Back to Journals » Infection and Drug Resistance » Volume 14
Comparison of Clinical Characteristics and Outcomes Between Positive and Negative Blood Culture Septic Patients: A Retrospective Cohort Study
Authors Yang L, Lin Y, Wang J, Song J, Wei B, Zhang X , Yang J, Liu B
Received 15 August 2021
Accepted for publication 30 September 2021
Published 12 October 2021 Volume 2021:14 Pages 4191—4205
DOI https://doi.org/10.2147/IDR.S334161
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Long Yang,1,* Yue Lin,2,* Junyu Wang,1,* Jianmei Song,3 Bing Wei,1 Xiangqun Zhang,1 Jun Yang,1 Bo Liu1
1Department of Emergency Medicine Clinical Research Center, Beijing Chao-Yang Hospital, Capital Medical University, & Beijing Key Laboratory of Cardiopulmonary Cerebral Resuscitation, Beijing, 100020, People’s Republic of China; 2Department of Radiology, China-Japan Friendship Hospital, Beijing, 100029, People’s Republic of China; 3Department of Microbiology Laboratory, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, 100043, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bo Liu
Department of Emergency Medicine Clinical Research Center, Beijing Chao-Yang Hospital, Capital Medical University, & Beijing Key Laboratory of Cardiopulmonary Cerebral Resuscitation, Beijing, 100020, People’s Republic of China
Tel/Fax +86-10-51718171
Email [email protected]
Background: Few studies have studied the relationship between blood culture and mortality in sepsis patients. The aim of this study was to compare the characteristics and outcomes of positive and negative blood culture sepsis.
Methods: We performed a study on 640 patients suffering from sepsis in Beijing Chao-Yang Hospital from October 2017 to December 2019. The primary findings revolved around length and expenditure of hospital stay, the possibility of suffering from acute respiratory distress syndrome (ARDS), and any requirements for mechanical ventilation. The secondary findings revolved around whether the patient died early (28-day) or late (28-to-90-day).
Results: A total of 592 of the 640 patients met the inclusion criteria for sepsis, with 274 of them having culture-positive results. The culture-positive patients were mostly elderly suffering from diabetes and at risk of cancer, with a higher white blood cell count, and higher procalcitonin. Additionally, they scored higher in their acute physiology and chronic health evaluation II score (15 vs.11, P=0.010), as well as in their predisposition, infection, response, and organ dysfunction (17 vs 11, P< 0.001) than the individuals in the culture-negative group. Culture-positive patients had a longer duration of hospital stay (14 vs 6, P< 0.001) and higher in-hospital mortality (14.6% vs 8.5%, P=0.019) than culture-negative ones. No significant difference in intensive care unit (ICU) mortality (45.7% vs.36.4%, P=0.254) or early mortality (9.5% vs 7.2%, P=0.321) was noted between the two groups. However, the culture-positive patients had increased late mortality (15.7% vs.6.9%, P=0.001), when compared with those with culture-negative results in the cohort. Furthermore, the culture-positive patients who received the appropriate antibiotics early had a lower mortality rate than the culture-negative patients (7.3% vs.14.2%, P=0.008).
Conclusion: Culture-positive patients had higher in-hospital mortality, comparable early mortality, and worse late mortality than the culture-negative patients. Early appropriate use of antibiotics might reduce mortality and improve clinical prognosis.
Keywords: blood culture, culture-positive, culture-negative, sepsis, antibiotics, mortality
Introduction
Sepsis is a major cause of morbidity and mortality across both developed and developing nations.1 Over the past decade, the incidence of sepsis has been increasing worldwide, and its morbidity and mortality are still unbelievably high.2 In the United States, severe sepsis and septic shock remain the dominating causes of ICU deaths, with about 90–300 million people per year dying as a result.3 ICU expenditure is therefore far above the affordable rate for the country.4 So, minimizing patient costs and reducing hospital mortality are the goals that doctors currently strive for.
It is well-known that bacteria are the main cause of sepsis pathogens5 and that early selection of appropriate antibiotics can improve chances of survival.6 There are several guidelines that widely recommend empirical application of broad-spectrum antibiotics, demonstrating that a timely and effective course of antibiotics can reduce mortality.7 Blood culture can be used to guide the adjustments of an empiric antibiotic treatment regimen,8 with positive blood culture having been suggested as a surrogate marker for bacteria load.9 Culture-positive patients are often considered to have a higher infection load and suffer from worse outcomes.10,11 Early and appropriate use of antibiotics can reduce mortality. However, microorganisms are unable to be cultivated in about one third to two thirds of sepsis patients,12 making it impossible to accurately apply antibiotics in the early stage, and therefore increasing the risk of death.
