Back to Journals » Therapeutics and Clinical Risk Management » Volume 15
Comparative study of the PD-L1 expression and CD8+ tumor-infiltrating lymphocyte between surgically resected and matched re-biopsy specimens in recurrent non-small cell lung cancer
Authors Shimizu K, Okita R , Saisho S, Maeda A, Nojima Y, Nakata M
Received 1 October 2018
Accepted for publication 19 March 2019
Published 1 May 2019 Volume 2019:15 Pages 605—612
DOI https://doi.org/10.2147/TCRM.S189320
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Katsuhiko Shimizu, Riki Okita, Shinsuke Saisho, Ai Maeda, Yuji Nojima, Masao Nakata
Department of General Thoracic Surgery, Kawasaki Medical School, Kurashiki, Okayama, Japan
Introduction: Numerous studies conducted until date have reported that the chemotherapy regimen could affect the programmed cell death ligand 1 (PD-L1) expression status in patients with non-small cell lung cancer (NSCLC).
Materials and methods: A total of 36 NSCLC patients for whom both the surgically resected specimens of the primary tumors and re-biopsy specimens of the recurrent tumors were available were enrolled in this study. The PD-L1 expression status and tumor-infiltrating CD8-positive T lymphocytes (CD8+TILs) count were measured in paired samples by immunohistochemistry. The concordance rate in the tumor immune microenvironment (TIME) classification based on the PD-L1 expression status and CD8+TILs count was analyzed.
Results: While the PD-L1 expression levels were similar between the surgical and re-biopsy specimens in 77.8% of cases, in 16.7% of cases, the expression levels were higher in the re-biopsy specimens. When the analysis was confined to patients who had received platinum-based chemotherapy, the percentage increased to 42.9%. The TIME classification changed in the re-biopsy specimens as compared to the surgical specimens in one-third of the patients, especially in those who had received chemotherapy previously. The TIME classification in the re-biopsy specimens more closely resembled that in the metastatic lymph nodes as compared to that in the primary tumor.
Conclusion: In patients with recurrent NSCLC, especially those who have received chemotherapy previously, a recent re-biopsy sample is required to determine whether PD-1/PD-L1 inhibitors should be used for treatment or not.
Keywords: non-small cell lung cancer, NSCLC, programmed cell death ligand 1 (PD-L1), tumor-infiltrating T lymphocytes, tumor immunemicroenvironment (TIME), re-biopsy
Introduction
Lung cancer is a major cause of death in many developed countries. Although surgery is a major component of treatment for localized and resectable lung cancer, patients often develop local and/or distant recurrence after curative operation.1,2 Several biomarkers have now been reported as predictors of survival and recurrence in patients with non-small cell lung cancer (NSCLC). Especially, immunological biomarkers in the tumor microenvironment are useful prognostic predictors, in addition to being promising targets for novel therapeutic approaches.3
The interaction between programmed cell death-1 (PD-1) and PD-1 ligand 1 (PD-L1) inhibits T-cell activation and proliferation.4,5 Several previous studies have reported that PD-L1 overexpression is a predictor of a poor clinical outcome in patients with NSCLC.6–8 On the other hand, PD-L1 overexpression has been shown to be correlated with an improved response to treatment with PD-1/PD-L1 inhibitors.9–12 Meanwhile, several previous studies have indicated that the presence of tumor-infiltrating CD8-positive T lymphocytes (CD8+TILs) is associated with a longer survival period in patients with colorectal, ovarian, and breast cancer.13–15 CD8+TIL count is a candidate biomarker for tumor-associated immune response, and most previous studies of CD8+TILs in NSCLC have reported an association of a high count with a favorable outcome.16–18
Recently, we studied the PD-L1 expression status and CD8+TIL count in the tumors of 170 lung adenocarcinoma patients who had undergone pulmonary resection as the initial treatment.19 We divided them into four subgroups according to the PD-L1 expression status and CD8+TIL count in the tumors and reported that the outcomes of patients with low PD-L1 expression levels and high CD8+TIL counts in the tumor were significantly better than those of patients with high PD-L1 expression levels and low CD8+TIL counts in the tumors. In the current study, we evaluated the concordance rate of the tumor immune microenvironment (TIME) classification based on the PD-L1 expression status and CD8+TIL count between surgically resected specimens and matched re-biopsy specimens in patients with recurrent NSCLC.
