Back to Journals » Clinical Ophthalmology » Volume 17
Comparison Between Pulsed and Continuous Accelerated Corneal Cross-Linking Protocols
Authors Omar Yousif M, Elkitkat RS , Abdelsadek Alaarag N, Moustafa Seleet M, Hassan Soliman A
Received 18 February 2023
Accepted for publication 10 May 2023
Published 16 May 2023 Volume 2023:17 Pages 1407—1413
DOI https://doi.org/10.2147/OPTH.S409178
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mohamed Omar Yousif,1,2 Rania Serag Elkitkat,1,3– 5 Noha Abdelsadek Alaarag,1 Mouamen Moustafa Seleet,1 Ashraf Hassan Soliman1
1Ophthalmology Department, Faculty of Medicine, Ain Shams University, Cairo, Egypt; 2Maadi Eye Subspecialty Center, Cairo, Egypt; 3Watany Eye Hospitals, Cairo, Egypt; 4Watany Research and Development Center, Cairo, Egypt; 5Ophthalmology Department, Faculty of Medicine, MTI University, Cairo, Egypt
Correspondence: Rania Serag Elkitkat, Email [email protected]; [email protected]
Purpose: To compare between two accelerated corneal cross-linking (A-CXL) protocols in the management of keratoconus (KC) as regard to the extent of corneal treatment.
Methods: This retrospective, comparative study included patients having mild to moderate, progressive KC. The study population was divided into two groups; group 1 enrolled 103 eyes of 62 patients who received pulsed light A-CXL (pl-CXL) at a power of 30 mW/cm2 with an irradiation time of 4 minutes, while group 2 comprised 87 eyes of 51 patients who received continuous light A-CXL (cl-CXL) at a power of 12 mW/cm2 with an irradiation time of 10 minutes. Recordings of the central and peripheral demarcation line depths (DD), and the maximum (DDmax) and minimum (DDmin) DD, using anterior segment optical coherence tomography, were compared between the two studied groups one month after the treatment protocol. Treatment stability was also evaluated pre and postoperatively (one year following surgery) by comparing the refractive and keratometric outcomes in both groups.
Results: The differences between the preoperative corneal thickness (minimum and central) and the epithelial thickness measurements between both groups were not statistically significant. Although group 1 had slightly larger central DD (223.4 ± 62.3 um), DDmax (240.4 ± 61.8 um), and DDmin (201 ± 54 um) than those of group 2 (221.8 ± 37 um, 229.1 ± 38.4 um, and 212 ± 37.2 um, respectively), the differences between both groups’ measurements were not statistically significant. Also, the two groups showed statistically insignificant differences regarding the subjective refraction and the average and maximum keratometry pre and postoperatively, denoting visual, refractive, and keratometric stability in both groups.
Conclusion: Longer duration cl-CXL seems to be as effective as pl-CXL regarding both postoperative stability and the extent of corneal tissue penetration by the ultraviolet treatment.
Keywords: keratoconus, corneal cross-linking, demarcation line anterior segment OCT, pulsed light corneal cross-linking, ultraviolet A corneal cross-linking
Introduction
Keratoconus (KC) is the most prevalent primary corneal ectasia and is characterized by progressive corneal thinning and steepening, visual distortion, and eventual central corneal scarring.1 The disease is often manifested in the second decade of life due to both genetic and environmental contributions, with recognized risk factors that include ocular allergy, eye rubbing, a positive family history, an Arabian or Asian ethnicity, and syndromic co-occurrences as Down, Ehlers–Danlos, and Marfan syndromes.2
Various management options for KC are available depending on the stage of the disorder and the goal of intervention.3 Eyeglasses and contact lenses are suitable for correction of refractive errors in the early stages in cases with stationary course, mainly with older ages. Implantable intrastromal corneal ring segments (ICRS) are useful for flattening the cornea and reducing aberrations, while surgical grafting of partial- or full-thickness corneal tissue is often the only option in advanced disease.4
Corneal collagen cross-linking (CXL) is a relatively recent procedure that has been established as a safe and effective means for halting the progression of KC.5,6 The intervention entails the administration of ultraviolet A (UVA) radiation (wavelength: 365 nm) in conjunction with riboflavin (vitamin B2) to the corneal surface, leading to a photochemical reaction which generates reactive oxygen species and new covalent bonds between the stromal collagen fibers, with an end result of increased corneal biomechanical strength.7,8
The standard protocol for CXL (also known as Dresden protocol or conventional CXL) has raised concerns regarding the long duration of treatment, higher rate of complications, and possibility of overtreatment, and has, thus, been largely replaced by newer accelerated protocols (A-CXL).9 Such protocols administer higher powers along shorter time intervals, while preserving constant delivery of total energy dose (TED).10,11 This is possible because the Bunsen Roscoe law states that the extent of the photochemical reaction is proportional to the TED regardless of the duration of delivery.12