Recent studies have shown that culture-negative and culture-positive sepsis patients are comparable in both characteristics and outcomes.13,14 Only a few studies have shown the epidemiology and outcomes of culture-negative sepsis.3,12 Few studies have studied the relationship between blood culture and mortality in sepsis patients. There is almost no published research discussing the distribution of pathogenic bacteria in different areas of the hospital and its impact on the mortality of patients with sepsis. Herein, we performed a retrospective study to compare the characteristics and outcomes of positive and negative blood culture in septic patients, study the characteristics of pathogenic bacteria in the different areas of the hospital and analyse early and late mortality in blood culture sepsis.
Methods and Materials
Study Design
This was a retrospective cohort study conducted in Beijing Chao-Yang Hospital. We included all 640 patients admitted to our hospital for sepsis with blood culture during the period October 2017 to December 2019. Sepsis with blood culture was defined according to The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3).15 Patients with fungal, viral, or parasitic infections, or tuberculosis were excluded. We only made a record of the bacteria infection. We also excluded those patients who refused to be treated or were transferred to other hospitals. Finally, we compared and analysed the differences between the blood culture-positive and blood culture-negative septic patients.
Data Collection and Definition of Variables
All patients with blood culture were recorded in the hospital system. An infection had to be clinically defined by at least two specialists. The SOFA (Sequential Organ Failure Assessment) score was used to evaluate sepsis in each patient with a suspected infection. According to The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3), an infection was defined as sepsis if the SOFA score was equal to or more than 2. The remaining 592 septic patients with blood culture met the inclusion criteria for sepsis and were ultimately included.
According to local practices, blood cultures were obtained within 3 hours of recognition from samples collected at two or more different anatomical sites.16 The blood culture bottles used were BD BACTEC 23F (aerobic medium) and 22F (anaerobic medium). Each bottle was incubated into the BacT/ALTER 3D instrument and waited for atleast 5 days. All isolated bacteria were identified using growth on differential agar and biochemical tests and identification with Phoenix 100 (Becton Dickinson, Franklin Lakes, USA). Clinical bloodstream infection(BSI) was defined as at least two sets of positive blood cultures from separate sites, one set positive for a Gram-positive pathogen or one set positive for a Gram-negative pathogen. The contamination was regarded as coagulase negative which isolated from only one of at least two sets of blood cultures. When indwelling catheters were used, one blood sample was obtained through the catheter, with the remainder taken from different peripheral venous sites. The major infection sites were grouped as the lower respiratory tract, urinary tract and abdominal infection, and the minor sites were grouped as isolated blood stream infection, skin and soft tissue infection, and the central nervous system.17
The collected data include demographics, underlying diseases, initial vital signs, infection sites, and clinical outcomes, such as ICU admission, duration of ICU stay and expenditure, and mechanical ventilation requirements. The date of the patient’s death was obtained from the hospital’s electronic medical record system. Early mortality was defined as 28-day mortality and late mortality was defined as 28-to-90-day mortality. The information on in-hospital mortality, ICU mortality, and early or late mortality was extracted. The proportion and fatality of the different pathogens were also recorded. Laboratory examinations, including white blood cell (WBC) counts, platelet (PLT), albumin (ALB), C-reactive protein (CRP), procalcitonin (PCT) and lactate (Lac) were also extracted from the data. When the initial antibiotics were changed to cover more extensive pathogens, an escalation of the antibiotics occurred. SOFA scores, Acute Physiology and Chronic Health Evaluation II (APACHE II) scores, Mortality in Emergency Department Sepsis (MEDS) scores and Predisposition, Infection, Response, and Organ dysfunction (PIRO) scores were calculated from the clinical data and used to assess the severity of the infection.
Additionally, the microbiological culture results and information on the required antibiotics were extracted from the electronic medical records. In the case of a disagreement concerning the discrimination of the exact pathogen, the final decision was taken by three experienced infection specialists after cautious discussion. All pathogenic bacteria included in the cohort were obtained in strict accordance with blood culture standards. Furthermore, receiving appropriate antibiotic prescriptions meant that the antibiotics could effectively treat the pathogens in time.
The primary outcomes were ICU mortality, ICU stay duration, and expenditure, as well as mechanical ventilation requirements. The secondary outcome variables were early mortality and late mortality between culture-positive and culture-negative sepsis patients.
