Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Comparing Anatomical and Functional Outcomes of Two Neovaginoplasty Techniques for Mayer-Rokitansky-Küster-Hauser Syndrome: A Ten-Year Retrospective Study with Swine Small Intestinal Submucosa and Homologous Skin Grafts
Authors Xu H, Hou S, Ruan Z , Liu J
Received 4 April 2023
Accepted for publication 29 June 2023
Published 4 July 2023 Volume 2023:19 Pages 557—565
DOI https://doi.org/10.2147/TCRM.S415672
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Hui Xu, Shuhui Hou, Zhengyi Ruan, Jianhua Liu
Department of Obstetrics and Gynecology, Shanghai Ninth People’s Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, People’s Republic of China
Correspondence: Zhengyi Ruan; Jianhua Liu, Department of Obstetrics and Gynecology, Shanghai Ninth People’s Hospital, School of Medicine, Shanghai Jiaotong University, No. 639, Zhi Zaoju Road, Shanghai, 200011, People’s Republic of China, Email [email protected]; [email protected]
Objective: This study aimed to compare the anatomical and functional outcomes of the modified McIndoe vaginoplasty for Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome using swine small intestinal submucosa (SIS) graft or homologous skin grafts.
Methods: A total of 115 patients with MRKHs who underwent neovaginoplasty between January 2012 and December 2021 were included in the study. Among them, 84 patients received vaginal reconstruction with SIS graft, whereas 31 neovaginoplasty underwent a skin graft procedure. The length and width of the neovagina were measured, and sexual satisfaction was evaluated using the Female Sexual Function Index (FSFI). The operation details, cost, and complications were also assessed.
Results: The SIS graft group had a significantly shorter mean operation time (61.13± 7.17min) and less bleeding during the operation (38.57± 9.46mL) compared to the skin graft group (92.1± 9.47min and 55.81± 8.28mL, respectively). The mean length and width of the neovagina in the SIS group were comparable to the skin graft group at 6 months follow-up (7.73± 0.57 cm versus 7.6± 0.62cm, P=0.32). The SIS group had a higher total FSFI index than the skin graft group (27.44± 1.58 versus 25.33± 2.16, P=0.001).
Conclusion: The modified McIndoe neovaginoplasty using SIS graft is a safe and effective alternative to homologous skin grafts. It results in comparable anatomical outcomes and superior sexual and functional outcomes. Overall, these results suggest that the modified McIndoe neovaginoplasty using SIS graft is preferred for MRKH patients who require vaginal reconstruction.
Keywords: neovaginoplasty, Mayer-Rokitansky-Küster-Hauser syndrome, skin graft, swine small intestinal submucosa biological graft
Introduction
Mayer-Rokitansky-Küster-Hauser syndrome is a congenital malformation that affects approximately 1 in 4000–5000 females and results in the variable absence of the upper two-thirds of the vagina while retaining normal secondary sexual characteristics.1 As a result, affected women often experience primary amenorrhea and/or periodic pelvic pain and may seek neovaginoplasty to improve sexual function and psychological well-being. Several therapeutic methods are currently available, including the non-surgical Frank intermittent pressure method2 and surgical approaches using a variety of such as autologous, allogeneic, and artificial materials- skin graft,3 peritoneum,4 buccal mucosa,5 sigmoid,6 amnion,7 and regenerated cellulose biomaterials.8 Successful vagina reconstructive procedures should result in a functional vagina with sufficient length and width, minimal scarring, require no long-term dilatation, and provide acceptable sexual satisfaction. However, there is no consensus on the ideal material or technique.
The McIndoe vaginoplasty, first employed by McIndoe in England,9 is a commonly used surgical technique that involves the creation of a canal covered by skin grafts in the anterior portion of the pelvic region, between bladder/urethra and rectum.
