Back to Journals » Clinical Ophthalmology » Volume 17
Comparing Predictive Accuracy of a Swept Source Optical Coherence Tomography Biometer and an Optical Low Coherence Reflectometry Biometer
Received 16 May 2023
Accepted for publication 13 July 2023
Published 25 July 2023 Volume 2023:17 Pages 2125—2131
DOI https://doi.org/10.2147/OPTH.S421504
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Clayton Blehm,1 Brad Hall2
1Gainesville Eye Associates, Gainesville, GA, USA; 2Sengi, Penniac, NB, Canada
Correspondence: Clayton Blehm, Gainesville Eye Associates, 2061 Beverly Road, Gainesville, GA, 30501, USA, Tel +1 770-532-4444, Email [email protected]
Purpose: To compare the refractive accuracy resulting from calculations based on measurements with a swept-source optical coherence tomography (SS-OCT) biometer compared to calculations based on measurements with an optical low coherence reflectometry (OLCR) biometer at one month postoperatively.
Methods: This was a retrospective comparative non-interventional study of preoperative biometry and postoperative refraction and visual acuity of 200 eyes. All eyes had preoperative biometry with both the Argos (Movu, a Santec company) and Lenstar LS900 (Haag-Streit AG) devices. Data were collected for mean postoperative prediction error (directional and absolute), preoperative mean K, delta K (corneal astigmatism), axial length, and anterior chamber depth.
Results: The mean directional prediction error was − 0.15 ± 0.47 D for Argos and − 0.31 ± 0.50 D for Lenstar LS900, and there was a statistically significant mean of the differences (0.16 ± 0.24 D; p < 0.001). The mean absolute prediction error was 0.35 ± 0.34 D for Argos and 0.42 ± 0.41 D for Lenstar LS900, and there was a statistically significant mean of the differences (− 0.07 ± 0.24 D; p < 0.001). Neither the differences in directional prediction error nor the differences in absolute prediction error were clinically significant.
Conclusion: The directional and absolute prediction accuracies were statistically significant, but not clinically different between the Argos and Lenstar LS900 devices. In addition, differences between preoperative K, AL, and ACD measurements were not clinically significant.
Keywords: Argos, Lenstar LS900, biometry, SS-OCT, OLCR
Plain Language Summary
When the natural lens becomes opaque, cataract surgery can be performed to replace the natural lens with a clear artificial intraocular lens (IOL). Precise and accurate measurements of the eye are required to select the most appropriate IOL power. These measurements can be done prior to surgery with devices called biometers. There are many different types of biometers available, each with different optical technologies. The purpose of this study was to compare the refractive accuracy resulting from calculations based measurements from two different biometers. The results of this study suggest that the refractive accuracies were similar between the two biometers.
Introduction
Cataract surgery patients have high expectations of good clinical outcomes postoperatively. A crucial element of obtaining good clinical outcomes is achieving the target postoperative refraction, which is dependent on precise and accurate preoperative biometry. Optical biometry enables effective evaluation of keratometry (K), axial length (AL), and anterior chamber depth (ACD). Several different types of optical biometers are available including partial coherence interferometry (PCI),1 optical low coherence interferometry (OLCI),2 swept-source optical coherence tomography (SS-OCT),3,4 and optical low coherence reflectometry (OLCR).5
The Lenstar LS900 (Haag-Streit AG) is an OLCR biometer, which measures AL, ACD, lens thickness (LT), and central corneal thickness (CCT) using a 820 nm superluminescent diode.6 White-to-white (WTW) measurements are obtained with color photography, while K readings are determined from 32 reference points on the anterior cornea.7 Studies have reported that the Lenstar LS900 provides accurate and precise biometery measurements,8,9 however dense cataracts can impact AL determinations.10
Biometers based on SS-OCT have good optical penetration and are less prone to measurement failure with dense cataracts compared to OLCR biometers.10 The Argos (Movu, a Santec company) utilizes SS-OCT biometry, and measures AL, ACD, CCT, K, LT, and WTW using a wavelength of 1060 nm.9 Keratometry is performed using a ring of 16 infrared light emitting diodes (LEDs). It uses refractive indices of 1.376 for the cornea, 1.410 for the lens, and 1.336 for the aqueous and vitreous.11 A sum of these segments is used to determine axial length, with the advantage that adjustments can be made to the axial length calculation for the variability of the lengths in each segment. The Argos has been reported to provide good refractive and clinical outcomes.6,12
With the various advances in optical biometer technology, it is important for surgeons to understand biometer performance, and especially refractive outcomes compared to other biometers. To date, there is minimal data comparing the predictive accuracy of the Lenstar LS900 to the Argos. The purpose of this study is to compare the refractive accuracy resulting from calculations based on Argos measurements compared to calculations based on Lenstar LS900 measurements.