Statistical Analysis
We classified the patients into two groups: positive and negative blood culture groups. We expressed the categorical variables as numbers (percentages). After using the Kolmogorov–Smirnov test and examining histograms to verify whether the normality and homogeneity assumptions had been satisfied, we expressed the normally distributed numerical variables as a mean (95% confidence interval (CI)) and the other numerical variables as the median (inter-quartile range). We compared the categorical variables using the χ2 test or Fisher exact test, the normally distributed quantitative variables with the t-test, and the other quantitative variables with the Mann–Whitney U-test. We used the Bonferroni correction for pairwise comparisons. In order to identify the independent predictors for culture-positive or culture-negative mortality, we performed a logistic regression analysis. The Kaplan–Meier survival curves with the Log rank test were stratified by the culture results. We used SPSS 25.0 (IBM Corp., USA) for statistical analyses, with a P value < 0.05 considered significant.
Results
Study Cohort and Patient Characteristics
In this cohort, 640 patients with blood culture were defined as having sepsis. 275 of these (43.0%) had severe sepsis and 108 (16.9%) had septic shock. We only recorded bacterial infections. Patients (n=36, 5.6%) with fungal, viral, or parasitic infections were excluded, as were those with tuberculosis. We also excluded patients who refused to be treated (n=4, 0.6%) and those transferred to other hospitals (n=8, 1.2%). Of the 592 patients that met the inclusion criteria, 274 (46.3%) were identified as culture-positive sepsis patients and 318 (53.7%) were identified as culture-negative sepsis patients. The majority of culture-positive patients were elderly and male (63.9%), and were more likely to have chronic obstructive pulmonary disease (COPD), hypertension, congestive heart failure (CHF), diabetes mellitus (DM) and tumours. In the culture-negative patients meanwhile, cerebrovascular disease (CVD) and chronic renal disease (CRD) were more common (Table 1 and Figure 1). When evaluating the source of the infections, the most frequent sites were the lower respiratory tract and urinary tract, in both the culture-positive and culture-negative groups. The differences between the two groups were obvious in lower respiratory tract infections (58.5% vs 45.9%, P=0.002) and urinary tract infections (23.0% vs 14.8%, P=0.01) (Table 1 and Figure 2).
 |
Table 1 Characteristics of the Study Cohort |
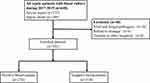 |
Figure 1 Flowchart of the study population. |
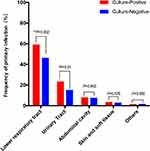 |
Figure 2 The primary infection sites of culture-positive and culture-negative groups. *P<0.05, **P<0.01. |
Clinical Characteristics and Severity of Illness
We compared the vital signs, laboratory examination results and severity scores between the culture-positive and culture-negative sepsis patients. Culture-positive patients had higher heart rates and respiratory rates, and a lower mean blood pressure (MBP) than the culture-negative patients. Culture-positive patients were also more likely to develop severe sepsis or septic shock. Culture-positive patients commonly had higher infection markers in their WBC counts and PCT, as well as having lower ALB. All these findings suggest that patients with positive blood culture are more likely to be malnourished and to contract severe infectious. The illness severity was assessed by the SOFA, MEDS, APACHE II and PIRO scores. The results showed that culture-positive patients had a higher SOFA score (4 vs.2, P<0.001), MEDS score (11 vs.7, P<0.001), APACHE II score (15 vs.11, P=0.010) and PIRO score (17 vs 11, P<0.001) than culture-negative patients (Table 2).