Swine small intestinal submucosa (SIS) is collagen-based extracellular matrix (ECM) material that has been used in various medical applications, including body wall repair,10–12 vascular grafts,13,14 hernia repair15 and some other use.16,17 SIS also serves as the growing base of host tissue and fosters the differentiation of autologous cells, resulting in new growth tissue that closely resembles host tissue.
In this retrospective study, we summarized our experience of modified McIndoe vaginoplasty using SIS graft and compared this technique with traditional McIndoe vaginoplasty using skin graft in terms of operative time, bleeding amount, long-term anatomical effect, and sexual satisfaction. We reported satisfactory sexual and functional outcomes in 46 patients who underwent neovaginoplasty using either a skin graft or a scar-free method using an SIS graft.
Materials and Methods
Patients
Between January 2012 and December 2021, 115 patients with Mayer-Rokitansky-Küster-Hauser syndrome (MRKHs) were treated for vaginal agenesis in our Department of Gynecology. Of these, 84 patients underwent vaginal reconstruction using SIS grafts, while 31 underwent vaginoplasty using a skin graft. The age of patients ranged from 16 to 40 years. All patients had a 46XX karyotype and had normal external genitalia and secondary sexual characteristics despite various degrees of vaginal agenesis. Sonography and magnetic resonance imaging assessed the general condition of ovaries and primordial uteri with/without functional endometrium. Urinary abnormalities were assessed by intravenous pyelogram. The clinical characteristics of the patients were recorded in Table 1. Before the operation, the mean vaginal depth was 1.29±0.84cm in the SIS group and 1±0.82cm in the skin graft group (Table 2). Informed consent was obtained from all patients before surgical procedures, and the final decision regarding the choice of procedure was made by the patient after receiving detailed counseling on the benefits and risks of the alternative methods. This study was approved by the Institutional review board of the Ethics Committee of the Ninth People’s Hospital of Shanghai Jiao Tong University School of Medicine, consistent with the Declaration of Helsinki and all patients signed the consent form.
 |
Table 1 Clinical Characteristics of the MRKH Patients in Two Groups |
 |
Table 2 Perioperative Data Related to Vaginoplasty in Mayer-Rokitansky-Küster-Hauser Syndrome Patients Using SIS Graft or Skin Graft |
Surgery
The modified McIndoe neovaginoplasty using SIS grafts
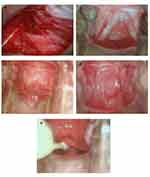
Patients were placed in a lithotomy position during the surgical procedure under general anesthesia. A small transverse incision, 2–3cm in size according to different patients, was made at the anatomical vestibule of the vagina. Subsequently, 200 mL of normal saline was injected between the urethra orifice and posterior perineum to enable further division. The neovaginal cavity, measuring 10cm in depth and 3 fingers in width along the anatomical vaginal route (Figure 1c–e), was created by blunt and sharp dissection with adequate hemostasis to prevent hematoma after the operation. To avoid accidental rectal injury during dissection, one finger of the operator was inserted into the patient’s anus.
COOK Biotech Incorporated’s small swine intestinal submucosa biological graft, also known as a biological tissue regeneration material, was used in the procedure. The selected size was 7*20cm. The graft was soaked in normal saline for 5–10 mins, then folded and sutured into a tube measuring 10cm long and 3.2cm in diameter with one end closed using 3/0 absorbable suture material (Ethicon, Somerville, NJ, USA) (Figure 1a and b). Next, the shaped SIS graft was wrapped around a vaginal mold was placed into the neovaginal cavity. Next, the closed end was fixed to the top of the neovagina using 3–0 absorbable Ethicon while the open end was sutured to the vestibular mucosa (Figure 1f). Finally, a rubber drainage strip was inserted between the graft and the vaginal cavity to drain the effusion from the wound.
A non-toxic polymer mold of various sizes was inserted into the vaginal cavity based on the depth and width of neovagina (Figure 1g). Finally, the mold was fixed with gauze and tapes remained in the vagina for seven days postoperatively.