Methods
This study was a retrospective chart review of the refractive accuracy from calculations based on measurements with a SS-OCT (Argos) and an OLCR biometer (Lenstar LS900). Study approval and waiver of informed consent was granted by an institutional review board (Salus IRB, approval number CB-21-001). A waiver was granted as this was a non-interventional retrospective chart review of anonymized data. All data were maintained with confidentiality. As this was a retrospective study, there was no requirement to register in a clinical trials database (such as clinicaltrials.gov). The tenets of the Declaration of Helsinki, International Harmonization (ICH) guidelines, and Good Clinical Practice (GCP) were followed.
Eligible charts were those from subjects between June 2021 and August 2022 with on-label treatment of cataracts with implantation of the AcrySof IQ IOL (Model SN60WF; Alcon Vision, LLC), postoperative corrected distance visual acuity (CDVA) of 20/30 or better in each eye, and those with both preoperative biometry and 1 month postoperative data. Charts were excluded from subjects with moderate or severe corneal pathology, corneal astigmatism greater than 4.00 D, prior corneal refractive surgery, or moderate or severe maculopathy.
All eyes in the study were measured with the Argos and Lenstar LS900 biometers preoperatively. Eyes were targeted for plano or first minus using the Barrett Universal II formula with each biometer. When the suggested powers from each biometer differed, the Argos recommended power was selected. One experienced surgeon (CB) performed microincision phacoemulsification in all eyes. All surgeries were performed using manual technique with a 2.2 mm diamond knife keratome from a superior approach at approximately the 160 degree axis. An approximate 5.0 mm capsulorhexis was created in all eyes. The primary endpoint was to compare the absolute prediction error between the Argos and Lenstar LS900. Absolute prediction error was determined for each eye using optimized lens constants for each biometer with the Barrett Universal II formula. Absolute prediction error was calculated as the difference in predicted spherical equivalent from each biometer to the postoperative manifest refraction spherical equivalent. Directional prediction error was calculated as the the difference in predicted spherical equivalent from each biometer to the postoperative manifest refraction spherical equivalent. Secondary endpoints included comparing preoperative K, AL, and ACD measurements, residual astigmatism, and monocular CDVA.
The statistical software R (version 4.1.2; The R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analysis. The software AstigMATIC13 was used for astigmatism analysis, using manifest refraction. The statistical significance of each parameter between devices was assessed using a paired t-test (for parametric data) or a Wilcoxon signed rank test (for non-parametric data). A p-value ≤ 0.05 was considered significant for all statistical tests. To control the family-wise error rate, p-values were adjusted using the Bonferroni correction. We estimated that the study would require a sample size of 138 eyes to achieve a power of 80% and a level of significance of 5% (two sided), for detecting a mean of the differences of 0.25 between pairs (non-inferiority margin), and assuming 0 expected differences between pairs and the standard deviation of the differences to be 1.0. The calculated sample size above was increased 50%, to 200 eyes to improve the reliability of results.
Results
A total of 200 consecutive eyes were identified for this study based on the inclusion and exclusion criteria above. The preoperative and patient demographics are summarized in Table 1.
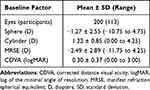 |
Table 1 Preoperative and Demographic Data |
Table 2 summarizes the comparisons between devices. Non-inferiority was confirmed before a superiority test was performed, using a non-inferiority margin of 0.25 D. The mean directional and absolute prediction errors for the Argos device were better than the Lenstar LS900 (by 0.16 D and 0.07 D respectively), and were statistically significant (p < 0.001). However, the differences in both the directional and absolute prediction errors were not clinically significant (Table 2). Figure 1 summarizes the distribution of absolute prediction error for the Argos and Lenstar LS900 devices. The percentages of eyes with a prediction error less than or equal to 0.5 D was 78.5% (157 eyes) for the Argos and 69.5% (139 eyes) for Lenstar LS900. A Bland-Altman plot of absolute prediction error is shown in Figure 2. On average, the Argos device measured a slightly higher steep K, corneal astigmatism, and mean K, larger anterior chamber depth, and shorter axial length, which were statistically significant, but not clinically significant.
 |
Table 2 Summary of Comparison Between Devices |
 |
Figure 1 Absolute prediction error for spherical equivalent. D = diopters. |
Table 3 summarizes the postoperative visual and refractive outcomes. Postoperative monocular visual acuity was excellent, with 93% of eyes 20/25 or better (Figure 3). The refractive outcomes were also good, with 83% of eyes having MRSE 0.5 D or less. The percentage of eyes with residual astigmatism 0.5 D or less was 54%. Astigmatism vector analysis is shown in Figure 4. The mean target induced astigmatism was 1.08 D, the mean surgically induced astigmatism was 0.82 D, and the correction index was 0.89, indicating a slight undercorrection.