 |
Table 2 Comparison of Laboratory Characteristic and Severity Between Culture Positive and Culture Negative Patients |
Multiple Highly Pathogenic Bacteria Aggravate the Poor Outcome of Culture-Positive Patients
We also studied the culture-positive pathogens throughout the hospital, ICU, and emergency department (ED), and found that Gram-negative bacteria surpassed Gram-positive bacteria in the throughout hospital, whereas the opposite results were found in ICU and ED. Of the total 274 culture-positive patients, 147 cases (53.6%) had Gram-negative bacteria and 127 cases (46.4%) had Gram-positive bacteria. Escherichia coli (16.8%) was the most commonly found Gram-negative bacteria, followed by Klebsiella pneumoniae (12.8%), Pseudomonas aeruginosa (3.3%) and Acinetobacter baumannii (2.6%). Among the Gram-positive bacteria, Enterococcus faecium (8.8%), Staphylococcus epidermidis (8.0%), Staphylococcus hominis (7.3%) and Staphylococcus aureus (5.5%) were the four most common. Of the 274 culture-positive cases, 69 patients died, where the total mortality caused by Klebsiella pneumoniae (26.1%), Escherichia coli (20.3%) and Staphylococcus aureus (15.9%) was higher than from other pathogenic bacteria. When comparing the net mortality, we found that Staphylococcus aureus had the highest net mortality (73.3%) in the throughout hospital, followed by Klebsiella pneumoniae (51.4%), Pseudomonas aeruginosa (44.4%) and Acinetobacter baumannii (42.9%) (Table 3 and Figure 3). When studying the bacteria in the ICU, the results showed that 37 patients, out of the total 81 patients there, had died. The proportion of Gram-positive bacteria (56.8%) was higher than Gram-negative bacteria (43.2%). Klebsiella pneumoniae (11.1%), Acinetobacter baumannii (8.6%) and Escherichia coli (7.4%) were the three most common Gram-negative bacteria, while Staphylococcus hominis (12.3%), Enterococcus faecium (9.9%) and Staphylococcus epidermidis (8.6%) were the three most common Gram-positive bacteria. When comparing total mortality, Klebsiella pneumoniae and Acinetobacter baumannii both had high mortality in the ICU. Although the occurrence of Staphylococcus aureus was only 4.9%, its total mortality rate was as high as 10.8%. The net mortality from bacteria in the ICU was similar to that found in the throughout hospital, with Klebsiella pneumoniae, Acinetobacter baumannii, Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus all resulting in high mortality (Table 4 and Figure 4). This result between the throughout hospital and ICU was similar. Although the data seemed to be different in Tables 3 and 4, the above four pathogens were similar in terms of high net mortality. The bacteria in the ED were also analysed, with the results showing that the quantity of Gram-positive bacteria was almost two times that of the Gram-negative bacteria. Different types of Staphylococci accounted for the vast majority of Gram-positive bacteria, with Staphylococcus epidermidis and Staphylococcus human accounting for the highest proportion. Although the proportion of Staphylococcus aureus was small, its mortality was very high. The highest proportions of Gram-negative bacteria remained Klebsiella pneumoniae, Escherichia coli and Acinetobacter baumannii, all of which resulted in a high mortality rate (Table 5 and Figure 5).
 |
Table 3 Frequency of Bacteria for the Positive Blood Culture Sepsis in the Throughout Hospital |
 |
Table 4 Frequency of Bacteria for the Positive Blood Culture Sepsis in the ICU |
 |
Table 5 Frequency of Bacteria for the Positive Blood Culture Sepsis in the ED |
Clinical Outcomes and Poor Prognosis Between Culture-Positive and Culture-Negative Sepsis
With regards clinical outcomes, the culture-positive group had a higher occurrence of ICU admission (29.6% vs 20.8%, P=0.013) and ICU length of stay (14 vs 6, P<0.001) than the culture-negative patients. There was no difference in ICU cost between the two groups (P=0.125). Culture-positive patients were more inclined to merge with ARDS than culture-negative patients (44.9% vs.34.9%, P=0.013). When comparing the median duration for mechanical ventilator use, the results showed that culture-positive patients had a higher requirement. There was no difference in non-invasive ventilation (3 vs 2, P=0.786), but a little difference in invasive ventilation (9 vs 4, P=0.018) between the two groups. In-hospital mortality was higher in the culture-positive group (14.6% vs 8.5%, P=0.019), but there was no difference in the ICU mortality (45.7% vs 36.4%, P=0.254) between the two groups. Late mortality (28–90 day) was significantly higher among the culture-positive sepsis patients (15.7% vs 6.9%, P=0.001), but early mortality (28-day) was comparable between both groups (9.5% vs 7.2%, P=0.321) (Table 6 and Figure 6). We plotted out the Kaplan-Meier survival curve to facilitate a visual comparison between the two groups (Figure 7). Since there were many confounding factors between both groups, it was impossible to intuitively compare the factors affecting mortality. A repeated regression analysis was carried out to analyse the factors affecting culture-positive and culture-negative mortality. Age, WBC, Lac, CRP, PCT, MEDS and PIRO were all risk factors for culture-positive mortality, while ALB was the protective factor. Of these, PIRO was the independent predictor (P=0.010). It was also determined that gender, age, Lac, PCT, MEDS and SOFA were all risk factors for culture-negative mortality. Age and ALB were seen to be the independent predictors for culture-negative sepsis (P<0.05) (Figure 8). We further performed a subgroup analysis on the culture-negative, culture-positive appropriate antibiotics and culture-positive inappropriate antibiotics groups. All 274 culture-positive patients received an antibiotics prescription on the first day of admission. The culture-positive subgroup that received an appropriate antibiotics prescription had a significantly lower mortality rate than both the culture-negative group (7.3% vs 14.2%, P=0.008) and the culture-positive subgroup that did not receive an appropriate antibiotics prescription (7.3% vs 17.9%, P<0.001). The culture-positive subgroup that did not receive an appropriate antibiotics prescription had a higher mortality rate than the culture-negative group, but this was not statistically significant (17.9% vs 14.2%, P=0.216) (Table 6 and Figure 9).
