Back to Journals » Clinical Ophthalmology » Volume 15
Comparing Effectiveness of Three Different Anti-VEGF Treatment Regimens for Neovascular Age-Related Macular Degeneration: Two Years’ Real-World Clinical Outcomes
Authors Horner F, Lip PL , Mohammed BR, Fusi-Rubiano W, Gokhale E , Mushtaq B, Chavan R
Received 17 February 2021
Accepted for publication 25 March 2021
Published 23 April 2021 Volume 2021:15 Pages 1703—1713
DOI https://doi.org/10.2147/OPTH.S305141
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
Faye Horner, Peck Lin Lip, Bashar R Mohammed, William Fusi-Rubiano, Eesha Gokhale, Bushra Mushtaq, Randhir Chavan
Birmingham & Midland Eye Centre, Birmingham, UK
Correspondence: Randhir Chavan
Birmingham & Midland Eye Centre, Sandwell & West Birmingham Hospitals NHS Trust, City Hospital Dudley Road, Birmingham, B18 7QH, UK
Tel +44 121 5543801
Fax +44 121 5076791
Email [email protected]
Purpose: To compare and report the 2-year treatment outcomes from 3 different anti-VEGF treatment regimens in treating neovascular aged-related macular degeneration (nAMD): Ranibizumab pro re nata (Ranibizumab-PRN); Ranibizumab treat and extend (Ranibizumab-T&E); Aflibercept fixed first year dosing (7 injections) with treat and extend in subsequent year (Aflibercept-Fixed).
Methods: All treatment-naïve nAMD patients who completed 24 months of monitoring from a single treatment center were included. Patients received the initial loading dose of three injections (4-weekly interval), followed by one of the 3 treatment regimens. Primary outcomes were changes in visual acuity (VA) and central retinal thickness (CRT). Secondary outcome was number of injections required in each year. Data analysis included last observation carried forward (LOCF) for patients with incomplete year-2 follow-up.
Results: A total of 249 eyes (230 patients) were studied: 121 Ranibizumab-PRN; 65 Ranibizumab-T&E, and 63 Aflibercept-Fixed. Baseline median VA (ETDRS letters) for Ranibizumab-PRN, Ranibizumab-T&E, and Aflibercept-Fixed was 53.9, 61.1, and 54.9 letters, achieving final VA of 54.9, 65.1, and 65.1 letters, respectively. Hence, the number of letters increased at the end of 24 months for each group was +1.0 (Ranibizumab-PRN), +4.0 (Ranibizumab-T&E), highest +10.2 in Aflibercept-Fixed group. Median number of injections over 2 years (year-1/year-2) was 5/1 for Ranibizumab-PRN, 9/6 for Ranibizumab-T&E, and 7/5 for Aflibercept-Fixed. Both Ranibizumab-T&E and Aflibercept-Fixed also shared the same reduction of median CRT (115 μm), higher than Ranibizumab-PRN (83 μm).
Conclusion: We report VA improvement from all three different treatment regimens with both Aflibercept-Fixed and Ranibizumab-T&E regimens achieving the same higher final VA. Aflibercept-Fixed dosing may have more favorable efficacy with the highest VA gain and comparatively lower dosing frequency whereas Ranibizumab-T&E may be more efficient than Ranibizumab-PRN regimen, according to our study.
Keywords: AMD, Ranibizumab, Aflibercept, protocols, PRN, treat & extend
Plain Language Summary
Frequent monthly injections in the eyes as an out-patient procedure have been the mainstay for treating active wet age-related macular degeneration (nAMD) world-wide. However, there is increasing wider adaption of different treatment schedules (away from monthly injections) to meet the efficacy and the increasing local AMD service demand. It has been uncertain and confusing regarding which treatment regimen delivers best efficacy and vision outcomes. We compared two licensed injection drugs (Ranibizumab and Aflibercept) and three commonly adapted injection treatment regimens (“As-Required regimen”, “Fixed-dosing interval regimen”, and “Treat-and-Extend” regimen). This is the first such informative comparison reporting the relevant visual outcomes and injection frequency, confirming relative superiority and efficacy of the three treatment regimens. Our cohort of longer term (2 years) results in a real-world practice could further aid clinicians to consolidate decisions in choosing a best-suited treatment regimen for individual patients during consultation.