Materials and methods
Patients and specimens
We retrospectively collected and reviewed the medical records of patients with histologically confirmed, recurrent NSCLC who had undergone curative resection between 2010 and 2016 at our hospital. Among these patients, patients whose surgical specimens and re-biopsy specimens after recurrence were available were enrolled in this study. Written informed consent for study of the excised tissue samples was obtained from each patient. This study was conducted with the approval of the Ethics Committee of Kawasaki Medical School (No.2798: Approved on September 30, 2017). The study was conducted according to the criteria set by the Declaration of Helsinki.
The TNM stage was determined according to the revised criteria published in 2009 (version 7; TNM-7), and the histological diagnoses of the tumors were based on the WHO classification published in 2015.20,21 Selection of the postoperative adjuvant chemotherapy regimen was based on consensus among the members of institution’s cancer board or on enrollment in a clinical trial. In practice, oral tegafur was selected for patients with stage I disease (T1bN0M0 and T2N0M0), and platinum-based chemotherapy was selected for patients with stage II or stage IIIA disease.
Immunohistochemical analysis and assessment
Four-micrometer-thick sequential histologic sections were prepared from representative formalin-fixed, paraffin-embedded tumor blocks. Immunohistochemical (IHC) analysis was performed using an automated immunostainer (Nexes; Ventana, Tucson, AZ, USA). The following primary antibodies were used according to the manufacturer’s instructions and according to a previously described protocol: a mouse monoclonal anti-PD-L1 antibody (1: 50, clone 28–8: ab205921; Abcam, Cambridge, MA, USA) and CD8 (1:50, clone C8/144B, Dako; Agilent Technologies, Inc., Santa Clara, CA, USA).10,16
The expression levels of each marker protein were examined and evaluated according to a previously reported original protocol. PD-L1 expression was categorized as positive when staining of the tumor-cell membrane (at any intensity) was present. PD-L1 high expression was observed at prespecified expression levels of 5% of all cells in a section that included at least 100 evaluable tumor cells.10 To evaluate the CD8+TIL count, 10 digital high-power field images of the tumor area were selected, and the absolute number of CD8+TILs in these images was determined.16
Tumor immune microenvironment classification
The TIME classification into four groups based on the PD-L1 expression status and CD8+TIL count has been previously proposed, as follows: type-I (high PD-L1 expression with high CD8+TIL count), type-II (low PD-L1 expression with low CD8+TIL count), type-III (high PD-L1 expression with low CD8+TIL count), and type-IV (low PD-L1 expression with high CD8+TIL count).19,22
Statistical analysis
All the statistical analyses were performed using the SPSS statistical software package (version 23.0; SPSS, Chicago, IL, USA). The Chi-square test or Fisher’s exact test was performed to compare the changes of TIME classification in the patients. In all cases, p<0.05 was considered as denoting statistical significance. The prognostic evaluation was performed based on the overall survival (OS).
Results
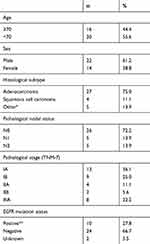
A total of 36 eligible patients with recurrent NSCLC were included in the present study (Table 1). The mean age at surgery was 66.4 years (range, 44–81years). The histologic subtype was adenocarcinoma in 27 patients, squamous cell carcinoma in 4 patients, and the other type of NSCLC in 5 patients. The TNM-7 cancer stages were IA, IB, IIA, IIB, and IIIA in 13, 9, 4, 2, and 8 patients, respectively. The median OS from surgery was 55.2 months and the 3-year OS rate was 76.8%.