 |
Table 6 Comparison of Outcomes Between Positive and Negative Blood Culture Sepsis |
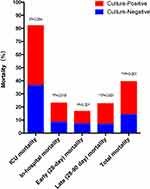 |
Figure 6 The mortality of ICU, in-hospital, early day (28-day) and late day (28–90-day) between culture-positive and culture-negative groups. *P<0.05, **P<0.01. |
 |
Figure 8 Odds ratio for culture-positive group and culture-negative group. (A) Odds ratio for culture-positive group. (B) Odds ratio for culture- negative group. |
 |
Figure 9 The clinical outcomes and mortality of culture-positive patients after receiving appropriate antibiotic. **P<0.01, ***P<0.001. |
Discussion
This cohort study shows that culture-positive patients are more likely to be admitted into an ICU and have a longer ICU stay than culture-negative patients. Culture-positive patients have a higher comorbidity burden, higher clinical severity, and higher in-hospital mortality rate than culture-negative patients. Additionally, they have comparable early mortality (28-day) but worse late mortality (28-to-90 day). Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Staphylococcus aureus all have strong mortality and aggravate poor prognoses in the ICU. The culture-positive subgroup that received an appropriate antibiotics prescription on the other hand, had a significantly lower mortality than the culture-negative group in this study.
The differences between these two groups are likely due to differences in patient populations, proportions of infection sites and resistance to antibiotics.18 The long periods under mechanical ventilation, including under invasive ventilators and non-invasive ventilators, as well as the length of stay in the ICU, and the higher in-hospital mortality rate that was observed in the culture-positive patients were likely attributed to the greater occurrence of risk factors. These risk factors included patients being older, and having more severe infections, worse malnutrition, and higher proportion of malignancies, as well as higher SOFA score, APACHE II score, MEDS score and PIRO score than the culture-negative patients. Previous retrospective studies have shown that culture-positive patients with intra-abdominal infections and lung infections are associated with poor clinical outcomes.19,20 Urinary tract infections on the other hand, are related to better clinical outcomes than other infections.21 However, the most commonly seen infection sites for both groups in our study were in the lower respiratory tract and urinary tract. The reason for this difference could be the different research scope in the studies. Previous studies included a wider scope for the culture patients, including blood, urine, stool, sputum, and pus, while our research only focused on the blood culture aspect. We also found that culture-negative patients with lung or urinary tract infections had lower mortality. This was consistent with the result that culture negativity might imply susceptibility to the initially prescribed antibiotic regimens, leading to lesser severity of the disease. In addition, the clinical outcomes could be associated with the infection sources as well as the management of the sepsis patients.18 Early comprehensive treatments, such as fluid resuscitation, appropriate use of antibiotics, nutritional support, and cleaning and care of infected sites, played a vital role in reducing mortality and improving clinical prognosis.
