Back to Journals » Therapeutics and Clinical Risk Management » Volume 16
Comparative Treatment Patterns and Outcomes of Fulvestrant versus Everolimus Plus Exemestane for Postmenopausal Metastatic Breast Cancer Resistant to Aromatase Inhibitors in Real-World Experience
Authors Li Y, Xie Y, Gong C, Zhao Y, Zhang J, Zhang S, Wang L , Chen S, Hu X , Wang B
Received 31 March 2020
Accepted for publication 12 June 2020
Published 30 June 2020 Volume 2020:16 Pages 607—615
DOI https://doi.org/10.2147/TCRM.S255365
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Yi Li,1,* Yizhao Xie,1,* Chengcheng Gong,1 Yannan Zhao,1 Jian Zhang,1 Sheng Zhang,1 Leiping Wang,1 She Chen,2 Xichun Hu,1 Biyun Wang1
1Department of Medical Oncology, Fudan University Shanghai Cancer Center, Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, People’s Republic of China; 2Key Laboratory of Glycoconjugate Research Ministry of Public Health, Department of Biochemistry and Molecular Biology, School of Basic Medical Sciences, Fudan University, Shanghai 200032, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Biyun Wang; Xichun Hu
Department of Medical Oncology, Fudan University Shanghai Cancer Center, #270 Dongan Road, Xuhui District, Shanghai 200032, People’s Republic of China
Email [email protected]; [email protected]
Background: Fulvestrant (FUL) and the combination of everolimus and exemestane (EVE-EXE) were the options to treat hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) metastatic breast cancer (MBC) patients who were refractory to aromatase inhibitors (AIs). The practical knowledge of treatment patterns and outcomes between the two regimens is essential for improving treatment decisions.
Methods: HR+/HER2− MBC patients, who were refractory to AI, were treated with FUL or EVE-EXE from June 2013 to June 2016 were included. Treatment patterns, progression-free survival (PFS), overall survival (OS), and toxicity were reported. Propensity score matching (PSM) was used to minimize potential confounders.
Results: A total of 168 patients were enrolled. Of 168 patients, 124 patients were treated with FUL and 44 patients received EVE-EXE. Patients who were treated with EVE-EXE were younger, more likely to have visceral, liver, multiple sites of metastases, and had received more prior chemotherapy. After adjusting for propensity score matching (PSM), no significant difference in PFS was found between two groups (P=0.419). However, in the subgroup of multiple metastatic sites, the median PFS was significantly improved in the EVE-EXE arm compared with FUL arm (6.1 vs 3.2 months, respectively, P=0.012). More patients in EVE-EXE arm discontinued treatment due to adverse events than in the FUL arm.
Conclusion: A substantial difference in treatment patterns was observed between the two arms. Clinical outcomes were comparable after PSM.
Clinicaltrials.gov Identifier: NCT03695341 (May 14, 2018).
Keywords: fulvestrant, everolimus, exemestane, metastatic breast cancer, endocrine therapy
Introduction
Breast cancer (BC) is the most common women malignancy worldwide.1 According to the China cancer statistics, the incidence and mortality of BC have shown an increasing trend.2 Considering that about 70% of the BC are hormone receptor-positive (HR+)/human epidermal growth factor 2 negative (HER2-), endocrine therapy (ET) has become the cornerstone in the treatment of this BC subtype.3 Third-Generation Aromatase inhibitors (AIs) have become the first choice for postmenopausal and high-risk premenopausal patients in their adjuvant treatment.4 Besides, AIs are considered the first treatment of choice for patients who develop metastasis.5 However, not all patients respond well to ET; and even those who do will inevitably relapse in the future. Fulvestrant (FUL) and the combination of everolimus (EVE) and exemestane (EXE) have been considered secondary treatment options.6,7 Moreover, CDK4/6 inhibitors combined with ET have shown efficacy in metastatic breast cancer (MBC) who were refractory to AIs.8 However, the inhibitors of CDK4/6 have just entered the Chinese market, which limits the clinical choices.