 |
Table 3 Postoperative Data |
 |
Figure 3 Postoperative cumulative corrected distance visual acuity (CDVA). |
 |
Figure 4 Astigmatism vector analysis. Abbreviation: D, diopters. |
Discussion
Cataract surgery is a refractive procedure, with an end goal of hitting the refractive target. It is currently estimated that in 73% of cases, surgeons are achieving the target spherical equivalent refraction (± 0.5 D).14 Preoperative formulas to select IOL power are heavily reliant on preoperative biometry measurements. Therefore, accurate biometry measurements are crucial to achieve good refractive outcomes. Our study compared the predictive accuracy of a SS-OCT biometer and an OLCR biometer.
The directional and absolute prediction errors were slightly better for the Argos compared to the Lenstar LS900 (by 0.16 D and 0.07 D respectively), but the differences were not clinically relevant. To the best of our knowledge, this is the first study to directly compare both the directional and absolute prediction error between the Argos and Lenstar LS900. In a retrospective study, Cummings et al15 reported the absolute prediction error between the Argos and Lenstar LS900 in 45 eyes. They observed minimal differences in mean absolute prediction error between the Argos (0.239 D) and Lenstar LS900 (0.244 D), which do not appear clinically relevant.
In addition to prediction error, we compared preoperative K measurements, AL, and ACD. Compared to the Lenstar LS900, the Argos tended to measure slightly higher steep K, corneal astigmatism, and mean K, larger anterior chamber depth, and shorter axial length, although these differences were not clinically significant. In a prospective, comparative case series, Montes-Mico6 also reported that the Argos tended to measure slightly higher steep K, flat K, and ACD, but slightly lower AL. The differences reported by Montes-Mico6 also do not appear to be clinically significant. In a prospective study, Shammas et al9 observed minimal differences for AL and ACD between the Argos and Lenstar LS900 devices. Cummings et al15 in their retrospective study also reported minimal differences for AL and ACD between the Argos and Lenstar LS900 devices. The results of our study and those of previous studies6,9,15 suggest that there are minimal differences in preoperative measurements (K, AL, ACD) between the Argos and Lenstar LS900.
The refractive outcomes in this study were good, with 83% of eyes having postoperative MRSE 0.5 D or less. Both the Argos and Lenstar LS900 biometers were used preoperatively, although where the suggested power differed between the devices, the Argos suggested power was preferred. The percentage of eyes with an absolute prediction error ≤ 0.5 D was 79% for the Argos and 70% for the Lenstar LS900. We were not able to find any studies directly comparing these figures between the 2 biometers. However, studies comparing the Argos to other biometers and the Lenstar LS900 to other biometers have reported similar results.4,7,12,16–20
A limitation of this study was the retrospective design. In addition, this study focused on the prediction accuracy for spherical equivalent. Future work should investigate the prediction accuracy for residual astigmatism. Visual acuity is estimated to decrease by 1.5 lines for every diopter of residual astigmatism.21 Therefore, it is important to also understand the performance of biometers for predicting residual astigmatism. Another limitation of this study was the use of the same eye to calculate the prediction error for both biometers. This could introduce bias, since the power suggested by the Argos device was preferred, although our results indicated clinically insignificant differences between the Argos and Lenstar LS900 device.
In conclusion, the directional and absolute prediction accuracies were clinically equivalent between the Argos and Lenstar LS900 devices. Differences between preoperative K, AL, and ACD measurements were not clinically significant.
Funding
This study was supported with an investigator-initiated study grant (67464611) from Alcon Vision, LLC, Fort Worth, TX, USA.