The traditional McIndoe neovaginoplasty using skin grafts
Most steps for creating the neovaginal cavity and placing the vaginal mold were similar. However, a 12*10cm split-thickness skin graft harvested from the lateral thigh was used instead of using the SIS graft. The skin graft was sutured and similarly fixed to the neovaginal cavity (Figure 2a and b).
 |
Figure 2 The surgical procedure of vaginal reconstruction for patients with MRKHS using skin graft. (a) Skin graft harvest. (b) Shape the skin graft into a cylinder. |
Follow-Up
All the post-operative patients were monitored monthly for the first three months. During this time, the depth and width of the neovagina and the appearance of the vaginal mucosa were assessed through visual and speculum examination. Patients in the SIS group were requested to wear mold for 12 months, while patients in the skin graft group were asked to wear it for 3 months. For patients with a sex partner, their sexual function was assessed utilizing Female Sexual Function Index (FSFI), a self-reported questionnaire commonly used to evaluate female sexual health.
Statistical Analysis
Clinical data were analyzed using SPSS 22.0 software. The data were expressed as mean±standard deviation (SD). In addition, the Student’s t-test or nonparametric Mann–Whitney U-test was used to compare quantitative variables. A P-value less than 0.05 was considered statistically significant.
Results
Clinical Outcomes
All patients tolerated the surgical procedure well. A total of 84 patients underwent vaginal reconstruction using SIS, while 31 underwent neovaginoplasty using skin graft. There were no significant differences in the baseline data recorded in Table 1 between these two groups. However, the skin graft group had a longer operating time and more bleeding due to the harvesting of a split-thickness skin graft compared to the SIS group (92.1±9.47 min versus 61.13±7.17 min, P=0.001 and 55.81±8.28mL versus 38.57±9.46mL, respectively, P=0.001). Conversely, the total cost of hospitalization was much higher in the SIS group due to the use of SIS graft (43045.11±2359.98CNY versus 23500.94±2020.29CNY, respectively, P=0.001). In addition, both groups of patients with functional endometrium underwent laparoscopic hysterectomy before vaginoplasty. For detailed results, please refer to Table 1 and Table 2.
Anatomical Outcomes
All the patients completed follow-up and effect evaluation at four different time points within 12 months post-surgery, with the final follow-up occurring in the 12th month after the procedure. During the speculum examination, the depth of neovagina in the SIS group or skin graft group was measured at 7.73±0.57cm and 7.6±0.62cm, respectively P=0.317, and with a width of three fingers. The vaginal mucosa in the SIS graft patients appeared ruddy, smooth, soft, moist, and elastic. In the SIS group, we observed that it took four to six months for the neovaginal mucosa to regenerate in the SIS group (Figure 3a–e) fully. During this period, the mucosa gradually changed from bright red to ruddy, ultimately resembling normal vaginal mucosa, and the vault of neovagina was the last to regenerate. Six patients in the SIS group developed multiple vaginal polyps, 1–3 in number and 0.5–1cm in diameter, during mucosal regeneration and underwent polypectomy at an outpatient department under local anesthesia. We recommended patients in the SIS group wear a vaginal mold constantly for at least 6 months except for cleaning once daily. After six months, they only need to wear it while sleeping. If the patients had a sexual partner and engaged in regular sexual activity twice a week, they could stop wearing the mold after 12 months. In the split-thickness skin graft group, patients were instructed to wear the mold continuously for 3 months and only at night for the following 3 months.
Functional Outcomes
During follow-up, sexual activity was reported by 51 patients (60.7%) in the SIS group and by 21 patients (67.7%) in the skin graft group within 6 to 24 months and 3–24 months after surgery, respectively. The sexual function was evaluated using the FSFI questionnaire and the results are shown in Table 3. The total FSFI score in the SISI group was 27.44±1.58 (range 24.5 to 30.8), with three patients scoring over 30 and one reporting a low total FSFI score below 25. The total FSFI score in the skin graft group was 25.33±2.16 (range 20.3 to 28.2), with no patients scoring over 30 and three patients reporting low total FSFI scores below 25 (Table 3). According to the FSFI questionnaire, the patients in the SIS group scored significantly higher in lubrication, orgasm, and satisfaction scores compared to those in the skin graft group (5.28±0.25 versus 4.45±0.36, P=0.000, 4.52±0.55 versus 4.02±0.51, P=0.001, 4.85±0.61 versus 4.42±0.63, P=0.012, respectively).