 | Table 1 Characteristics of the patients enrolled in this study (n=36) |
Characteristics of the re-biopsy samples
Characteristics of the post-recurrence treatments and of the re-biopsy samples are summarized in Table 2. Of the re-biopsies, 47.2% (n=17) were collected from the lungs or bronchi and 52.8% (n=19) from metastatic sites. Metastatic organs included the lymph node (n=5), chest wall or pleura (n=4), adrenal glands (n=3), bone (n=3), GI tract (n=1), and soft tissue (n=1). Samples were obtained by surgery in 21 patients (58.3%) and by biopsy in 15 patients (41.7%). The median interval from surgery to the re-biopsy was 32.4 months (range, 4.9–114.5), with 80.6% of the paired samples collected >24 months apart. During the interval between the initial surgery and re-biopsy, 36.1% (n=13) of the patients received no treatment (Group A), while 63.9% of patients received chemotherapy or epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) therapy. The most common chemotherapy regimens used were oral tegafur (n=12, Group B), platinum-based chemotherapy (n=7, Group C), and EGFR-TKI (n=4, Group D). None of the patients was treated with PD-1/PD-L1 inhibitors.
 | Table 2 Characteristics of the re-biopsy specimens |
Comparison of PD-L1 expression and CD8+TIL status among paired samples
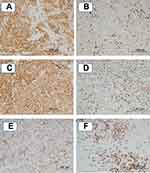
Representative sections showing PD-L1 expression and CD8+TILs are depicted in Figure 1. The PD-L1 expression levels were similar between the primary tumor and re-biopsy specimens in 28 patients (77.8%), whereas they were higher in the re-biopsy specimens in 6 patients (16.7%) and lower in the re-biopsy specimens in 2 patients (5.5%). Among the 7 patients who had received platinum-based chemotherapy (Group C), the PD-L1 expression level was higher in the re-biopsy specimens in 3 patients (42.9%). On the other hand, the CD8+TIL count did not differ between the primary tumor and re-biopsy specimens in 26 patients (72.2%), whereas it was higher in the re-biopsy specimens in 3 patients (8.3%) and lower in the re-biopsy specimens in 7 patients (19.5%). In the patients who had received oral tegafur chemotherapy (Group B), the CD8+TIL count was lower in the re-biopsy specimens in 4 patients (36.4%). (Table 3)
 | Table 3 PD-L1 expression status and CD8+TIL count in re-biopsy specimens as compared to the primary tumors |
Changes of TIM classification
The TIME classification remained unchanged between the specimens in 24 cases (66.7%), whereas it changed in the re-biopsy specimens as compared to specimens of the primary tumors in 12 (33.3%) patients. The TIME classification changed in 23.1% in Group A, in 41.7% in Group B, in 57.1% in Group C, and in 50.0% in Group D (Figure 2A). Although there was a tendency toward a higher rate of change in Group C, the differences among the groups were not statistically significant.
Heterogeneity of the TIM classification among the primary tumors, metastatic lymph node, and re-biopsy specimens
Of the 36 eligible patients enrolled in this study, 10 had lymph node metastasis at the time of the surgery (Table 1). We next compared the TIME classification among the primary tumor, one representative metastatic lymph node (the largest metastatic lymph node), and a re-biopsy specimen in each case. Comparisons of paired primary tumor and metastatic lymph node specimens showed that the TIME classification was the same in 7 patients (70.0%), but discordant in 3 patients (30.0%) (Figure 2B). The discordance between primary tumor and re-biopsy specimen was shown in 7 patients (70.0%); furthermore, discordance between the metastatic lymph node and re-biopsy specimens was observed in 4 patients (40.0%). Thus, the TIME classification of the re-biopsy specimens more closely observed resembled that of the metastatic lymph nodes as compared to that of the primary tumor.
Discussion
Recently, several studies have attempted to identify predictive biomarkers to help identify populations that are likely to respond to immunotherapy to PD-1/PD-L1 inhibitor, such as 1) PD-L1 expression status, 2) count of TILs, 3) mismatch-repair (MMR) protein expression status, tumor mutation and neoantigen burdens, 4) oncogene mutations, 5) radiographic markers, and 6) clinical pathological features.23
In 2017, we divided lung adenocarcinoma patients into four different TIME subgroups based on the PD-L1 expression status and CD8+TIL count, in an attempt to ascertain the prognosis and select the appropriate treatment.19 In this study, we used paired tumor samples from the same patients collected at different times. The purpose of this study was to compare the TIME classification based on the PD-L1 expression status and CD8+TIL count between the primary sites and recurrence sites. Until date, there are few reports on the changes in the PD-L1 expression status between surgically resected specimens of the primary tumors and paired re-biopsy specimens of the recurrent tumors. Another purpose of this study was to determine whether the chemotherapy regimen affects the TIME classification.