Introduction
Age-related macular degeneration is the commonest cause of registered blindness affecting the elderly population in the western world.1 Since the introduction of several licensed intravitreal anti-vascular endothelial growth factor (anti-VEGF) agents as the frontline treatment for neovascular age-related macular degeneration (nAMD), many patients have unequivocally benefited with extended years of preserved or improved useful vision.2,3 While the significant visual improvement was clearly welcomed by patients, the short-acting efficacy of anti-VEGF agents has demanded very frequent treatments and assessments in order to prevent vision loss. Indeed, the original clinical trials (MARINA and ANCHOR) recommended monthly intravitreal injections of Ranibizumab for the first treatment year to achieve the clinical benefits.2,3 As clinical trials rarely reflect the complexity of clinical practice, alternative dosing regimens have been rapidly modified and adopted in real-world clinical practices to meet the heavy treatment burden by reducing the numbers of injections, clinic review visits, whilst achieving similar visual gain. Clinical trials such as the PrONTO study on Ranibizumab demonstrated the efficacy of a variable dosing regimen (PRN, pro re nata) approach based on disease activity as an alternative to the monthly dosing regimen.4 Subsequently, a “Treat and Extend” (T&E) approach also gained popularity in terms of efficacy compared to PRN approach.5,6 The latest treatment regimen modification was based on VIEW 1 and 2 studies confirming non-inferiority of Aflibercept fixed dose regimen in comparison with monthly Ranibizumab.7 As the differences at molecular level and mechanisms of actions of the few available anti-VEGF agents have been well studied, this knowledge helps clinicians to further relate and understand the differences in clinical effectiveness of each agent, from many published clinical studies.8
In the majority of clinical practices, patients with newly diagnosed nAMD often receive an initial loading phase of three-monthly anti-VEGF injections. Thereafter, there are three different commonly modified treatment regimens in follow-up plan: firstly, the PRN approach advocates retreatment only when there is evidence of disease activity; secondly, the T&E regimen aims to continue injections despite disease inactivity, but dosing interval increases with time; thirdly, fixed dosing of 2 monthly injections, regardless of disease activity in the first year.
Since 2016, our tertiary eye treatment center have made available all three different anti-VEGF treatment regimens to patients with newly diagnosed nAMD using the two NICE-approved anti-VEGF agents: Ranibizumab-PRN, Ranibizumab-T&E, and Aflibercept-Fixed dosing. Herein, we present the related clinical outcomes of these patients with 2-year follow-up period. To the best of our knowledge, this is the first study based on a single treatment center providing a direct comparison of all three commonly applied nAMD treatment regimens, offering the relevance of real-world clinical experience of a long follow-up period.
Methods
We conducted a retrospective data collection of all treatment-naïve nAMD patients who commenced intravitreal anti-VEGF injections between December 2015 and December 2016 at Sandwell and West Birmingham hospitals NHS Trust, UK. Data capturing was based on electronic medical notes (Medisoft Ltd, Leeds, UK) which included demographic information, the primary outcome measures of visual acuity (VA) and central retinal thickness (CRT) and secondary outcome measure was number of injections per year.
Best-corrected visual acuity was measured with LogMAR or Snellen visual acuity charts. Spectral-domain optical coherence tomography (OCT, 3D-OCT 2000, Topcon, Tokyo) was performed at every clinic visit, fundus fluorescein angiography was undertaken to confirm the initial nAMD diagnosis. Patients who met NICE guidelines for treatment were informed of the three treatment regimens and proceeded to informed consent for their treatment regimen preference or based on clinician’s decision if patients had no preference.
We studied the two commonly used intravitreal anti-VEGF agents for nAMD treatment as per NICE guidelines: Ranibizumab 0.5 mg (Genentech Inc, South Sand Francisco, California, USA, licensed in 2008) and Aflibercept 4 mg (Bayer Pharma, Berlin, Germany licensed in 2013). All intravitreal injections were performed with aseptic technique in dedicated sterile rooms as out-patient procedures.