 |
Table 3 Comparison of the Sexual Functional Outcomes in the Two Groups |
Complications
During surgery, only one patient in the SIS group suffered a rectal injury, which was immediately repaired without any fistula formation. No such case occurred in the skin graft group. In addition, no urethra or bladder injury was observed in either group. The major postoperative complications were vaginal shrinkage (n=1 in the SIS group) and granulation tissue formation (n=6 in the SIS group). The case of shrinkage was resolved using dilation, while outpatient procedures under local anesthesia were performed to excise granulation tissues. No shrinkage cases occurred in the skin graft group within 12 month follow-up period. The detailed results are shown in Table 4.
 |
Table 4 Comparison of the Anatomical Outcomes of the Two Groups After Surgery (6 Months After Surgery) |
Discussion
The SIS graft comprises type I collagen, type III collagen, type IV collagen, fibronectin, mucopolysaccharides, and leukopolysaccharides, each playing a unique role in tissue repair.18–20 SIS grafts possess several characteristics: (1) SIS graft has a porous structure that allows host cells to adhere and increase into the graft. As the SIS graft degrades, tissue collagen deposition and differentiation promote tissue regeneration and reconstruction in the defect area. (2) SIS grafts are cells and blood vessel-free, resulting in a transient immune response dominated by TH2 lymphocytes, leading to good histocompatibility without immune rejection.21 (3) The growth of host tissues and the degradation of the biological mesh occur simultaneously after implantation. The biological mesh is eventually entirely replaced by host tissue, thereby repairing the organ function without foreign matter remaining in the body.15 Therefore, we utilized SIS grafts in the modified McIndoe technique.
Numerous surgical or nonsurgical methods have been reported and evaluated in women with vagina agenesis caused by Mayer-Rokitansky-Küster-Hauser syndrome.3–5,7,8,22 However, an “ideal” method significantly superior to others and can be recommended universally as the “gold standard” has not yet been identified. Successful reconstruction is essential for a patient’s physical and mental well-being.23 The choice of method should consider multiple factors, such as patient preparedness, economic concerns, expectations, and the surgeon’s training. Patients should be thoroughly informed about potential complications, costs, and advantages/disadvantages of alternative methods. The ultimate goal of patients and physicians is to create a neovagina with sufficient length and width, normal anatomic axial direction, and adequate moisture for satisfactory sexual activities.
According to the American College of Obstetricians and Gynecologists (ACOG) Committee, Frank’s nonsurgical progressive dilation technique remains the first-line approach for MRKHS patients.24 However, the problems associated with the relatively long dilator use and the relatively low sexual functional outcomes cannot be ignored.25,26 In particular, the high risk of non-compliance due to time-consuming and tedious daily dilation may be the major issue of nonsurgical methods.27 Therefore, surgical techniques are a promising second-line choice for patients who have failed the nonsurgical method or cannot maintain compliance. Among various surgical methods of vaginoplasty, the McIndoe technique and Davydov’s operation are the most popular and widely adopted. Davydov’s method, which may perform through laparoscopic technique or laparotomy alternatively, can achieve adequate neovagina length and sexual function. However, it is relatively traumatic and requires more advanced surgical skills and abdominal surgery. In a study conducted by Dong et al between 2010 and 2013, 28 patients with MRKHS were compared using two methods of laparoscopic-assisted peritoneal vaginoplasty, including Davydov’s