Regarding the A-CXL protocols, the use of pulsed light fractionation (pl-CXL) has been suggested to be more preferable than its continuous application (cl-CXL) counterpart, as it allows for episodes of re-oxygenation of the stroma, a condition that is believed to be essential for the occurrence of an effective photochemical reaction.13
The demarcation line depth (DD), which is the border between the treated and untreated corneal stroma, has long been regarded as an indicator of the effect of ultraviolet treatment.14 The DD has been shown to vary according to the duration of irradiance,15 the use of pl-CXL versus cl-CXL,13 the removal or non-removal of the corneal epithelium,16 and the concentration of the riboflavin used.17 Measurements using anterior segment optical coherence tomography (AS-OCT) have been shown to yield reproducible results and to correlate with the findings of corneal confocal images.18,19
In this work, we studied the DD in the eyes of two groups of KC patients treated by two different A-CXL protocols. The primary outcome of the study was to determine if there was a difference in the extent of corneal treatment, while the secondary outcome was to compare the visual and refractive behavior between the enrolled patients in both groups.
Materials and Methods
This retrospective, comparative study was conducted on the electronic medical records of KC patients who sought medical advice at the Ophthalmology Clinic, Ain Shams University, in the time interval between January 2020 and August 2022, and who performed the treatment protocol at Maadi Eye Subspeciality Center, Cairo, Egypt. The study adhered to the tenets of the Declaration of Helsinki and was approved by the ethical committee of Ain Shams University (FMASU-R-188/2022). The institutional review board of Ain Shams University granted a waiver of informed consents, owing to the retrospective nature of the study.
Case definition for enrolling patients in the present study was based on the Global Consensus on KC and Ectatic Diseases.2 Eyes with mild to moderate, progressive disease were included in the study. Progression was defined as an increase within 1 year in the steepest keratometry or manifest cylinder by 1 diopter (D) or more, or in the manifest refraction spherical equivalent by 0.5 D or more. Eyes that had undergone previous ocular procedures, those with a history of infectious keratitis, and those with opacification or apical hydrops were excluded. Eyes that belonged to patients receiving systemic steroids or cytotoxic therapy, and those with collagen disorders were also excluded from the selection process (owing to the possibly delayed healing process).
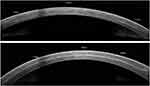
The electronic medical files of the enrolled candidates were searched for relevant data, which included history taking (to reassure that each patient fulfilled the inclusion and exclusion criteria), pre and postoperative subjective refraction “Corrected Distance Visual Acuity (CDVA) and spherical equivalent”, details of the CXL treatment protocol parameters, and data regarding the preoperative corneal and epithelial thickness and postoperative DD (one month after the procedure) were obtained from AS-OCT (Optovue, Fremont, USA) utilizing the corneal high-resolution mode and the caliper tool. (Figure 1) The central and peripheral (at 6 mm optical zone) DD were measured, and the maximum (DDmax) and minimum (DDmin) DD were determined. Furthermore, patients’ pre and postoperative (one year following CXL) average keratometry (K avg) and maximum keratometry (K max) were obtained from Sirius topographer (CSO Italia) with software version Phoenix 3.2.1.60.
The eyes were divided into two groups based on the treatment parameters; group 1 included patients who received epi-off pl-CXL (pulses interval 1.3 seconds) at a power of 30 mW/cm2 with an irradiation time of 4 minutes and a total treatment time of 8 minutes, while group 2 received epi-off cl-CXL at a power of 12 mW/cm2 with an irradiation time of 10 minutes - both corresponding to equivalent TED. The two treatment protocols were performed by two experienced, equal caliber corneal consultants (MOY and AHS, respectively).
Comparisons were performed between both groups regarding the subjective refraction data, keratometric data, and the DD data obtained one month following treatment.
Statistical Analysis
Data was coded and tabulated in an Excel Sheet (Microsoft, USA). The Statistical Package for Social Science (IBM SPSS Version 25) was used for statistical analysis. The Shapiro–Wilk test was applied to measure departure from normality, with the distribution of quantitative data described in terms of mean (± SD) in case of normal distribution, median with interquartile range (IQR) in case of non-normally distributed data, and percentage in case of qualitative variables. The means of variables between the two groups were compared using the two-sample t-test to calculate the probability value (p-value). Statistical significance was considered if p-value was equal or less than 0.05.