Protocols for Treatment Regimens
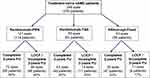
Figure 1 is a flow-diagram detailing our three study protocols: Ranibizumab-PRN, Ranibizumab-T&E, and Aflibercept-Fixed dosing and their related clinic review plans in keeping with our local departmental guidelines at that time. All protocols followed the initial loading-phase of three doses of monthly intravitreal injections. Thereafter, protocols differ in injection intervals by PRN (advocates retreatment only for disease activity with close monthly clinic review), or T&E (continues injections despite disease inactivity, but increasing or decreasing injection intervals commonly by 2 weeks depending on disease activity), or fixed dosing (follows a specified pattern of 2 monthly injections regardless of disease activity or inactivity).
Ranibizumab PRN protocol: commenced with three intravitreal Ranibizumab 0.5 mg monthly injections (loading doses), first reassessment was 4 weeks post- last injection. Further 2 or 3 injections (4 weekly) at doctor’s discretion were planned if there was evidence of disease activity (macular hemorrhage, intra-retinal fluid (IRF), or sub-retinal fluid (SRF)). Review interval was extended by 2 weeks for each episode of disease inactivity confirmed in assessment clinic.
Ranibizumab T&E protocol: commenced with three intravitreal injections of Ranibizumab 0.5 mg at 4-weekly interval (loading doses) with incorporation of “One-stop injection assessment clinic” plan where pre-injection VA/tonometry/OCT were performed on the same day of each planned injection. The first “One Stop” clinic module started on the day of the 3rd intravitreal injection (end of loading phase). A clinician remotely reviewing the “One-stop clinic” results made the decision for re-injection interval.. Injection interval was maintained at 4-weekly interval if there was evidence of persistent active disease. Injection interval was increased by 2 weeks with each episode of disease inactivity. Patients continued with 2-weekly interval extension of injections up to 12-week-interval, after which assessment without injections would be planned if inactivity phase remained. Re-injection of 4-weekly interval would be resumed at any stage with evidence of disease reactivation, and “T&E” module reapplied. All patients had an opportunity to have a “proper” face-to-face appointment with a clinician every 6 months.
Aflibercept Fixed protocol: commenced with three intravitreal injections of Aflibercept at 4-weekly interval (loading doses), followed by further four injections 2 monthly over the first treatment year (equivalent to receiving 7 fixed injections in the first year). In the second year, patients were treated with Aflibercept as per the same T&E protocol detailed previously. Clinic review/assessment was planned 2 monthly.
Our local departmental policy was to discharge patients who had not received any injection for the last 12 months either because of disease stability or deemed no further treatment benefit gain due to establishment of irreversible structural fovea damage/scarring.
Ethics and Statistical Analysis
This retrospective cohort was conducted at Birmingham & Midland Eye Centre, Birmingham, United Kingdom with the ethical approval from the Institutional Review Board (Sandwell and West Birmingham Research and Development review board) in accordance with the “Good Clinical Practice” regulations in the United Kingdom and adhered to the tenets of the Declaration of Helsinki. Informed written consents were obtained from all patients for investigations and treatment procedures, as part of the routine and standard clinical care in our real-world clinical practice. All statistical analyses were performed using Microsoft Excel Professional Plus 2016 (Microsoft Corporation, Redmond, WA, USA) or IBM SPSS software (SPSS Inc., Chicago, IL, USA). All VA measurements, Snellen and logMAR (logarithm of the minimum angle of resolution) units were converted into ETDRS (Early Treatment Diabetic Retinopathy Study) letter score for statistical analysis.
Distribution of data sets was evaluated using the Shapiro–Wilk test. All normally distributed data were represented as mean ± standard deviation (SD) and non-normally distributed data were expressed as median [interquartile range (IQR)].
Difference in the cohort average VA and CRT for each of the 3 groups was compared using a Mann–Whitney U-test. Difference in average VA within each cohort at different time points was compared using the Wilcoxon signed rank test.