Differently to culture-positive patients, the clinical prognosis of culture-negative patients was also not good despite their late mortality being lower. One reason for this might be the failure to cultivate special pathogenic bacteria in time, thus delaying the early use of antibiotics. However, why should patients presenting clinical sepsis have a culture-negative infection? There were five main reasons. First, cultures lack the sensitivity to identify all bacteria,22 with possible reasons including sampling error, insufficient volume for blood cultures, poor transport conditions, and slow-growing or fastidious bacteria. Second, the patients may have been prescribed empirical antibiotics at local clinics before the sepsis or septic shock developed.23 Third, the proportion of sepsis or septic shock caused by atypical pathogens, including fungal, parasites and viral infections, might be increasing in patients.24,25 In terms of effective definitive antimicrobial therapy, these atypical pathogen infections might be similar to culture-positive infections. Common microbiological methods usually fail to identify the microorganism. Fourth, some patients suffering with culture-negative sepsis or septic shock might have got it from non-infectious causes, such as metabolic disorders, tissue injuries, inflammatory diseases, adverse effects from drugs, malignancies, or subarachnoid haemorrhages.26,27 Fifth, certain infections are less common in culture-negative patients than in culture-positive patients. This is in part due to the nature of some infections, for example, liver abscesses are less likely to be negative.28
In our research, we also further investigate the influence of different pathogenic bacteria on mortality, something which has rarely been studied or analysed in previously published studies. We found that Gram-positive and Gram-negative bacteria roughly equalled the proportions found in the throughout hospital’s culture-positive bacteria. Of the total 147 Gram-negative bacteria, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii accounted were the four most prevalent. For the Gram-positive bacteria, Enterococcus faecium and different kinds of Staphylococcus were the main pathogenic bacteria. Although appropriate antibiotics were used during the early stages based on the blood culture results, the late mortality and total mortality of the culture-positive group were still relatively high. The reasons behind the high mortality, such as older age, severity of illness, malignancies, and even antibiotic resistance, could be complex. Resistant Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, Acinetobacter baumannii, Enterococcus faecium and Staphylococcus aureus could all aggravate the late mortality of patients with sepsis. These results were similar to those found among the patients in the ICU. The appropriate use of antibiotics significantly reduced the mortality, especially when compared to the inappropriate use of antibiotics or culture-negative sepsis, but the total and net mortality of the sepsis patients in the ICU remained high, which is also related to older age, illness severity, malignancies, and antibiotic resistance. However, in the hospital’s ED, the number of Gram-positive bacteria was found to be almost double that of Gram-negative bacteria. Of which various kinds of Staphylococci, such as Staphylococcus epidermidis, Staphylococcus hominis, Staphylococcus capitis, Staphylococcus longum and Staphylococcus haemolyticus, accounted for the vast majority of Gram-positive bacteria. The proportion of Staphylococcus aureus that accounted for high mortality was only 6.5%, while the frequency of other kinds of Staphylococcus and Enterococcus faecium that accounted for lower mortality was as high as 50.7%. Due to the blood culture time being long and the results not being reported quickly enough, there was no evidence of using appropriate antibiotics against Gram-positive bacteria in the early stages as this may have delayed working in the golden period of treatment. Thus, although some Staphylococcus and Enterococcus faecium were deemed less lethal, the delay in the use of antibiotics against Gram-positive cocci increased the early mortality of septic patients. In addition, the severity of the patients’ conditions in the ED also increased mortality and poor prognosis. More importantly, our aim was to make a pathogen spectrum as soon as possible by studying the distribution characteristics of pathogens, so as to provide guidance for early appropriate use of antibiotics, improve treatment plans, strengthen patients care to reduce cross-infection, and even prevent the drug-resistant. The distribution characteristics of these pathogen samples were very important data, which could be expanded and made a blood culture-positive pathogen spectrum. For critically ill patients with sepsis, antibiotics could be used empirically based on this pathogen spectrum before blood culture results were obtained. For those who had obtained blood culture results, the appropriate use of antibiotics could be instructed, thereby avoiding the inappropriate use of antibiotics, significantly reducing mortality and improving prognosis. These were essential to improve clinical treatment capabilities and save patients’ lives.
What are the implications of our study? We compared and analysed the clinical characteristics, illness severity, and early or late mortality between blood culture-positive and blood culture-negative sepsis patients. We found that elderly males with culture-positive sepsis were more likely to have chronic diseases and tumours, as well as more likely to suffer malnourishment and severe infections. We also found that Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii and different kinds of staphylococcus composed the majority of culture-positive bacteria and posed a higher mortality rate. Despite the timely use of appropriate antibiotics and other comprehensive treatments for cultured special pathogens, culture-positive septic patients still displayed a higher mortality rate. This might be related to older age, illness severity, malignancies, or antibiotic resistance. Previous studies have shown that in an effort to improve outcomes in culture-negative or culture-positive sepsis, the “Surviving Sepsis Campaign” guidelines recommend early administration of broad-spectrum antibiotics.6 From the onset of septic shock, every hour of delay in the administration of effective antibiotics results in increased mortality.29 Our research also confirmed this fact. For those septic patients admitted to the ED, failure to cultivate specific Gram-positive cocci in the early stages delayed the timing for the prescription of appropriate antibiotics, and thereby increased the mortality rate. Meanwhile, both the culture-negative septic patients and the culture-positive patients who did not receive appropriate antibiotics had a high mortality rate. Therefore, early and appropriate antimicrobial therapy is shown to be necessary for these patients, and aids the improvement of clinical outcomes.
