization lists were computer-generated, and each patient was sequentially assigned to receive either ALT or XLT in a 1:1 ratio. In order to maintain masking, a drug dispensing person was assigned at each center and the medications were provided to the patients in identical, opaque bottles, with masked labels. Whenever both eyes were eligible, only one eye was considered for statistical analysis.
We chose the magnitude of the IOP reduction from baseline between groups as the main variable for sample size calculation. For a sample power of 80% (β value of 0.20) and α value (type I error) of 0.05, we would need 17 patients in each group to detect a 2 mmHg difference between groups (assuming a SD of 2 mmHg).
Descriptive analysis was used to present the demographic and clinical data. Categorical data were analyzed with the chi-square test. Comparison between groups was performed with independent t-test for normally distributed data and with Mann–Whitney test for those non-normally distributed. For the non-inferiority analysis, it was assumed that ALT could be declared non-inferior to XLT if the two-sided 95% CIs for the difference between adjusted treatment means were entirely within the interval from −1.5 to +1.5 mmHg. Statistical significance was set at P<0.05. Computerized analysis was performed using the R software, version 3.4.4.
Results
A total of 61 patients were screened and 45 patients were randomized to the two treatment groups: ALT (22) and XLT (23). Fifteen patients were excluded because they did not meet the eligibility criteria and one withdrew consent. Additionally, one patient from the ALT group had no adherence to the treatment and did not complete the 12-week follow-up period, so he was excluded from the sample. All these considered, a total of 44 patients completed the study. Demographic data – including baseline IOP – did not differ between groups at baseline and are presented in Table 2.
 | Table 2 Comparison of clinical and demographic characteristics between groups |
A statistically significant reduction in IOP from baseline was observed in both treatment groups at all timepoints, while no statistically significant difference between groups was detected (Table 3). By week 12, observed mean IOP reduction was −7.95 and −7.89 mmHg in the ALT and in the XLT groups, respectively (P=0.60). Thus, mean treatment difference between the ALT and the XLT groups was −0.06 mmHg (95% CI: −0.97, 0.85) and fell within the interval set for therapeutic non-inferiority.
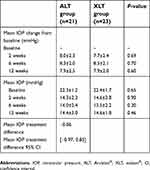 | Table 3 Comparison of IOP parameters between groups at all timepoints |
There was no statistically significant difference between the two groups in terms of safety profiles. Considering the drug-related side effects, the only side effects that were found were conjunctival/palpebral hyperemia: four patients in the ALT group and five patients in the XLT group. All cases were classified as mild and none of these patients had to stop medication. On the other hand, regarding the presence of adverse events, there was one case of myocardial infarction in the ALT group during the follow-up of this study, which was considered as an adverse event not related to the tested drug.
Discussion
The use of PAs eyedrops is frequently employed as first-line therapy for glaucoma management because of their IOP-lowering efficacy, safety profile and posology. That said, latanoprost was the first PA to be approved for glaucoma treatment and now there are plenty of generic formulations commercially available worldwide. In the present study, we sought to compare clinical effects between the Bausch & Lomb latanoprost 0.005% ophthalmic solution (ALT) versus the reference drug (XLT) and found no difference between the IOP-lowering effect and the safety profile of both solutions.
At this point, we believe it is important to briefly discuss the main clinical implications of our findings. Whenever a drug loses its patent protection, generic products may become commercially available – such as what happened with the latanoprost 0.005% ophthalmic solution – and approval protocols may differ between regulatory agencies. For example, the approval for marketing of a generic product both in Europe and in the United States is granted after the revision of an “abbreviated” dossier, not containing all the preclinical and clinical information required for a new chemical entity.14 That frequently raises questions whether the generic formulations have significant variations in physical properties and drug concentrations16 and mainly if they are as effective and safe as the reference drug.