Last observation carried forward (LOCF) analysis was performed in our study in order to reduce attrition bias. LOCF was a concept used in other published literature of similar interest where the last recorded episode of VA and CRT (in Year-2, before 24-month in our study) was applied in final data analyses for patients who had incomplete Year-2 data due to missed last follow-up visits for various reasons.9
Results
We identified a total of 261 eyes, 241 patients, newly diagnosed with nAMD who commenced intravitreal anti-VEGF treatment between December 2015 and December 2016. Having excluded patients who did not complete the initial anti-VEGF loading phase and early exit of treatment protocols (in Year-1), this cohort included 249 eyes of 230 patients with mean age balanced across all three groups: 79.9 ± 8.8 years in Ranibizumab-PRN, 79.7 ± 7.4 years in Ranibizumab-T&E, and 79.0 ± 7.1 years in Aflibercept-Fixed group.
Figure 2 depicts distribution of patient numbers in each three treatment protocols: a total of 121 eyes (114 patients) in Ranibizumab-PRN group, 65 eyes (60 patients) in Ranibizumab-T&E, and 63 eyes (56 patients) in Aflibercept-Fixed group. The chart also showed the proportion of patients who had completed the 2-year follow-up and those who missed the last follow-up clinics accounting for LOCF numbers of each subgroup: 49/121 eyes (40%) in PRN Ranibizumab-PRN, 13/65 eyes (20%) in Ranibizumab-T&E, and 11/63 eyes (17%) in Aflibercept-Fixed group.
Visual Acuity Analysis
Table 1A shows visual acuity results of each treatment group at various time points leading to 24 months. Ranibizumab-PRN and Aflibercept-Fixed groups shared a similar low baseline median VA (ETDRS letters) of 53.9 letters and 54.9 letters respectively, whilst baseline VA for Ranibizumab-T&E was higher: 61.1 letters. However, at 24 months, Ranibizumab-PRN group failed to achieve gaining only +1 letter, whereas Aflibercept-Fixed and Ranibizumab-T&E groups, although they started off from different baseline VA, had managed to achieve the same final VA of 65.1 letters (Figure 3A). Hence concluding Aflibercept-Fixed had the highest VA gain of +10.2 letters compared to +4.0 letters gain by Ranibizumab-T&E group.
 |
Table 1 Evaluation of Visual Acuity Over 24 Months of the Three Treatment Protocols: Ranibizumab-PRN; Ranibizumab-T&E, and Aflibercept-Fixed |
Table 1B shows subgroup VA analyses giving the opportunity to compare the true VA results in patients who had completed full 24-months follow-up against the results inclusive of LOCF. The sub-analysis on final VA was notably different in Ranibizumab-PRN group only where there was an increase of +4.7 letters in “analysis-without-LOCF” versus +1 letters in the “cohort analysis inclusive of LOCF”. There was no such difference observed in either Ranibizumab-T&E nor Aflibercept-Fixed sub-analysis, proportionated to the smaller LOCF numbers in these two groups.
Anatomical Outcomes
All three treatment protocols showed a significant reduction of median CRT over 24 months from baseline 349 µm to 266 µm in Ranibizumab-PRN, 380 µm to 265 µm in Ranibizumab-T&E, and 352 µm to 237 µm in Aflibercept group (Figure 3B). Aflibercept-Fixed achieved better final CRT as compared to the other groups.
Figure 4A shows proportions of patients achieving different visual strata of interest for each treatment group at different time points. At the post-loading visit, the proportion of patients that achieved the best visual stratum (≥75 letters, Snellen equivalent of 6/9 or better) had increased in all groups to 14.7% (Ranibizumab-PRN), 27.4% (Ranibizumab-T&N), and 25.8% (Aflibercept-Fixed), which was similar to the 24 month result of 15.7%, 29.2%, and 22.2% respectively. The worse visual strata (<25 letters) was involved in all three groups at 24 months but was not in the initial baseline of Ranibizumab-T&E and Aflibercept-Fixed groups.
The largest percentage of patients gaining ≥15 letters at 24-months was in Aflibercept-Fixed group (27.0%), compared to 21.5% in Ranibizumab-T&E, and 15.7% in Ranibizumab-PRN. In contrast, Ranibizumab-T&E group had the lowest percentage of patients losing ≥15 letters at 24 months (12.3%) compared to the Aflibercept-Fixed (19.0%) and Ranibizumab-PRN (19.8%) groups (Figure 4B).