Results
The present study was conducted on KC patients with mild to moderate, progressive disease. Group 1 included 103 eyes of 62 patients, and group 2 included 87 eyes of 51 patients. The male/female ratio was 1.1/1 and 1.2/1 and the mean (SD) of the enrolled participants’ age was 26.9 (± 6.8) years, and 28.6 (± 7.6) years in groups 1 and 2, respectively. The differences between both groups regarding age and gender were statistically insignificant.
Patients’ mean preoperative CDVA and spherical equivalent for group 1 were 0.84 (± 1.12) and −3.75 D (±1.07), respectively, while the values for group 2 were 0.87 (±1.32) and −4.25 D (±1.75), respectively, with statistically insignificant differences between both groups. There were also statistically insignificant differences between the pre and post operative subjective refraction values for both groups, where the mean postoperative CDVA was 0.81 (±0.78) for group 1 and 0.83 (± 1.21) for group 2, and the means spherical equivalent was −4.15 (±0.95) for group 1 and −4.56 (±1.64) for group 2.
Table 1 demonstrates the comparative results of preoperative corneal thickness parameters and postoperative DD levels in both groups. The differences between the corneal thickness (minimum and central) and the epithelial thickness measurements between both groups were statistically insignificant. In regard to the DD parameters, the study results showed that group 1 patients had slightly larger central DD (223.4 ± 62.3 um), DDmax (240.4 ± 61.8 um), and DDmin (201 ± 54 um) than those of group 2 (221.8 ± 37 um, 229.1 ± 38.4 um, and 212 ± 37.2 um, respectively), yet the differences between both groups’ measurements were statistically insignificant (p = 0.8337, 0.1404, and 0.1102, respectively).
 |
Table 1 Comparison Between the Preoperative Corneal Thickness Parameters and the Postoperative Demarcation Line Depth (DD) Values in the Two Enrolled Groups |
In regard to patients’ K avg and K max readings, the results declared statistically insignificant differences between the pre and postoperative (one year following CXL) values in both groups, denoting keratometric stability, where patients of group 1 had pre and postoperative K avg values of 46.98 D ± 4.64 and 46.62 D ± 4.22, respectively, and pre and postoperative K max values of 50.24 D ± 4.12 and 49.88 D ± 3.73, respectively, and patients of group 2 similarly had statistically insignificant differences between the pre and postoperative K avg and K max values (47.64 D ± 3.27, 46.91 D ± 3.91, 49.39 D ± 4.31, and 48.78 D ± 3.29, respectively).
Discussion
In this study, we have compared two accelerated CXL protocols (pl-CXL and cl-CXL) in a relatively large number of eyes regarding the visual, refractive, and keratometric stability and regarding the extent (depth) of the treated cornea. Our study results revealed statistically insignificant differences between the two groups regarding the visual, refractive, and keratometric outcomes postoperatively. Although the eyes which received the pl-CXL had a deeper DD than those exposed to cl-CXL, the difference between the two groups was statistically insignificant.
The DD has been shown to be affected by a variety of factors. Ng et al15 have demonstrated that the A-CXL protocol achieves significantly reduced central DD (mean = 203 um) than the conventional Dresden protocol (mean = 295 um). It is to be noted, however, that their A-CXL group only included a small sample size of 15 eyes and that the TED dose used in their work for A-CXL was 5.4 J/cm2 as compared to the higher TED (7.2 J/cm2) used in our study. This difference could explain the larger mean central DD in our sample (223.4 and 221.8 um for groups 1 and 2, respectively). The same explanation could be provided for the study by Pircher et al20 that was conducted on only 40 eyes with an A-CXL protocol (5.4 J/cm2) and showed a mean DD of 200 um. This confirms that the higher TED is associated with a deeper DD.
Contrastingly, Mesen et al21 showed that there were no statistically significant differences between conventional CXL and A-CXL regarding both DD and progression in a relatively large cohort of patients, but both groups in this study had significantly larger DD and lower number of progressed eyes than the transepithelial group. The latter finding of shallower DD with transepithelial CXL as compared to epi-off protocols is also confirmed by the studies of Abdel-Radi et al22 and Salah et al.16 This solidifies the theory that the deeper penetration of light is associated with more effective CXL and that epithelial removal seems to be integral to the process.