method.28 The results showed that the operation time was 73±11 minutes, with an intraoperative blood loss of 63±10 mL, and anal exsufflation time after surgery was 28±6 hours in the Davydov group. The length of neovagina was 9.6±0.5 cm; the Female Sexual Function Index scale score was 28.5±1.7. On the other hand, the classic McIndoe technique involves taking a skin graft from the patient’s thighs, one of the most frequently performed surgical procedures for MRKHs. While it is a reliable, safe, and effective treatment that provides satisfactory and functional vaginas in most patients, it leaves an obvious scar on the body surface and requires assistance from a plastic surgeon. Some less commonly used techniques include bowel vagina reconstruction using sigmoid or ileum segments, the gradual dilatation Vecchietti technique, and Sheares vaginoplasty using perineal skin flap. Although bowel vaginoplasty creates a self-lubricated vagina without needing prolonged dilation, this technique is significantly more complex and traumatic, with a higher risk of complications and recurrence.29 The Vecchietti technique requires special devices,30 and the Sheares vaginoplasty has been associated with cosmetic concerns about damage to the vulvar appearance.31 Therefore, a surgical approach that is less traumatic, less complex, more versatile, and associated with favorable outcomes would be the optimal choice. Our modified McIndoe technique satisfies these requirements and provides satisfactory and functional vaginas in most patients while minimizing complications and preserving the patient’s aesthetic appearance.
In this study, we aimed to improve the surgical procedure for neovaginoplasty by replacing the traditional skin graft with a biological graft to achieve satisfactory anatomical and functional results with minimal scars. The modified McIndoe using SIS graft was compared with the traditional skin graft method based on various parameters. Because the SIS graft should take a long time to complete the mucous membrane of the vagina, especially at the tip of the vagina, this process often takes 6 months or longer, and according to the SIS patch vaginoplasty performed before 2012, the increased mold placement time of the surgical SIS group could effectively prevent the premature removal of the vagina. So patients in the SIS group requested to wear the mold for 12 months, while patients in the skin graft group were asked to wear it for 3 months, this could not influence the results. The SIS graft group showed advantages over the skin graft group, with significantly less bleeding volume and operative time. No statistically significant differences were observed in postoperative morbidity. However, the major drawback of neovaginoplasty using SIS graft is the higher cost of the SIS graft. And we found that there were some polyps specifically that grew in the SIS graft, the main reason may be that Polyp is a vegetation produced by mucosal tissue, while SIS graft provides mucoalized vagina, the friction with the mold may cause repeated stimulation, and finally lead to small polyps on the top. Even, the SIS graft still showed a great advantage.
We also compared the vaginal depth and FSFI index during follow-up in the two groups. The mean depth and width of the artificial vagina in both groups did not show significant differences. Shrinkage was maintained low due to the correct mold placement under our guidance. Regarding functional outcomes, the modified McIndoe neovaginoplasty using SIS graft showed higher total FSFI scores and subseries in lubrication, orgasm, and satisfaction. The main reason may be biological SIS grafts provide a more lubricant and moister neovagina compared with the skin graft. The total FSFI scores in our study are comparable to most of the previous studies of vaginoplasty in China32 but lower than some reports aboard.3 The variation may be due to differences in race and culture.