Treatment Exposure
Figure 5 recorded the mean number of injections performed for each treatment group over 24 months: lowest total of 6 in Ranibizumab-PRN, highest 15 in Ranibizumab-PRN, and 12 in Aflibercept-Fixed group. Sub-analysis per year showed Ranibizumab-T&E group required 9 [IQR 8–10] injections in Year-1, 6 [IQR 3–8] in Year-2; Aflibercept-Fixed group had 7 [IQR 7–7] injections in Year-1, 5 [IQR 3–7] in Year-2, and Ranibizumab-PRN group had significantly less: 5 [IQR 3–7] injections in year-1, only 1 [IQR 0–5] injection in Year-2.
 |
Figure 5 Frequency of intravitreal injections per year recorded for each treatment regimen group. Abbreviations: R-PRN, Ranibizumab-PRN; R-T&E, Ranibizumab-T&E; A-Fixed, Aflibercept-Fixed. |
Discussion
The published clinical outcomes on modified treatment regimens are commonly based on either PRN or T&E treatment regimens.4–6 Recently, several real-world studies comparing regimens10–13 and comparing different anti-VEGF14,15 have also been published. The heterogeneity and diversity of these studies complicate results’ comparison and interpretation, especially when published data were generated from multiple institutes10,14 with very varied treatment access and at different time points.11–13,15
Our cohort provided direct comparative results of three different anti-VEGF treatment regimens for nAMD patients based on a single-center real-world clinical practice. We report VA improvement from all three different treatment regimens with Aflibercept-Fixed-dosing and Ranibizumab-T&E regimens achieving similar VA-gain superior to Ranibizumab-PRN regimen. However, Aflibercept-Fixed-dosing was comparatively more efficient in dosing frequency compared to Ranibizumab-T&E. Interestingly, although they started with different baseline VA, both Aflibercept-Fixed-dosing and Ranibizumab-T&E regimens achieved the same level of VA (65 letters) at 24 months (Table 1, Figure 3A). Indeed, Aflibercept-Fixed-dosing had a lower baseline visual acuity (54.9 letters) than the Ranibizumab-T&E group (61.1 letters), which could account for the apparent greater difference in VA-gain. However, the efficacy of Aflibercept-Fixed-dosing cannot be denied when Ranibizumab-PRN group, which shared the same low baseline VA (with Aflibercept-Fixed), failed to achieve the same final VA level gaining only one letter (Table 1A). An interesting spike of VA gain was noted in the Aflibercept-Fixed group at 24 months and for Ranibizumab-T&E group at 12 months (Figure 3A). This could be due to the fact that some of our retrospective VA “fixed-time” reading-points coincided at around 4 weeks post-injection (most likely within “efficacy period”) whilst some VA readings fell at a much longer post-injection period. In addition, as this was a retrospective real-world cohort, unconscious selection bias when initiating treatment was a possibility, based on the treating clinician’s preference and disease category, for example Aflibercept might be the preferred anti-VEGF agent for treating vascularized retinal pigment epithelial detachments.
Our study corroborates other real-world studies specifically comparing T&E versus PRN confirming a superior outcome of T&E over PRN regimens.10–13 In the earlier years of modifying and adopting various treatment regimens, PrONTO study as the extension of pivotal clinical trial on Ranibizumab monotherapy did demonstrate the efficacy of Ranibizumab-PRN dosing regimen based on disease activity, reporting slightly higher +11.1 letters VA-gain and average of 9.9 injections over this time at 24 months as an alternative to the initially suggested monthly dosing regimen.4 Similarly, the VIEW 1 and 2 studies found non-inferiority of Aflibercept fixed 2-monthly dosing in comparison with monthly Ranibizumab.7 The popularity of T&E regimen later emerged when the TREND study reported noninferior results for Ranibizumab T&E versus monthly regimens (6.2 letters gain versus 8.1 letters) at 12 months5 and in TREX-AMD study comparing Ranibizumab T&E versus monthly regimens which reported statistically insignificant greater VA gain of +10.5 letters vs +8.7 letters respectively at 24-months follow-up.6
In the large audit based on real-world Electronic-Medical-Record data (EMR) published by UK Aflibercept-Users-group from 17 centers with patients receiving a similar Aflibercept-fixed-dosing regimen, they reported significantly less VA gain of +2.8 letters at 24 months (vs +10.2 letters gain in our study), also achieving a lower final mean VA of 59.1 letters (vs +65.1 letters in our study).15 In comparison to injection frequency, whilst both studies committed to applying the same number of fixed dosing of 7-injections in year-1, Aflibercept UK EMR group reported a lower mean 3.7 injections in year-2 compared to our 5 injections.15 This could be due to the difference in Year-2 injection protocol where we kept a T&E approach in year-2 for Aflibercept-Fixed group whilst their results were based on varied Year-2 protocols used by 17 UK-centers. In summary, the comparatively favorable results from Aflibercept-fixed-dosing group in our cohort could be attributed to our lower baseline VA (hence potential scope of higher VA gain), and demonstrated that “Treat & Extend” approach in Year-2 may be a more effective pathway in maintaining higher final visual outcome in this patient group.