Previous studies pitting pl-CXL against cl-CXL have mostly weighed in favor of pl-CXL. Mazzotta et al23 showed that although pl-CXL and cl-CXL (both with TED of 7.2 J/cm2) had similar functional outcomes, pl-CXL had deeper penetration. However, the sample size was only 10 eyes in each group and the authors used a power of 30 mW/cm2 for a duration of 4 minutes for the cl-CXL group (as compared to our used power of 12 mW/cm2 in 10 minutes). Using the same energy parameters as Mazzota et al,23 Moramarco et al,13 and Peyman et al24 arrived at the same conclusion in larger analyses. This supports the findings that the longer duration of total treatment time may also be associated with deeper DD.25
One of the main strengths of the present study is the large sample size (the largest to date, to the authors’ knowledge). Our study also confirmed previous findings18 regarding the reliability of AS-OCT in measuring DD one month following A-CXL for detecting the effectiveness of treatment.
In our study, the DD was used as a proxy for the extent of CXL and hence for the presumed halting of progression. This rationale has been challenged in some of the previous studies.20,21,26 Those studies, however, attempted to establish correlation patterns between DD and keratometric parameters rather than direct assessment of disease progression. Only the study by Mesen et al21 assessed the progression and found it to be significantly lower in eyes that underwent epithelial removal and had deeper DD than eyes undergoing a transepithelial approach and had a superficial patchy line. Future longitudinal long-term studies are needed to directly measure the effect of different A-CXL protocols with different DDs on the risk of KC progression.
It is noteworthy that the DD cannot be considered as a sole reliable parameter for the effectiveness of CXL protocols. Hence, we also compared the pre and postoperative visual, refractive, and keratometric performances, which indicated stability in both groups. However, the DD still remains an important marker for the extent of treatment.
Our study is not without limitations. The retrospective nature provides less evidence than prospective, longitudinal studies. Moreover, we used both eyes in some patients, which raises some concerns regarding the possible existence of systemic factors in the same patient that would possibly affect both eyes. However, the two eyes of the same KC patient may develop different responses to treatment in many instances. Future longitudinal studies, maybe also using one eye from each patient, may reinforce or contradict our study results.
Conclusions
In conclusion, the present study showed that longer duration cl-CXL seems to be as effective as pl-CXL regarding the visual, refractive, and keratometric outcomes and also regarding the extent of corneal tissue penetration by the treatment in a large group of eyes treated by A-CXL.
Data Sharing Statement
Available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consents
This study was approved by the ethical committee of Ain Shams University (FMASU-R-188/2022). The institutional review board of Ain Shams University granted a waiver of informed consents, owing to the retrospective nature of the study. Patients’ data were kept anonymous with utmost confidentiality.
Consent for Publication
All the material included in this paper can be published, and the person(s) providing consent have been shown the article contents to be published.
Acknowledgments
The authors would like to thank the statistician Mr. Hesham Elkady for the performance of the statistical analysis of the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
None to be declared.
References
1. Romero-Jiménez M, Santodomingo-Rubido J, Wolffsohn JS. Keratoconus: a review. Cont Lens Anterior Eye. 2010;33(4):157–166. doi:10.1016/j.clae.2010.04.006
2. Gomes JAP, Tan D, Rapuano CJ, et al. Global consensus on Keratoconus and ectatic diseases. Cornea. 2015;34(4):359–369. doi:10.1097/ICO.0000000000000408
3. Mohammadpour M, Heidari Z, Hashemi H. Updates on managements for Keratoconus. J Curr Ophthalmol. 2018;30(2):110–124. doi:10.1016/j.joco.2017.11.002
4. Jhanji V, Sharma N, Vajpayee RB. Management of keratoconus: current scenario. Br J Ophthalmol. 2011;95(8):1044–1050. doi:10.1136/bjo.2010.185868
5. Vohra V, Tuteja S, Chawla H. Collagen cross linking for Keratoconus. In: StatPearls. Treasure Island (FL): StatPearls Publishing Copyright; 2021.