Fulvestrant, a pure antiestrogen, binds to the estrogen receptor (ER) and accelerates its degradation. It also inhibits receptor dimerization, which may also decrease estrogen-independent signaling.9 Previous clinical trials indicated that FUL (250 mg) and EXE are equally active and well-tolerated in MBC who experienced progression or relapse during treatment with a nonsteroidal aromatase inhibitor (NSAI).6 Moreover, CONFIRM study10 compared the FUL 250 mg with FUL 500 mg in ER+ MBC, finding a significant prolongation in median progression-free survival (PFS) when using a higher dosage (HR=0.80; P=0.006); and the dosage was not related to increased toxicity.
Everolimus, rapamycin derivative that inhibits mTOR through allosteric binding to mTORC1 thus preventing the activation of the PI3K-AKT-mTOR pathway, has been demonstrated to improve the antitumor effect of ET in BC.11,12 BOLERO-2, a Phase III study, found significantly higher PFS interval in HR+/HER2- MBC refractory to NSAI who treated with EVE plus EXE compared to those receiving EXE monotherapy.7 The combination of EVE-EXE was approved for the treatment of postmenopausal women with HR+/HER2- MBC who did not respond to NSAI due to the significant improvement on PFS.
The currently available clinical evidence supports using either FUL or EVE+EXE to treat HR+/HER2- MBC who experienced progression or recurrence during AI treatment. However, due to lacking direct comparisons of the two regimens, optimizing the disease management in patient’s refractory on AIs is quite challenging. The practical knowledge of treatment patterns and outcomes of the two options is critical for improving everyday treatment decisions. The aim of this study was to access the treatment choices in the real-world and investigate the treatment outcome of FUL and EVE-EXE for HR+/HER2- MBC patients’ progression on AI.
Patients and Methods
Patients
One hundred sixty-eight HR+/HER2- MBC patients who experienced progression or recurrence during treatment with AI and were consequently treated with FUL or EVE-EXE in metastatic setting from June 2013 to June 2016 in Fudan University Shanghai Cancer Center (FUSCC) were enrolled. "The disease was defined refractory if the recurrence occurred during or within 12 months after the end of adjuvant treatment; the disease progression was defined if progression was observed during or within 1 month after the end of treatment for metastatic disease".7 AI did not have to be the most recent treatment before FUL or EVE-EXE. HR was considered “positive” when the immunohistochemical (IHC) analysis confirmed that at least 10% of nuclei were stained. HER2-negative was scored as 0 or +1 by IHC analysis or +2 but the negative result of fluorescence in situ hybridization. Metastatic diseases were confirmed by biopsy or imaging.
Patients who were receiving FUL or EVE-EXE combined with chemotherapy or targeted treatment were excluded. Medical records were used to collect data about the characteristics of patients and their treatment outcomes.
The Ethics Committee and Institutional Review Board of Fudan University Shanghai Cancer Center approved this study. This study is retrospectively registered with ClinicalTrials.gov (NCT03695341)
Treatment
Patients received FUL 500 mg intramuscularly on d1, 15, 29, and every 28 days thereafter. For patients receiving the combination of EVE and EXE, 25mg EXE was given orally once a day. EVE was usually started at 10 mg or 5 mg daily, according to physicians’ choices. All the premenopausal patients received concurrent luteinising hormone-releasing hormone analogues (LHRHa). Treatment could be interrupted, reduced, or permanently discontinued for the physicians’ decisions in the clinical practice.
Outcome Measurements
The primary outcome measurement was PFS, which was defined as the date from treatment initiation to disease progression recorded for the first time, death or final follow-up. Overall survival (OS) and safety were the second outcome measurements. OS was defined as the time interval between treatment initiation and death or final follow-up. Efficacy was evaluated every 2–3 months until disease progression. Disease progression was obtained based on version 1.1 of Response Evaluation Criteria in Solid Tumors (RECIST).
Adverse events (AEs) were retrospectively collected from medical records and laboratory test results. All AEs were graded by using the National Cancer Institute Common Terminology Criteria for adverse events (NCI-CTCAE) version 4.0.