Our study has several limitations. First, some culture-negative results came from inappropriate sampling. In retrospective studies, improper operation by the staff of the microbiology laboratory, contamination caused by incomplete environmental disinfection, and even quality problems of certain blood culture reagents and so on might all lead to improper handling of samples. This might be the cause of possible contamination. However, the majority of the samples were obtained by experienced physicians and nurses, and this is less likely to be a significant contributing factor to our study. Second, our study was composed of single-centre retrospective research, and therefore cannot represent the universality of all studies. Third, there was heterogeneity among our cohort study and some underlying confounders may have influenced on our results. We should therefore carry out further research to reduce any confounding factors in the future. Our study also had some strengths. We analysed the distribution and characteristics of the pathogenic bacteria in different areas of the hospital, and explained in detail how pathogenic bacteria affect mortality and clinical outcomes. This may be helpful for prescribing appropriate antibiotics. We also used a logistic regression analysis to determine the influencing factors for culture-positive and culture-negative patients, and drew a forest plot to facilitate intuitive research; something which has rarely been seen before.
Conclusion
We found that culture-positive patients were more likely to be admitted to an ICU, have longer ICU lengths of stay, higher mechanical ventilation requirements and more likely to merge with ARDS than the culture-negative patients. Culture-positive patients had a higher comorbidity burden, higher clinical severity, and higher in-hospital mortality, as well as comparable early mortality (28-day) but worse late mortality (28-to-90 day) than culture-negative patients. Additionally, early blood culture results could provide a basis for the appropriate use of antibiotics, and early appropriate use of those antibiotics might reduce mortality and improve clinical prognosis. Large scale studies are still required in order to confirm these results.
Abbreviations
ARDS, acute respiratory distress syndrome; ICU, intensive care unit; SOFA, Sequential Organ Failure Assessment; APACHE II, Acute Physiology and Chronic Health Evaluation II; MEDS, Mortality in emergency department sepsis; PIRO, Predisposition, Infection, Response, and Organ dysfunction; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure; DM, diabetes mellitus; CVD, cerebrovascular disease; CRD, chronic renal disease; MBP, mean blood pressure; WBC, white blood cells; CRP, C-reactive proteins; PCT, procalcitonin; ALB, albumin; ED, Emergency Department; Lac, lactate; PLT, platelet.
Highlights
- Studies on the comparison of characteristics and outcomes between positive and negative blood culture septic patients are rare. Our research provides guidance for reducing mortality and improving clinical prognosis.
- The distribution and characteristics of pathogenic bacteria in different areas of the hospital may be helpful for prescribing appropriate antibiotics.
- A forest plot was drawn to facilitate intuitive research for culture-positive and culture-negative patients which has rarely been seen before.
Data Sharing Statement
The dataset that was used to support the finding of this study will be made available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participant
This study was approved by the Institutional Review Board of affiliated hospital of Capital Medical University, Beijing, China (CCMU-R2021077) and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
Acknowledgments
We would like to thank Beijing Chao-Yang Hospital for facilitating the study and covering the data collection costs. We would also like to acknowledge all data collectors, supervisors, and respondents without whom this research would not have been realized.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare that there are no conflicts of interest regarding this work.
References
1. Russell JA. Management of sepsis. N Engl J Med. 2006;355:1699.
2. Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200. doi:10.1016/S0140-6736(19)32989-7
3. Kethireddy S, Bilgili B, Sees A, et al. Culture-negative septic shock compared with culture-positive septic shock: a Retrospective Cohort Study. Crit Care Med. 2018;46(4):506. doi:10.1097/CCM.0000000000002924
4. Chou EH, Mann S, Hsu T-C, et al. Incidence, trends, and outcomes of infection sites among hospitalizations of sepsis: a nationwide study. PLoS One. 2020;15:e0227752. doi:10.1371/journal.pone.0227752
5. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546. doi:10.1056/NEJMoa022139
6. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165.
7. Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235. doi:10.1056/NEJMoa1703058
8. Berild D, Mohseni A, Diep LM, Jensenius M, Ringertz SH. Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs. J Antimicrob Chemother. 2006;57(2):326. doi:10.1093/jac/dki463
9. Mancini N, Carletti S, Ghidoli N, Cichero P, Burioni R, Clementi M. The era of molecular and other non-culture-based methods in diagnosis of sepsis. Clin Microbiol Rev. 2010;23(1):235. doi:10.1128/CMR.00043-09
10. Phua J, Ngerng W, See K, et al. Characteristics and outcomes of culture-negative versus culture-positive severe sepsis. Crit Care. 2013;17:R202. doi:10.1186/cc12896
11. Yang S-C, Liao K-M, Chen C-W, Lin W-C. Positive blood culture is not associated with increased mortality in patients with sepsis-induced acute respiratory distress syndrome. Respirology. 2013;18:1210. doi:10.1111/resp.12121
12. Gupta S, Sakhuja A, Kumar G, McGrath E, Nanchal RS, Kashani KB. Culture-negative severe sepsis: nationwide trends and outcomes. Chest. 2016;150:1251. doi:10.1016/j.chest.2016.08.1460
13. Sigakis MJG, Jewell E, Maile MD, Cinti SK, Bateman BT, Engoren M. Culture-negative and culture-positive sepsis: a comparison of characteristics and outcomes. Anesth Analg. 2019;129:1300. doi:10.1213/ANE.0000000000004072
14. Brun-Buisson C, Doyon F, Carlet J, et al. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for severe sepsis. JAMA. 1995;274:968. doi:10.1001/jama.1995.03530120060042
15. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315:801. doi:10.1001/jama.2016.0287
16. Lee A, Mirrett S, Reller LB, Weinstein MP. Detection of bloodstream infections in adults: how many blood cultures are needed? J Clin Microbiol. 2007;45(11):3546. doi:10.1128/JCM.01555-07
17. Liu A, Yo C-H, Nie L, et al. Comparing mortality between positive and negative blood culture results: an inverse probability of treatment weighting analysis of a multicenter cohort. BMC Infect Dis. 2021;21(1):182. doi:10.1186/s12879-021-05862-w
18. Li Y, Guo J, Yang H, Li H, Shen Y, Zhang D. Comparison of culture-negative and culture-positive sepsis or septic shock: a systematic review and meta-analysis. Crit Care. 2021;25(1):167. doi:10.1186/s13054-021-03592-8
19. Labelle AJ, Arnold H, Reichley RM, Micek ST, Kollef MH. A comparison of culture-positive and culture-negative health-care-associated pneumonia. Chest. 2010;137(5):1130. doi:10.1378/chest.09-1652
20. Karvellas CJ, Abraldes JG, Zepeda-Gomez S, et al. The impact of delayed biliary decompression and anti-microbial therapy in 260 patients with cholangitis-associated septic shock. Aliment Pharmacol Ther. 2016;44(7):755. doi:10.1111/apt.13764
21. Abe T, Ogura H, Kushimoto S, et al. Variations in infection sites and mortality rates among patients in intensive care units with severe sepsis and septic shock in Japan. J Intensive Care. 2019;7(1):28. doi:10.1186/s40560-019-0383-3
22. Lever A, Mackenzie I. Sepsis: definition, epidemiology, and diagnosis. BMJ. 2007;335(7625):879. doi:10.1136/bmj.39346.495880.AE
23. Scheer CS, Fuchs C, Gründling M, et al. Impact of antibiotic administration on blood culture positivity at the beginning of sepsis: a prospective clinical cohort study. Clin Microbiol Infect. 2019;25(3):326. doi:10.1016/j.cmi.2018.05.016
24. Lin G-L, McGinley JP, Drysdale SB, Pollard AJ. Epidemiology and immune pathogenesis of viral sepsis. Front Immunol. 2018;9:2147.
25. Delaloye J, Calandra T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence. 2014;5(1):161. doi:10.4161/viru.26187
26. Cohen J, Brun-Buisson C, Torres A, Jorgensen J. Diagnosis of infection in sepsis: an evidence-based review. Crit Care Med. 2004;32(Supplement):S466. doi:10.1097/01.CCM.0000145917.89975.F5
27. Vincent J-L, Opal SM, Marshall JC, Tracey KJ. Sepsis definitions: time for change. Lancet. 2013;381:774. doi:10.1016/S0140-6736(12)61815-7
28. Tsai F-C, Huang Y-T, Chang L-Y, Wang J-T. Pyogenic liver abscess as endemic disease, Taiwan. Emerg Infect Dis. 2008;14(10):1592. doi:10.3201/eid1410.071254
29. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589. doi:10.1097/01.CCM.0000217961.75225.E9
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