All these considered, there are few studies evaluating different types of populations that previously assessed generic formulations of latanoprost 0.005% ophthalmic solution versus XLT regarding IOP-lowering effect and safety profile, and the results are conflicting. Considering the studies that found a better performance for XLT when compared to the generic formulation, Golan et al18 found a tendency for better IOP control for XLT when compared to Glautan (an Israeli generic formulation) in a population of 19 POAG and HO patients at 1-month follow-up and Narayanaswamy et al19 found XLT to have better IOP control than Latoprost (an Indian generic formulation) in a population of 30 POAG and HO patients at a 24-week crossover protocol. On the other hand, considering the studies that found no difference between XLT and the generic formulation, Diagourtas et al20 found no difference between two Greek latanoprost 0.005% formulations (Latz and Xalaprost) and XLT in a 60 POAG and OH patients 16-week trial and Digiuni t al,14 found no difference between an Italian latanoprost 0.005% formulation (manufactured by Mipharm) and XLT in a 184 POAG and OH patients 12-week trial. Our results corroborate the findings of the latter two studies since we found no difference in IOP-lowering effect and safety profile between ALT and XLT. Our results also corroborate the results of Allaire et al21 that demonstrated no difference in safety and efficacy between a Bausch & Lomb latanoprost test formulation and XLT in POAG and OH patients in a 266 patients 6-week trial. It is important, though, to highlight that, although these previously mentioned results considered different drug formulations, they also evaluated very different populations and this might contribute to conflicting results. As far as we know, this is the first study to compare the effects of latanoprost and a generic formulation in a South American population.
The present study has some limitations that should be addressed. The main limitation is the small sample size. Nonetheless, the previous studies that found a difference between the generic latanoprost 0.005% ophthalmic solution and the reference drug had even smaller populations.18,19 Additionally, although we can draw some conclusions regarding ALT, we cannot extrapolate those findings to other generic formulations not used in this study.
Conclusion
In this trial we found that ALT can be considered non-inferior to the reference drug XLT regarding IOP-lowering efficacy at 12 weeks in POAG and OH patients. Additionally, ALT could not only be considered as effective as ALT in achieving a statistically significant reduction in IOP at 2, 6 and 12 weeks, but also no difference was found regarding safety profiles between both solutions.
Abbreviations list
BCVA, best-corrected visual acuity; GON, glaucomatous optic neuropathy; OH, ocular hypertension; PA, prostaglandin analog; POAG, primary open-angle glaucoma; RNFL, retinal nerve fiber layer; VF, visual field.
Ethics approval
This study protocol was approved by the ethics committee and the institutional review board of the Federal University of São Paulo. The reference number of approval is 2.034.869.
Acknowledgment
None of the investigators are Bausch & Lomb employees.
Author contributions
All authors contributed to conception and design, data acquisition or data analysis and interpretation, drafting the article or critically revising it for important intellectual content, final approval of the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved.
Disclosure
Diego Torres Dias, Rubens Belfort Jr, and Tiago Santos Prata report grants from Bausch & Lomb, during the conduct of the study. The authors report no proprietary interest and no other conflicts of interest in this work.
References
1. Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363(9422):1711–1720. doi:10.1016/S0140-6736(04)16257-0
2. Kingman S. Glaucoma is second leading cause of blindness globally. Bulletin of the World Health Organization. 2004;82(11):887–888.
3. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi:10.1016/j.ophtha.2014.05.013
4. Prum BE
5. Whitson JT. Glaucoma: a review of adjunctive therapy and new management strategies. Expert Opinion on Pharmacotherapy. 2007;8(18):3237–3249. doi:10.1517/14656566.8.18.3237
6. van der Valk R, Schouten JS, Webers CA, et al. The impact of a nationwide introduction of new drugs and a treatment protocol for glaucoma on the number of glaucoma surgeries. Journal of Glaucoma. 2005;14(3):239–242.