Statistical Analysis
We analyzed treatment choices for the total cohort. Chi-square tests were used to compare the baseline characteristics of the two arms except for age. Student’s t-test was used to compare ages in the two arms. The Kaplan-Meier method was used to estimate PFS and OS and compared using a Log rank test. By adjusting the Cox proportional hazards model of the two arms, the hazard ratios (HRs) with two-sided 95% confidence interval (CIs) were calculated. Exploratory analyses were performed with the Log rank test using the following variables: age, menopausal status, number of metastatic sites (1–2 vs ≥3), disease-free interval (DFI), visceral metastases, lines of ET, endocrine sensitivity (primary vs secondary), and lines of chemotherapy. Multiple metastatic sites were diagnosed if more than three metastatic sites were observed. Primary endocrine resistance was defined if a relapse occurred during the first 2 years of adjuvant ET, or a patient experienced disease progression within the first six months of first-line ET. Secondary endocrine resistance was defined as follows: a relapse during adjuvant ET after the first 2 years, or relapse within 12 months of completing adjuvant ET, or progress more than 6 months after initiating ET for MBC.13
To balance confounding factors, propensity score matching (PSM) was used for one-to-one nearest neighbor matching without replacement to match the cohort representing patients receiving either regimen. Variables shown as independent predictors of treatment outcome or were significantly different between the two groups were used for matching. Using a non-replaceable 1: 2 matching protocol (greedy matching algorithm), with a caliper width equal to 0.2 of the standard deviation of the logarithm of the propensity score.14
The Chi-square test was used to compare AEs in the two arms. The software SPSS 22.0 (IBM Corporation, Armonk, New York, the US) was used for data analysis. P values less than 0.05 were considered significant.
Results
Baseline Characteristics
Between June 2013 and June 2016, 168 eligible patients were included in this analysis. Among the 168 patients, 124 (73.8%) were treated with FUL, while 44 (26.2%) patients received EVE-EXE. Baseline characteristics were shown in Table 1. Patients who received EVE-EXE were younger than those treated with FUL (respectively, median 53.4 vs 58.1 years, P=0.013). Besides, more multiple metastatic sites were observed in the EVE-EXE group compared to the FUL group (61.4% vs 32.3%, P=0.002). Also, the percentages of patients who had visceral or liver metastases and experienced more later-lines of chemotherapy were higher in the EVE-EXE group than the FUL group.
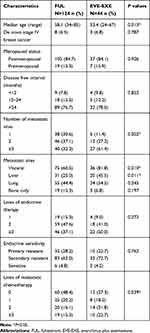 |
Table 1 Baseline Characteristics of Patients, According to Treatment Choices |
Considering the differences in patients’ baseline characteristics between the two groups, PSM was performed to balance baseline characteristics. Five variables were selected for PSM by the aforementioned criteria; the variables included age, lines of metastatic chemotherapy, number of metastatic sites, visceral metastasis, and liver metastasis. With the use of PSM, 88 patients treated with FUL were matched with 44 patients who received EVE-EXE. After matching, the characteristics were well balanced between the two groups. Baseline characteristics after matching were listed in Table 2.
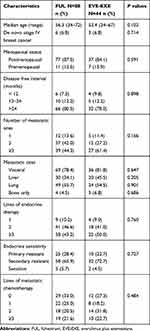 |
Table 2 Baseline Characteristics After Propensity-Score Matching |
Treatment Efficacy
After a median follow-up time of 21.1 months in the FUL group and 22.6 months in the EVE-EXE group. In the FUL group, 101 out of 124 patients experienced disease progression, and 42 passed away, while in the EVE-EXE group, 41 patients experienced progression, and 17 passed away, resulting in a median PFS of 4.7 months (95% CI 3.2–6.2) in the FUL group and a median PFS of 6.0 months (95% CI 3.8–8.4) in the EVE-EXE group. OS data for both groups were immature at the time of analysis. A trend of prolongation of PFS was observed in EVE-EXE group, however, it was not statistically significant (HR, 1.044; 95% CI, 0.723–1.507; P=0.818) (Figure 1A). Considering that unbalanced covariates may be potential sources of confounding when comparing the efficacy, we conducted PSM analysis to balance the covariates. However, we did not find a significant difference for PFS although patients receiving EVE-EXE had a numerical increase (4.0 vs 6.1months, respectively; HR, 1.173; 95% CI, 0.797–1.727; P=0.419) after conducting PSM (Figure 1B). An exploratory analysis confirmed the consistency of the results across all subgroups except for the subgroup of 3 or more metastatic sites (Figure 2). In this subgroup, 39 patients received FUL treatment, and 27 patients received EVE-EXE treatment. The median PFS in EVE-EXE group was significantly better than in FUL group (6.1 vs 3.2 months, respectively; HR=0.508; 95% CI, 0.299–0.862; P=0.012) (Figure 3)
Safety
The safety results of the two groups were shown in Table 3. FUL was better tolerated compared to EVE-EXE. Most frequent all-grade adverse events included joint disorders (5%) and injection site reactions (4%). No grade 3 or more AEs were observed; however, AEs in patients who received EVE-EXE were more common and severe. All all-grade non-hematological AEs with an incidence of more than 10% as follows: stomatitis (56.8%), fatigue (25%), infection (25%), rash (18.2%), edema (13.6%), cough (13.6%), diarrhea (11.4%) and noninfectious pneumonitis (11.4%). Hyperglycemia (22.7%), elevated alanine aminotransferase (15.9%), elevated aspartate aminotransferase (15.9%), anemia (13.6%) and thrombocytopenia (11.4%) were common laboratory abnormalities with the incidence of greater than 10%. Stomatitis was the most common grade 3 AEs. No grade 4 AEs were observed.