In this study, the PD-L1 expression level in the re-biopsy specimen was higher as compared to that in the primary tumors in 17% of patients. However, when this analysis was confined to patients who had received platinum-based chemotherapy, the percentage of cases in which the PD-L1 expression increased in the re-biopsy specimens increased from 17% to 43%. On the other hand, the CD8+TIL count decreased in the re-biopsy specimens in 20% of cases. However, when the analysis included only patients who had received oral tegafur, this percentage increased to 36%. The TIME classification changed from the primary tumor to re-biopsy specimens in one-third of patients. When this analysis included only patients who had received chemotherapy, the percentage increased to >40%.
The discordance rate of the PD-L1 expression status between the paired samples was 22.2%. This could be attributable to either heterogeneity of the TIME within tumors or change of the tumor biology by chemotherapy. The discordance rate of PD-L1 expression between paired samples obtained at difference time-points observed in the present study is consistent with that reported from other studies.24–28 On the other hand, the current study revealed that the TIME classification in re-biopsy specimens more closely resembled that of the metastatic lymph nodes as compared to that of the primary tumors. These results suggest that in case re-biopsy proves difficult, it might be better to refer to the biological status of the metastatic lymph nodes than that to that of the primary tumor for determining the most appropriate chemotherapy regimen.
Oral tegafur (UFT and S1) is often selected as adjuvant chemotherapy in patients with various cancers, including gastric, lung, and pancreatic cancer. Postoperative adjuvant chemotherapy with UFT is the standard treatment for stage I NSCLC patients in Japan.29,30 Until date, there are few reports on the changes of the PD-L1 expression levels or CD8+TIL count after treatment with oral tegafur. In an in-vitro study of 5-FU treatment, Van Der Kraak et al reported that the PD-L1 expression increased following the treatment in gastrointestinal cancer cell lines.31 In our study, PD-L1 expression in the re-biopsy specimen increased in only one patient who had received oral tegafur, although the CD8+TIL count decreased in 33% of patients. Considering these results, we concluded that oral tegafur does not upregulate PD-L1 expression.
Platinum-based chemotherapy is often selected as first-line chemotherapy in patients with advanced NSCLC and as adjuvant chemotherapy in resected stage II or IIIA NSCLC patients.32–34 In a study of ovarian cancer, increase of the PD-L1 expression and TILs was noted following neoadjuvant chemotherapy (mainly carboplatin and paclitaxel).35 In the case of head and neck squamous cell carcinoma, alteration of PD-L1 expression was noted during treatment, particularly following cisplatin-containing chemotherapy, in baseline PD-L1-negative patients.36 In NSCLC cell lines, cisplatin upregulated PD-L1 expression, and the enhancement of PD-L1 expression in the cancer cell lines occurred in a drug dose-dependent manner, and the depletion of PD-L1 significantly reduced cisplatin resistance.37 Considering these results, it may be surmised that platinum-based chemotherapy upregulates PD-L1 expression. Our finding of increase of the PD-L1 expression in the re-biopsy in 43% of patients who had received platinum-based chemotherapy was consistent with these reports.
EGFR-TKIs are often selected as first-line chemotherapy in advanced NSCLC patients with tumors harboring mutated EGFR.38,39 In a previous study, the EGFR-TKI gefitinib reduced the tumor PD-L1 expression via inhibiting NF-κB, in EGFR-mutant NSCLC, both in vitro and in vivo.40 However, in our study, the re-biopsy specimens showed a decrease of the tumor PD-L1 expression in only 1 of 4 patients treated with EGFR-TKIs.
Our study had several limitations. First, the number of patients enrolled in the study was small, reflecting the difficulties in obtaining an appropriate number of paired specimens for such studies. Second, for IHC analyses of PD-L1 expression and CD8+TILs, we used relatively standardized scoring systems. However, different PD-L1 scoring protocols have been used to determine the appropriateness of treatment with each of the PD-1/PD-L1 inhibitors available in the market. Although a standardized stromal CD8+TIL scoring system is currently available, there is as yet no standardized CD8+TIL scoring protocol for lung cancer.