6. Kobashi H, Rong SS. Corneal collagen cross-linking for keratoconus: systematic review. Biomed Res Int. 2017;2017:8145651. doi:10.1155/2017/8145651
7. Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg. 2003;29(9):1780–1785. doi:10.1016/S0886-3350(03)00407-3
8. Santhiago MR, Randleman JB. The biology of corneal cross-linking derived from ultraviolet light and riboflavin. Exp Eye Res. 2021;202:108355. doi:10.1016/j.exer.2020.108355
9. Koller T, Mrochen M, Seiler T. Complication and failure rates after corneal crosslinking. J Cataract Refract Surg. 2009;35(8):1358–1362. doi:10.1016/j.jcrs.2009.03.035
10. Moramarco A, Mastrofilippo V, Romano MG, et al. Efficacy and safety of accelerated corneal cross-linking for progressive Keratoconus: a 5-year follow-up study. J Refract Surg. 2020;36(11):724–730. doi:10.3928/1081597X-20200819-01
11. Sherif AM. Accelerated versus conventional corneal collagen cross-linking in the treatment of mild keratoconus: a comparative study. Clin Ophthalmol. 2014;8:1435–1440. doi:10.2147/OPTH.S59840
12. Chan C. Corneal cross-linking for Keratoconus: current knowledge and practice and future trends. Asia-Pacific J Ophthalmol. 2020;9(6):557–564. doi:10.1097/APO.0000000000000335
13. Moramarco A, Iovieno A, Sartori A, et al. Corneal stromal demarcation line after accelerated crosslinking using continuous and pulsed light. J Cataract Refract Surg. 2015;41(11):2546–2551. doi:10.1016/j.jcrs.2015.04.033
14. Seiler T, Hafezi F. Corneal cross-linking-induced stromal demarcation line. Cornea. 2006;25(9):1057–1059. doi:10.1097/01.ico.0000225720.38748.58
15. Ng ALK, Chan TCY, Lai JSM, et al. Comparison of the central and peripheral corneal stromal demarcation line depth in conventional versus accelerated collagen cross-linking. Cornea. 2015;34(11):1432–1436. doi:10.1097/ICO.0000000000000626
16. Salah Y, Omar K, Sherif A, et al. Study of demarcation line depth in transepithelial versus epithelium-off accelerated cross-linking (AXL) in Keratoconus. J Ophthalmol. 2019;2019:3904565. doi:10.1155/2019/3904565
17. Ozek D, Kemer OE, Ozer PA. Corneal stromal depth of the demarcation line in ‘accelerated corneal cross-linking’ with different concentrations of riboflavin solutions. Int Ophthalmol. 2019;39(6):1329–1335. doi:10.1007/s10792-018-0951-x
18. Thorsrud A, Sandvik GF, Hagem AM, et al. Measuring the depth of crosslinking demarcation line in vivo: comparison of methods and devices. J Cataract Refract Surg. 2017;43(2):255–262. doi:10.1016/j.jcrs.2017.01.003
19. Tzamalis A, Vinciguerra R, Romano V, et al. Intraobserver reproducibility and interobserver agreement of demarcation line depth measurements following corneal cross linking. Eur J Ophthalmol. 2020;30(4):635–642. doi:10.1177/1120672119835116
20. Pircher N, Lammer J, Holzer S, et al. Correlation between central stromal demarcation line depth and changes in K values after corneal cross-linking (CXL). Graefes Arch Clin Exp Ophthalmol. 2018;256(4):759–764. doi:10.1007/s00417-018-3922-z
21. Mesen A, Bozkurt B, Kamis U, et al. Correlation of demarcation line depth with medium-term efficacy of different corneal collagen cross-linking protocols in Keratoconus. Cornea. 2018;37(12):1511–1516. doi:10.1097/ICO.0000000000001733
22. Abdel-Radi M, Eldaly Z, Abdelmotaal H, et al. Correlation between corneal demarcation line depth in epithelium-off and trans-epithelium accelerated corneal cross linking and keratoconus progression. Int J Ophthalmol. 2020;13(6):907–912. doi:10.18240/ijo.2020.06.08
23. Mazzotta C, Traversi C, Paradiso AL, et al. Pulsed light accelerated crosslinking versus continuous light accelerated crosslinking: one-year results. J Ophthalmol. 2014;2014:604731. doi:10.1155/2014/604731
24. Peyman A, Nouralishahi A, Hafezi F, et al. Stromal demarcation line in pulsed versus continuous light accelerated corneal cross-linking for Keratoconus. J Refract Surg. 2016;32(3):206–208. doi:10.3928/1081597X-20160204-03
25. Mazzotta C, Traversi C, Caragiuli S, et al. Pulsed vs continuous light accelerated corneal collagen crosslinking: in vivo qualitative investigation by confocal microscopy and corneal OCT. Eye. 2014;28(10):1179–1183. doi:10.1038/eye.2014.163
26. Gatzioufas Z, Balidis M, Kozeis N. Is the corneal stromal demarcation line depth a true indicator of corneal collagen crosslinking efficacy? J Cataract Refract Surg. 2016;42(5):804. doi:10.1016/j.jcrs.2016.02.043
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.