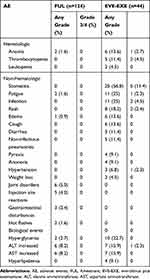 |
Table 3 Drug-Related AEs |
In the FUL group, four patients (3.2%) experienced discontinuations due to AEs. However, patients who received EVE-EXE had a relatively high rate of dose adjustments due to AEs. Stomatitis was the most common AE that causes discontinuation (N=5). 18.2% of patients in the EVE-EXE group discontinued treatment for AEs. 33 patients (75%) started a standard dose of 10 mg EVE per day, and 11 patients (25%) started at 5 mg per day. In patients who initiated 10mg EVE, five patients stopped treatment for intolerable toxicity. Six patients reduced to 5mg and in 1 patient the discontinuation occurred because of AEs. Among patients who were started with 5 mg daily, ten patients remained at 5 mg, while only one patient increased to 10mg after a few weeks of treatment and 2 patients stopped treatment because of AEs.
Discussion
For HR+/HER2- MBC, which is the most prevalent subtype in advanced-stage disease, ET is the recommended initial treatment. AIs are an important treatment option for the adjuvant or advanced setting. Nevertheless, treatment options for patients who are refractory to AIs are limited. EVE-EXE and FUL have been confirmed effective in MBC patients pretreated with AIs; yet, the comparative clinical evidence of those treatments in patients who experienced progression or recurrence after treatment with AI, are still very limited. Besides, the treatment choices made in a real-world setting, and the outcomes have not yet been evaluated.
We presented data on patient characteristics and outcomes in daily practice for HR+/HER2- MBC pretreated with AI. As far as we know, this is the first study focusing on treatment choice, EVE-EXE or FUL for HR+/HER2-MBC pretreated with AI. Our study demonstrated that patients with visceral, liver, multiple sites of metastases, or later-lines of chemotherapy were more likely to receive EVE-EXE than FUL in real life. It seems that physicians prefer to give EVE-EXE to patients with severe metastasis and large tumor burden compared with FUL. Besides, FUL was more commonly used in clinical practice during 2013–2016 than EVE-EXE as patients enrolled in the FUL group were nearly three times than EVE-EXE.
Our findings suggested no difference in PFS between the two endocrine regimens. To examine whether the unbalanced characteristics might affect the results, we used the PSM to balance these covariates. However, after matching, there was still no difference, except for subgroup of 3 or more metastatic sites. In this subgroup, EVE-EXE was superior to FUL in terms of PFS. Patients with multiple metastatic sites may express greater tumor heterogeneity. Only decreased estrogen-independent signaling may not lead to better therapeutic results. Previous studies have demonstrated that patients’ receiving ET had higher p-mTOR expression in the metastatic tumor lesions than the primary lesion.15 Thus, the mTOR pathway could be further activated in patients who had multiple metastases. Therefore, we believe that EVE-EXE may be more effective in patients with a large tumor burden.