Conclusion
Comparisons of paired specimens of the primary tumor and re-biopsy specimens showed that the TIME classification changed from the primary to the recurrent tumor in one-third of the patients, especially in patients who had received tegafur or platinum-based chemotherapy. Our study results suggest that a recent re-biopsy sample is required in patients with recurrent NSCLC, especially those who have previously received chemotherapy, to determine whether PD-1/PD-L1 inhibitors should be used for treatment.
Acknowledgments
We thank Ms. Keiko Isoda and the staff of the Tissue Culture & Immunology and the Tissue Biology & Electron Microscopy Research Centers (Kawasaki Medical School) for providing technical assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Asamura H, Goya T, Koshiishi Y, et al. A Japanese lung cancer registry study: prognosis of 13,010 resected lung cancers. J Thorac Oncol. 2008;3(1):46–52. doi:10.1097/JTO.0b013e31815e8577
2. Sawabata N, Miyaoka E, Asamura H, et al. Japanese lung cancer registry study of 11,663 surgical cases in 2004: demographic and prognosis changes over decade. J Thorac Oncol. 2011;6(7):1229–1235. doi:10.1097/JTO.0b013e318219aae2
3. Shimizu K, Okita R, Nakata M. Clinical significance of the tumor microenvironment in non-small cell lung cancer. Ann Transl Med. 2013;1:20. doi:10.3978/j.issn.2305-5839.2013.06.01
4. Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034.
5. Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54(4):307–314. doi:10.1007/s00262-004-0593-x
6. Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94(1):107–116. doi:10.1038/labinvest.2013.130
7. Shimoji M, Shimizu S, Sato K, et al. Clinical and pathologic features of lung cancer expressing programmed cell death ligand 1 (PD-L1). Lung Cancer. 2016;98:69–75. doi:10.1016/j.lungcan.2016.04.021
8. Sun JM, Zhou W, Choi YL, et al. Prognostic significance of PD-L1 in patients with non-small cell lung cancer: a large cohort study of surgically resected cases. J Thorac Oncol. 2016;11(7):1003–1011. doi:10.1016/j.jtho.2016.04.007
9. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi:10.1056/NEJMoa1504627
10. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi:10.1056/NEJMoa1507643
11. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi:10.1016/S0140-6736(15)01281-7
12. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi:10.1056/NEJMoa1606774
13. Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964.
14. Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–213. doi:10.1056/NEJMoa020177
15. Ali HR, Provenzano E, Dawson SJ, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12 439 patients. Ann Oncol. 2014;25(8):1536–1543. doi:10.1093/annonc/mdu191
16. Kinoshita T, Muramatsu R, Fujita T, et al. Prognostic value of tumor-infiltrating lymphocytes differs depending on histological type and smoking habit in completely resected non-small-cell lung cancer. Ann Oncol. 2016;27(11):2117–2123. doi:10.1093/annonc/mdw319
17. Teng F, Meng X, Wang X, et al. Expressions of CD8+TILs, PD-L1 and Foxp3+TILs in stage I NSCLC guiding adjuvant chemotherapy decisions. Oncotarget. 2016;7(39):64318–64329. doi:10.18632/oncotarget.11793
18. Donnem T, Hald SM, Paulsen EE, et al. Stromal CD8+ T-cell density- a promising supplement to TNM staging in non-small cell lung cancer. Clin Cancer Res. 2015;21(11):2635–2643. doi:10.1158/1078-0432.CCR-14-1905
19. Shimizu K, Okita R, Saisho S, et al. Prognostic value of Cox-2 and PD-L1 expression and its relationship with tumor infiltrating lymphocytes in resected lung adenocarcinoma. Cancer Manag Res. 2017;9:741–750. doi:10.2147/CMAR.S146897
20. Goldstraw P, Crowley J, Chansky K, et al. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2(8):706–714. doi:10.1097/JTO.0b013e31812f3c1a
21. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 world health organization classification of lung tumors. J Thorac Oncol. 2015;10(9):1243–1260. doi:10.1097/JTO.0000000000000630
22. Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T cell infiltration and PD-L1. Cancer Res. 2015;75(11):2139–2145. doi:10.1158/0008-5472.CAN-15-0255
23. Teng F, Meng X, Kong L, Yu J. Progress and challenges of predictive biomarkers of anti PD-1/PD-L1 immunotherapy: a systematic review. Cancer Lett. 2018;414:166–173. doi:10.1016/j.canlet.2017.11.014
24. Ilie M, Long-Mira E, Bence C, et al. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol. 2016;27(1):147–153. doi:10.1093/annonc/mdv489
25. Ilie M, Hofman V, Dietel M, Soria JC, Hofman P. Assessment of the PD-L1 status by immunohistochemistry: challenges and perspectives for therapeutic strategies in lung cancer patients. Virchows Arch. 2016;468(5):511–525. doi:10.1007/s00428-016-1910-4
26. Kitazono S, Fujiwara Y, Tsuta K, et al. Reliability of small biopsy samples compared with resected specimens for the determination of programmed death-ligand 1 expression in nonesmall-cell lung cancer. Clin Lung Cancer. 2015;16(5):385–390. doi:10.1016/j.cllc.2015.03.008
27. McLaughlin J, Han G, Schalper KA, et al. Quantitative assessment of the heterogeneity of PD-L1 expression in nonesmall-cell lung cancer. JAMA Oncol. 2016;2(1):46–54. doi:10.1001/jamaoncol.2015.3638
28. Mansfield AS, Murphy SJ, Peikert T, et al. Heterogeneity of programmed cell death-ligand 1 expression in multifocal lung cancer. Clin Cancer Res. 2016;22(9):2177–2182. doi:10.1158/1078-0432.CCR-15-2246
29. Kato H, Ichinose Y, Ohta M, et al. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med. 2004;350(17):1713–1721. doi:10.1056/NEJMoa032792
30. Hamada C, Tanaka F, Ohta M, et al. Meta-analysis of postoperative adjuvant chemotherapy with tegafur-uracil in non-small-cell lung cancer. J Clin Oncol. 2005;23(22):4999–5006. doi:10.1200/JCO.2005.09.017
31. Van Der Kraak L, Goel G, Ramanan K, et al. 5-Fluorouracil upregulates cell surface B7-H1 (PD-L1) expression in gastrointestinal cancers. J Immunother Cancer. 2016;18(4):65. doi:10.1186/s40425-016-0163-8
32. Fennell DA, Summers Y, Cadranel J, et al. Cisplatin in the modern era: the backbone of first-line chemotherapy for non-small cell lung cancer. Cancer Treat Rev. 2016;44:42–50. doi:10.1016/j.ctrv.2016.01.003
33.
34. Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552–3559. doi:10.1200/JCO.2007.13.9030
35. Mesnage SJL, Auguste A, Genestie C, et al. Neoadjuvant chemotherapy (NACT) increases immune infiltration and programmed death-ligand 1 (PD-L1) expression in epithelial ovarian cancer (EOC). Ann Oncol. 2017;28(3):651–657. doi:10.1093/annonc/mdw625
36. Ock CY, Kim S, Keam B, et al. Changes in programmed death-ligand 1 expression during cisplatin treatment in patients with head and neck squamous cell carcinoma. Oncotarget. 2017;8(1):97920–97927. doi:10.18632/oncotarget.18542
37. Zhang P, Ma Y, Lv C, et al. Upregulation of programmed cell death ligand 1 promotes resistance response in non-small-cell lung cancer patients treated with neo-adjuvant chemotherapy. Cancer Sci. 2016;107(11):1563–1571. doi:10.1111/cas.13072
38. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi:10.1056/NEJMoa0909530
39. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405). Lancet Oncol. 2005;11(2):121–128. doi:10.1016/S1470-2045(09)70364-X
40. Lin K, Cheng J, Yang T, Li Y, Zhu B. EGFR-TKI down-regulates PD-L1 in EGFR mutant NSCLC through inhibiting NF-κB. Biochem Biophys Res Commun. 2015;463(1–2):95–101. doi:10.1016/j.bbrc.2015.05.030
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.