7. Aptel F, Cucherat M, Denis P. Efficacy and tolerability of prostaglandin analogs: a meta-analysis of randomized controlled clinical trials. Journal of Glaucoma. 2008;17(8):667–673. doi:10.1097/IJG.0b013e3181666557
8. Toris CB, Camras CB, Yablonski ME. Effects of PhXA41, a new prostaglandin F2 alpha analog, on aqueous humor dynamics in human eyes. Ophthalmology. 1993;100(9):1297–1304.
9. Ziai N, Dolan JW, Kacere RD, Brubaker RF. The effects on aqueous dynamics of PhXA41, a new prostaglandin F2 alpha analogue, after topical application in normal and ocular hypertensive human eyes. Archives of Ophthalmology. 1993;111(10):1351–1358.
10. Camras CB. Comparison of latanoprost and timolol in patients with ocular hypertension and glaucoma: a six-month masked, multicenter trial in the United States. The United States Latanoprost Study Group. Ophthalmology. 1996;103(1):138–147. doi:10.1016/S0161-6420(96)30749-5
11. Orzalesi N, Rossetti L, Invernizzi T, Bottoli A, Autelitano A. Effect of timolol, latanoprost, and dorzolamide on circadian IOP in glaucoma or ocular hypertension. Investigative Ophthalmology & Visual Science. 2000;41(9):2566–2573.
12. Orzalesi N, Rossetti L, Bottoli A, Fogagnolo P. Comparison of the effects of latanoprost, travoprost, and bimatoprost on circadian intraocular pressure in patients with glaucoma or ocular hypertension. Ophthalmology. 2006;113(2):239–246. doi:10.1016/j.ophtha.2005.10.045
13. Zore M, Harris A, Tobe LA, et al. Generic medications in ophthalmology. The British Journal of Ophthalmology. 2013;97(3):253–257. doi:10.1136/bjophthalmol-2012-302245
14. Digiuni M, Manni G, Vetrugno M, et al. An evaluation of therapeutic noninferiority of 0.005% latanoprost ophthalmic solution and xalatan in patients with glaucoma or ocular hypertension. Journal of Glaucoma. 2013;22(9):707–712. doi:10.1097/IJG.0b013e318259b47c
15. Chambers WA. Ophthalmic generics–are they really the same? Ophthalmology. 2012;119(6):1095–1096. doi:10.1016/j.ophtha.2012.03.033
16. Angmo D, Wadhwani M, Velpandian T, Kotnal A, Sihota R, Dada T. Evaluation of physical properties and dose equivalency of generic versus branded latanoprost formulations. International Ophthalmology. 2017;37(2):423–428. doi:10.1007/s10792-016-0280-x
17. Cantor LB. Ophthalmic generic drug approval process: implications for efficacy and safety. Journal of Glaucoma. 1997;6(5):344–349. doi:10.1097/00061198-199710000-00011
18. Golan S, Rosenfeld E, Shemesh G, Kurtz S. Original and generic latanoprost for the treatment of glaucoma and ocular hypertension: are they really the same? Clinical and Experimental Pharmacology & Physiology. 2015;42(2):220–224. doi:10.1111/1440-1681.12329
19. Narayanaswamy A, Neog A, Baskaran M, et al. A randomized, crossover, open label pilot study to evaluate the efficacy and safety of Xalatan in comparison with generic Latanoprost (Latoprost) in subjects with primary open angle glaucoma or ocular hypertension. Indian Journal of Ophthalmology. 2007;55(2):127–131. doi:10.4103/0301-4738.30707
20. Diagourtas A, Kagelaris K, Oikonomakis K, Droulias A, Kokolakis N, Papaconstantinou D. Prospective study comparing Xalatan((R)) eye drops and two similar generics as to the efficacy and safety profile. European Journal of Ophthalmology. 2018;28(4):378–384. doi:10.1177/1120672117747030
21. Allaire C, Dietrich A, Allmeier H, et al. Latanoprost 0.005% test formulation is as effective as Xalatan(R) in patients with ocular hypertension and primary open-angle glaucoma. European Journal of Ophthalmology. 2012;22(1):19–27. doi:10.5301/ejo.5000041
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