In the EFECT study, FUL250mg showed similar efficacy with EXE (HR=0.963, P=0.653).6 CONFIRM study further demonstrated the superior efficacy of FUL 500mg than FUL 250mg in ER+ MBC patients pretreated with AI (HR=0.80, P=0.006).10 Moreover, the BOLERO-2 trial indicated that EVE-EXE could significantly increase the median PFS than EXE (HR,0.45; P=0.0001).7 Despite the limited validity of empirical cross trials comparisons, the apparent magnitude of PFS benefits EVE-EXE exceeds FUL 500 mg.
In our study, EVE-EXE did not exert its full activity because of the relatively high occurrence of dose interruptions or reductions due to AEs. Treatment discontinuations due to AEs were observed in 18.2% of patients who received EVE-EXE, while only 3.2% of patients discontinued treatment in the FUL group. Besides, in the EVE-EXE group, EVE was initially started at 5 mg per day in 11 patients (25%). In patients who initiated the treatment at 10mg, 6 patients had a dose reduction to 5 mg. Compared with a standard dose of 10mg daily, dose interruption affected the result of the treatment. The results of EVA study suggested that the reduction of the EVE dose below a dose-intensity of 5mg daily was not recommendable, with the lowest ORRs observed in the group of patients (18.2%) while the ORR was very similar in patients who received the highest (>7.5mg daily) and the intermediate (>5, ≤7.5 mg daily) DI (33.5% and 34.7%, respectively).16 In our study, the combination regimens EVE-EXE were less likely to be used in the routine practice because of relatively severe toxicity. Thus, careful monitoring of patients and increased physician awareness of the safety profile of EVE are warranted. Recently, a Phase II trial SWISH17 investigated dexamethasone-based mouthwash in the prevention of EVE-related stomatitis in HR+/HER2- MBC. At the beginning of day 1 cycle 1, patients were treated with EVE 10 mg plus EXE 25 mg per day, with 10 mL of alcohol-free dexamethasone 0.5 mg/5 mL oral solution. The results demonstrated that by the eighth week, the incidence of grade 2 or worse stomatitis was 2% versus 33% reported in BOLERO-2. With the early intervention and reasonable control of the AEs, the drug may have a better effect.
A previous network meta-analysis,18 which compared the efficacy of EVE-EXE with that of FUL 250 and 500 mg in MBC indicated that patients received EVE-EXE had higher PFS than FUL alone (HR: 0.47; 95% credible interval (CrI): 0.38–0.58 compared with FUL 250 mg; HR: 0.59; 95% CrI: 0.45–0.77 compared with FUL 500 mg). Besides, the study showed that EVE-EXE had a greater clinical benefit than FUL 250 and 500 mg in a subgroup of patients previously treated with AI.
Similar results were obtained in our study. Besides, nearly half of the patients enrolled in our study received more than three lines of endocrine therapy, while patients selected in the meta-analysis18 received front lines of ET. In the real-world study, the compliance of patients was lower than in clinical trials. Finally, the occurrences of dose interruptions or reduction were higher in the EVE-EXE group than the FUL group in the present study.
A large number of studies have shown that endocrine monotherapy has limited efficacy in patients with disease progression or relapse after treatment with ET, thus suggesting more effective combined treatment regimens in routinely used for such patients. In the PALOMA 3 study,8 palbociclib combined with FUL in HR+/HER2- MBC who experience progression after ET resulted in more prolonged PFS than FUL alone (HR=0.42, P<0.001). However, the newly published OS data indicated that the combination of palbociclib and FUL did not meet the prespecified threshold. In subgroup analysis, among patients who had previous ET sensitivity, treatment with palbociclib and FUL resulted in more prolonged OS than FUL alone (HR, 0.72; 95% CI, 0.55–0.94).19 The final data support the use of palbociclib and FUL in patients with disease recurrence during ET after at least 2 years of adjuvant treatment or patients who received ET alone for metastatic disease with clinical benefit. Nevertheless, among patients without sensitivity to previous ET, combination therapy of palbociclib and FUL did not significantly prolong the OS (HR, 1.14; 95% CI, 0.71–1.84).19 Therefore, for other patients (those who develop relapse during or within 12 months after the end of adjuvant therapy or in patients who did not benefit from first-line ET), the palbociclib combination with ET, FUL or EVE-EXE were options for treatment.
In China, targeting drugs such as inhibitors of CDK4/6 were not available on the market before the last patient enrolled in our study, which limits our clinical choices. Promisingly, palbociclib has been recently listed in China giving patients more choice to achieve better efficacy.