The limitation of the present study is its non-randomized design. Further randomized studies are necessary to compare the two surgical methods. Additionally, future studies could explore further comparisons of other common procedures, such as the Davydov procedure and other methods. Psychological studies to evaluate postoperative mental health problems in patients are also necessary.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kolle A, Taran F-A, Rall K, et al. Neovagina creation methods and their potential impact on subsequent uterus transplantation: a review. BJOG. 2019;126(11):1328–1335. doi:10.1111/1471-0528.15888
2. Morcel K, Lavoué V, Jaffre F, et al. Sexual and functional results after creation of a neovagina in women with Mayer-Rokitansky-Kuster-Hauser syndrome: a comparison of nonsurgical and surgical procedures. Eur J Obstet Gynecol Reprod Biol. 2013;169(2):317–320. doi:10.1016/j.ejogrb.2013.03.005
3. Gari A. Mclndoe Neovagina in patients with mullerian agenesis: a single center experience. Pak J Med Sci. 2017;33(1):236–240. doi:10.12669/pjms.331.11867
4. Uncu G, Özerkan K, Ata B, et al. Anatomic and functional outcomes of paramesonephric remnant-supported laparoscopic double-layer peritoneal pull-down vaginoplasty technique in patients with Mayer-Rokitansky-Kuster-Hauser syndrome: uncu modification. J Minim Invasive Gynecol. 2018;25(3):498–506. doi:10.1016/j.jmig.2017.10.015
5. Teng Y, Zhu L, Chong Y, et al. The modified McIndoe technique: a scar-free surgical approach for vaginoplasty with an autologous micromucosa graft. Urology. 2019;131:240–244. doi:10.1016/j.urology.2019.05.020
6. Kisku S, Varghese L, Kekre A, et al. Bowel vaginoplasty in children and young women: an institutional experience with 55 patients. Int Urogynecol J. 2015;26(10):1441–1448. doi:10.1007/s00192-015-2728-3
7. Fotopoulou C, Sehouli J, Gehrmann N, et al. Functional and anatomic results of amnion vaginoplasty in young women with Mayer-Rokitansky-Kuster-Hauser syndrome. Fertil Steril. 2010;94(1):317–323. doi:10.1016/j.fertnstert.2009.01.154
8. Anagani M, Agrawal P, Meka K, et al. Novel minimally invasive technique of neovaginoplasty using an absorbable adhesion barrier. J Minim Invasive Gynecol. 2020;27(1):206–211. doi:10.1016/j.jmig.2019.02.025
9. Mc IA. The treatment of congenital absence and obliterative conditions of the vagina. Br J Plast Surg. 1950;2(4):254–267.
10. Badylak S, Kokini K, Tullius B, et al. Strength over time of a resorbable bioscaffold for body wall repair in a dog model. J Surg Res. 2001;99(2):282–287. doi:10.1006/jsre.2001.6176
11. Prevel CD, Eppley BL, Summerlin D-J, et al. Small intestinal submucosa: utilization for repair of rodent abdominal wall defects. Ann Plast Surg. 1995;35(4):374–380. doi:10.1097/00000637-199510000-00008
12. Badylak S, Kokini K, Tullius B, et al. Morphologic study of small intestinal submucosa as a body wall repair device. J Surg Res. 2002;103(2):190–202. doi:10.1006/jsre.2001.6349
13. Sanchez Puccini P, Briceno Triana JC. Visco-elasto-plastic modeling of small intestinal submucosa (SIS) for application as a vascular graft. J Mech Behav Biomed Mater. 2018;88:386–394. doi:10.1016/j.jmbbm.2018.08.044
14. Jaramillo J, Valencia-Rivero KT, Cedano-Serrano FJ, et al. Design and evaluation of a structural reinforced small intestinal submucosa vascular graft for hemodialysis access in a porcine model. Asaio j. 2018;64(2):270–277. doi:10.1097/MAT.0000000000000618
15. Stoll MR, Cook JL, Pope ER, et al. The use of porcine small intestinal submucosa as a biomaterial for perineal herniorrhaphy in the dog. Vet Surg. 2002;31(4):379–390. doi:10.1053/jvet.2002.33596