Disclosure
Brad Hall reports that he has received consulting fees from Ace Vision Group outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Vogel A, Dick BH, Krummenauer F. Reproducibility of optical biometry using partial coherence interferometry: intraobserver and interobserver reliability. J Cataract Refract Surg. 2001;27:1961–1968. doi:10.1016/S0886-3350(01)01214-7
2. Hoffer KJ, Shammas HJ, Savini G, Huang J. Multicenter study of optical low-coherence interferometry and partial-coherence interferometry optical biometers with patients from the United States and China. J Cataract Refract Surg. 2016;42:62–67. doi:10.1016/j.jcrs.2015.07.041
3. Montes-Mico R, Pastor-Pascual F, Ruiz-Mesa R, Tana-Rivero P. Ocular biometry with swept-source optical coherence tomography. J Cataract Refract Surg. 2021;47:802–814. doi:10.1097/j.jcrs.0000000000000551
4. Yang CM, Lim DH, Kim HJ, Chung TY. Comparison of two swept-source optical coherence tomography biometers and a partial coherence interferometer. PLoS One. 2019;14:e0223114. doi:10.1371/journal.pone.0223114
5. Hoffer KJ, Shammas HJ, Savini G. Comparison of 2 laser instruments for measuring axial length. J Cataract Refract Surg. 2010;36:644–648. doi:10.1016/j.jcrs.2009.11.007
6. Montes-Mico R. Evaluation of 6 biometers based on different optical technologies. J Cataract Refract Surg. 2022;48:16–25. doi:10.1097/j.jcrs.0000000000000690
7. Reitblat O, Levy A, Kleinmann G, Assia EI. Accuracy of intraocular lens power calculation using three optical biometry measurement devices: the OA-2000, Lenstar-LS900 and IOLMaster-500. Eye. 2018;32:1244–1252. doi:10.1038/s41433-018-0063-x
8. Shetty N, Kaweri L, Koshy A, Shetty R, Nuijts RM, Sinha Roy A. Repeatability of biometry measured by three devices and its impact on predicted intraocular lens power. J Cataract Refract Surg. 2021;47:585–592. doi:10.1097/j.jcrs.0000000000000494
9. Shammas HJ, Ortiz S, Shammas MC, Kim SH, Chong C. Biometry measurements using a new large-coherence-length swept-source optical coherence tomographer. J Cataract Refract Surg. 2016;42:50–61. doi:10.1016/j.jcrs.2015.07.042
10. Vasavada SA, Patel P, Vaishnav VR, et al. Comparison of optical low-coherence reflectometry and swept-source OCT-based biometry devices in dense cataracts. J Refract Surg. 2020;36:557–564. doi:10.3928/1081597X-20200612-03
11. Wang Q, Chen M, Ning R, et al. The precision of a new anterior segment optical coherence tomographer and its comparison with a swept-source OCT-based optical biometer in patients with cataract. J Refract Surg. 2021;37:616–622. doi:10.3928/1081597X-20210610-02
12. Omoto MK, Torii H, Masui S, Ayaki M, Tsubota K, Negishi K. Ocular biometry and refractive outcomes using two swept-source optical coherence tomography-based biometers with segmental or equivalent refractive indices. Sci Rep. 2019;9:6557. doi:10.1038/s41598-019-42968-3
13. Gauvin M, Wallerstein A. AstigMATIC: an automatic tool for standard astigmatism vector analysis. BMC Ophthalmol. 2018;18:255. doi:10.1186/s12886-018-0920-1
14. Lundstrom M, Dickman M, Henry Y, et al. Risk factors for refractive error after cataract surgery: analysis of 282,811 cataract extractions reported to the European Registry of Quality Outcomes for cataract and refractive surgery. J Cataract Refract Surg. 2018;44:447–452. doi:10.1016/j.jcrs.2018.01.031
15. Cummings AB, Naughton S, Coen AM, Brennan E, Kelly GE. Comparative analysis of swept-source optical coherence tomography and partial coherence interferometry biometers in the prediction of cataract surgery refractive outcomes. Clin Ophthalmol. 2020;14:4209–4220. doi:10.2147/OPTH.S278589
16. Fabian E, Wehner W. Prediction accuracy of total keratometry compared to standard keratometry using different intraocular lens power formulas. J Refract Surg. 2019;35:362–368. doi:10.3928/1081597X-20190422-02
17. Tamaoki A, Kojima T, Hasegawa A, et al. Clinical evaluation of a new swept-source optical coherence biometer that uses individual refractive indices to measure axial length in cataract patients. Ophthalmic Res. 2019;62:11–23. doi:10.1159/000496690
18. Choi JY, Choi A, Kwon H, Jeon S. Accuracy of IOL power calculation formulas for quadrifocal acrysof IQ PanOptix TFNT implantation in patients with previous corneal refractive surgery: comparison of SS-OCT-based biometers. J Refract Surg. 2021;37:836–841.
19. Reitblat O, Assia EI, Kleinmann G, Levy A, Barrett GD, Abulafia A. Accuracy of predicted refraction with multifocal intraocular lenses using two biometry measurement devices and multiple intraocular lens power calculation formulas. Clin Exp Ophthalmol. 2015;43:328–334. doi:10.1111/ceo.12478
20. Song JS, Yoon DY, Hyon JY, Jeon HS. Comparison of ocular biometry and refractive outcomes using IOL Master 500, IOL Master 700, and Lenstar LS900. Korean J Ophthalmol. 2020;34:126–132. doi:10.3341/kjo.2019.0102
21. Lehmann RP, Houtman DM. Visual performance in cataract patients with low levels of postoperative astigmatism: full correction versus spherical equivalent correction. Clin Ophthalmol. 2012;6:333–338. doi:10.2147/OPTH.S28241
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