This study has several limitations, including potentially missing data, and a small sample size. Besides, all patients were selected from FUSCC, which may lead to a selection bias. Even though PSM simulates randomization, it can correct only known confounders and is no substitute for a randomized clinical trial.
Conclusions
Our study provides first-hand real-world data of treatment pattern and outcome in treatment choice between FUL and EVE-EXE for HR+/HER2- MBC pretreated with AI, which may provide evidence for the clinical oncologist to the clinical decision-making. After adjusting for confounding factors, the efficacy was comparable between FUL and EVE-EXE. Patients with multiple metastatic sites may benefit from EVE-EXE. In terms of safety, FUL seemed to be better-tolerated. Our results needed to be confirmed by further randomized controlled clinical trials.
Data Sharing Statement
All datasets analyzed for this study are included in the article. The figures and tables will be available by contacting the corresponding author. The data can be used permanently after the article is published.
Ethics Approval and Consent to Participate
All data were anonymized to comply with the provisions of personal data protection legislation. Due to the retrospective nature of this study and due to the fact that only historical medical data were collected, written informed consent was not required. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
The authors would like to thank doctors, nurses, patients, and their family members for supporting our study.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
3. Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5). doi:10.1093/jnci/dju055
4. Gradishar WJ, Anderson BO, Balassanian R, et al. Breast cancer, version 4.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(3):310–320. doi:10.6004/jnccn.2018.0012
5. Rugo HS, Rumble RB, Macrae E, et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American society of clinical oncology guideline. J Clin Oncol. 2016;34(25):3069–3103. doi:10.1200/JCO.2016.67.1487
6. Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26(10):1664–1670. doi:10.1200/JCO.2007.13.5822
7. Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–529. doi:10.1056/NEJMoa1109653
8. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, Phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. doi:10.1016/S1470-2045(15)00613-0
9. Osborne CK, Wakeling A, Nicholson RI. Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action. Br J Cancer. 2004;90(Suppl 1):S2–S6. doi:10.1038/sj.bjc.6601629
10. Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28(30):4594–4600. doi:10.1200/JCO.2010.28.8415
11. Efeyan A, Sabatini DM. mTOR and cancer: many loops in one pathway. Curr Opin Cell Biol. 2010;22(2):169–176. doi:10.1016/j.ceb.2009.10.007
12. Boulay A, Rudloff J, Ye J, et al. Dual inhibition of mTOR and estrogen receptor signaling in vitro induces cell death in models of breast cancer. Clin Cancer Res. 2005;11(14):5319–5328. doi:10.1158/1078-0432.CCR-04-2402
13. Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2)dagger. Ann Oncol. 2014;25(10):1871–1888. doi:10.1093/annonc/mdu385
14. Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54(4):387–398. doi:10.1016/S0895-4356(00)00321-8
15. Beelen K, Hoefnagel LD, Opdam M, et al. PI3K/AKT/mTOR pathway activation in primary and corresponding metastatic breast tumors after adjuvant endocrine therapy. Int J Cancer. 2014;135(5):1257–1263. doi:10.1002/ijc.28769
16. Cazzaniga ME, Airoldi M, Arcangeli V, et al. Efficacy and safety of everolimus and exemestane in hormone-receptor positive (HR+) human-epidermal-growth-factor negative (HER2-) advanced breast cancer patients: new insights beyond clinical trials. The EVA study. BREAST. 2017;35:115–121. doi:10.1016/j.breast.2017.06.043
17. Rugo HS, Seneviratne L, Beck JT, et al. Prevention of everolimus-related stomatitis in women with hormone receptor-positive, HER2-negative metastatic breast cancer using dexamethasone mouthwash (SWISH): a single-arm, Phase 2 trial. Lancet Oncol. 2017;18(5):654–662. doi:10.1016/S1470-2045(17)30109-2
18. Bachelot T, McCool R, Duffy S, et al. Comparative efficacy of everolimus plus exemestane versus fulvestrant for hormone-receptor-positive advanced breast cancer following progression/recurrence after endocrine therapy: a network meta-analysis. Breast Cancer Res Treat. 2014;143(1):125–133. doi:10.1007/s10549-013-2778-5
19. Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379(20):1926–1936. doi:10.1056/NEJMoa1810527
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