16. Lin HK, Godiwalla SY, Palmer B, et al. Understanding roles of porcine small intestinal submucosa in urinary bladder regeneration: identification of variable regenerative characteristics of small intestinal submucosa. Tissue Eng Part B Rev. 2014;20(1):73–83. doi:10.1089/ten.teb.2013.0126
17. Greca FH, Noronha L, Bendhack M, et al. Use of small intestine submucosa as ureteral allograft in pigs. Int Braz J Urol. 2004;30(4):327–34; discussion 335. doi:10.1590/S1677-55382004000400013
18. Badylak SF. The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol. 2002;13(5):377–383. doi:10.1016/S1084952102000940
19. Hodde J, Janis A, Ernst D, et al. Effects of sterilization on an extracellular matrix scaffold: part I. Composition and matrix architecture. J Mater Sci Mater Med. 2007;18(4):537–543. doi:10.1007/s10856-007-2300-x
20. Hodde J, Janis A, Hiles M. Effects of sterilization on an extracellular matrix scaffold: part II. Bioactivity and matrix interaction. J Mater Sci Mater Med. 2007;18(4):545–550. doi:10.1007/s10856-007-2301-9
21. Kim MS, Ahn HH, Shin YN, et al. An in vivo study of the host tissue response to subcutaneous implantation of PLGA- and/or porcine small intestinal submucosa-based scaffolds. Biomaterials. 2007;28(34):5137–5143. doi:10.1016/j.biomaterials.2007.08.014
22. Fedele L, Frontino G, Restelli E, et al. Creation of a neovagina by Davydov’s laparoscopic modified technique in patients with Rokitansky syndrome. Am J Obstet Gynecol. 2010;202(1):33.e1–6. doi:10.1016/j.ajog.2009.08.035
23. Dabaghi S, Zandi M, Ilkhani M. Sexual satisfaction in patients with Mayer-Rokitansky-Küster-Hauser syndrome after surgical and non-surgical techniques: a systematic review. Int Urogynecol J. 2019;30(3):353–362. doi:10.1007/s00192-018-3854-5
24. Committee on Adolescent Health Care. ACOG Committee Opinion No. 728: Müllerian agenesis: diagnosis, management, and treatment. Obstet Gynecol. 2018;131(1):e35–e42. doi:10.1097/AOG.0000000000002458
25. Callens N, Weyers S, Monstrey S, et al. Vaginal dilation treatment in women with vaginal hypoplasia: a prospective one-year follow-up study. Am J Obstet Gynecol. 2014;211(3):228.e1–228.e12. doi:10.1016/j.ajog.2014.03.051
26. Holt R, Slade P. Living with an incomplete vagina and womb: an interpretative phenomenological analysis of the experience of vaginal agenesis. Psychol Health Med. 2003;8(1):19–33. doi:10.1080/1354850021000059232
27. Herlin M, Bay Bjørn A-M, Jørgensen LK, et al. Treatment of vaginal agenesis in Mayer-Rokitansky-Küster-Hauser syndrome in Denmark: a nationwide comparative study of anatomical outcome and complications. Fertil Steril. 2018;110(4):746–753. doi:10.1016/j.fertnstert.2018.05.015
28. Dong X, Xie Z, Jin H. 腹腔镜 Vecchietti 与 Davydov 阴道成形术治疗 MRKH 综合征的对比研究 [Comparison study between Vecchietti’s and Davydov’s laparoscopic vaginoplasty in Mayer-Rokitansky-Küster-Hauser syndrome]. Zhonghua Fu Chan Ke Za Zhi. 2015;50(4):278–282. Chinese.
29. Georgas K, Belgrano V, Andreasson M, et al. Bowel vaginoplasty: a systematic review. J Plast Surg Hand Surg. 2018;52(5):265–273. doi:10.1080/2000656X.2018.1482220
30. Adamiak-Godlewska A, Skorupska K, Rechberger T, et al. Urogynecological and sexual functions after vecchietti reconstructive surgery. Biomed Res Int. 2019;2019:2360185. doi:10.1155/2019/2360185
31. Fliegner JR. Long-term satisfaction with Sheares vaginoplasty for congenital absence of the vagina. Aust N Z J Obstet Gynaecol. 1996;36(2):202–204. doi:10.1111/j.1479-828X.1996.tb03286.x
32. Liu X, Liu M, Hua K, et al. Sexuality after laparoscopic peritoneal vaginoplasty in women with Mayer-Rokitansky-Kuster-Hauser syndrome. J Minim Invasive Gynecol. 2009;16(6):720–729. doi:10.1016/j.jmig.2009.07.018
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.