In comparing efficacy of anti-VEGF agents, several real-world studies reported non-inferiority of Afilbercept compared to Ranibizumab.14–16 Our cohort however showed both Aflibercept-Fixed and Ranibizumab-T&E achieved the same VA outcomes, clearly more superior to Ranibizumab-PRN group, suggesting that the relevance and importance might lie in dosing regimen rather than anti-VEGF choice. In particular, our Aflibercept-Fixed protocol had also incorporated T&E approach in Year-2 as in the Ranibizumab-T&E protocol.
As our study is based on real-world data, it is in our interest to provide sub-analysis taking into account some “missing real-world data” to deliberate its effect on final results. Herein, we included separate LOCF analysis to compensate for missing data, thereby minimizing attrition bias. The impact of applying LOCF was also reported by Frennesson and Nilsson.9 In our cohort, the results’ difference was only evident in Ranibizumab-PRN group which had a significantly higher number of patients (40%) who ceased clinic attendance before 24 months. Sub-analysis on VA gain in Ranibizumab-PRN group showed a greater difference of +4.7 letters in patients who “completed-24-months” versus +1 letter gain when LOCF analysis was applied (Table 1B). This finding confirms a potential over-estimation of VA-gain without applying LOCF analysis, or indeed a potential under-estimation of VA gain when analysis included LOCF patients. However, we were glad to report no similar discrepancy in VA results in the Ranibizumab-T&E and the Aflibercept-Fixed dosing groups in sub-analyses, as the patients lost-to-follow-up (LOCF) in these groups were relatively few (Figure 3B). In our cohort analysis, we hence provided the benefits to readers in further understanding the impact of LOCF and missing data which bear closer relevance in reflecting real-world clinical results.
With such an array of potential treatment options, it can be difficult to counsel patients on choice of anti-VEGF agent and advise on which treatment regimen to follow. In a real-world clinical practice, the decision very often lies with patient’s preference and the capacity of the treatment center to deliver the chosen treatment regimen. For example, fixed-dosing and TE regimens have the advantage to patients with predictable hospital visits, whereas PRN approach limits to active-disease-injection only, hence avoiding the unnecessary risk of exposure to serious complications related to intravitreal injections. As the long-term benefits of intravitreal anti-VEGF treatment with T&E approach are increasingly recognized to achieve relatively superior functional outcomes, it could also serve as the likely choice to meet treatment demand and to improve service capacity compared to any other treatment regimen tested. Supported by our cohort data, it is not a surprise that T&E concept has recently been approved as a recognized license extension for Aflibercept protocol in the UK.17
Our study has some limitations. Our real-world results were based on retrospective data collection with no best-corrected visual acuity recording at each clinic visit, and inconsistency of VA being recorded with two different methods (Snellen and LogMAR) and later conversion to ETDRS letters for analysis. There would have been selection bias in choosing the treatment protocol as this was not randomized as in a clinical trial but merely based on patients’ and clinicians’ discretion and preference. Despite this, our cohort data were the first to present comparative results of three different treatment regimens based on one single center providing the advantage of consistent results from uniform treatment protocols, eliminating data variation and interpretation difficulty generated from multiple study centers of different time points using different treatment regimens.10–15
In conclusion, we compared three commonly adopted anti-VEGF treatment regimens for patients diagnosed with nAMD based on a single center clinical practice. Our results confirmed the better efficacy of Aflibercept-Fixed and Ranibizumab-T&E regimens and T&E was more efficient than PRN regimen when considering Ranibizumab therapy. Our cohort of long-term results in a real-world practice could further aid clinicians to consolidate decision-making in choosing the best-suited treatment regimen for individual patients during consultation.
Disclosure
BRM received educational travel sponsorship from Bayer and Novartis. BM is on the advisory board for Alimera, Novartis, Bayer and Alcon and reports grants and personal fees from Alimera, Novartis and Bayer outside the submitted work. RC received speaker fees and travel grants from Novartis, Bayer, and Allergan. The other authors declare no conflicts of interest.
References
1. Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA. 2004;291(15):1900–1901. doi:10.1001/jama.291.15.1900
2. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi:10.1056/NEJMoa054481
3. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi:10.1056/NEJMoa062655
4. Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year-2 of the PrONTO Study. Am J Ophthalmol. 2009;148(1):43–58. doi:10.1016/j.ajo.2009.01.024
5. Silva R, Berta A, Larsen M, et al. Treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: results with ranibizumab from the TREND study. Ophthalmology. 2018;125(1):57–65. doi:10.1016/j.ophtha.2017.07.014
6. Wykoff CC, Ou WC, Brown DM, et al. Randomized trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: 2-year results of the TREX-AMD study. Ophthalmol Retina. 2017;1(4):314–321. doi:10.1016/j.oret.2016.12.004
7. Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. doi:10.1016/j.ophtha.2012.09.006
8. Platania CBM, Di Paola L, Leggio GM, et al. Molecular features of interaction between VEGFA and anti-angiogenic drugs used in retinal diseases: a computational approach. Front Pharmacol. 2015;29(6):248.
9. Frennesson CI, Nilsson SEG. A three-year follow-up of ranibizumab treatment of exudative AMD: impact on the outcome of carrying forward the last acuity observation in drop-outs. Acta Ophthalmol. 2014;92(3):216–220. doi:10.1111/aos.12091
10. Rufai SR, Almuhtaseb H, Paul RM. A systematic review to assess the ‘treat-and extend’ dosing regimen for neovascular age related macular degeneration using ranibizumab. Eye. 2017;31:1337–1344. doi:10.1038/eye.2017.67
11. Aurell S, Sjovall K, Paul A, Moren A, Granstam E. Better visual outcome at 1 year with antivascular endothelial growth factor treatment according to treat-and-extend compared with pro re nata in eyes with neovascular age-related macular degeneration. Acta Ophthalmol. 2019;97:519–524. doi:10.1111/aos.13989
12. Augsburger M, Sarra GM, Imesch P. Treat and extend versus pro re nata regimens of ranibizumab and aflibercept in neovascular age-related macular degeneration: a comparative study. Graefes Arch Clin Exp Ophthalmol. 2019;257(9):1889–1895. doi:10.1007/s00417-019-04404-0
13. Hatz K, Prünte C. Treat and extend versus Pro Re Nata regimens of ranibizumab in neovascular age‐related macular degeneration: a comparative 12 month study. Acta Ophthalmol. 2017;95:e67–e72. doi:10.1111/aos.13031
14. Lotery A, Griner R, Ferreira A, Milnes F, Dugel P. Real-world visual acuity outcomes between ranibizumab and aflibercept in treatment of neovascular AMD in a large US data set. Eye. 2017;31(12):1697–1706. doi:10.1038/eye.2017.143
15. Lee AY, Lee CS, Egan CA, et al. UK AMD/DR EMR REPORT IX: comparative effectiveness of predominantly as needed (PRN) ranibizumab versus continuous aflibercept in UK clinical practice. Br J Ophthalmol. 2017;101(12):1683–1688. doi:10.1136/bjophthalmol-2016-309818
16. Almuhtaseb H, Johnston RL, Talks JS, Lotery AJ. Second-year visual acuity outcomes of nAMD patients treated with aflibercept: data analysis from the UK Aflibercept users group. Eye. 2017;31(11):1582–1588. doi:10.1038/eye.2017.108
17. Ross AH, Downey L, Devonport H, et al. Recommendations by a UK expert panel on an aflibercept treat-and-extend pathway for the treatment of neovascular age-related macular degeneration. Eye. 2020;34(10):1825–1834. doi:10.1038/s41433-019-0747-x
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.