Back to Journals » Clinical Ophthalmology » Volume 12
Comparative study of straight vs angled incision in 27-gauge vitrectomy for epiretinal membrane
Authors Yomoda R, Sasaki H, Kogo J , Shiono A, Jujo T , Sekine R , Tokuda N , Kitaoka Y, Takagi H
Received 10 August 2018
Accepted for publication 15 October 2018
Published 26 November 2018 Volume 2018:12 Pages 2409—2414
DOI https://doi.org/10.2147/OPTH.S183456
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Ryo Yomoda, Hiroki Sasaki, Jiro Kogo, Akira Shiono, Tatsuya Jujo, Reio Sekine, Naoto Tokuda, Yasushi Kitaoka, Hitoshi Takagi
Department of Ophthalmology, St Marianna University School of Medicine, Kawasaki, Kanagawa, Japan
Purpose: The purpose of this study was to compare straight and angled incisions in 27-gauge microincision vitrectomy in patients with epiretinal membrane (ERM).
Methods: Seventy-three eyes of 68 patients with ERM who underwent straight (35 eyes) or angled incision (38 eyes) for 27-gauge microincision vitrectomy were retrospectively evaluated.
Results: No statistically significant difference was found between the two groups in postoperative logarithm of minimal angle of resolution best-corrected visual acuity. The intraocular pressure and rate of hypotony 1 day postoperatively did not differ between the straight- and angled-incision groups (intraocular pressure: 11.5 vs 13.4 mmHg, respectively; rate of hypotony: 20% vs 8%, respectively). Surgical wound closing occurred by postoperative day 10 in both groups.
Conclusion: A straight incision is as safe and useful in ERM vitrectomy as an angled one.
Keywords: 27-gauge vitrectomy, MIVS, epiretinal membrane, straight incision, angled incision, anterior segment OCT
Introduction
After the introduction of the first 17-gauge vitreous cutter for vitrectomy in 1971,1 marked improvements have been made in the instrumentation. In 2002, a sutureless 25-gauge (25G) vitrectomy system was introduced by Fujii et al,2 and thereafter, Eckardt3 reported the 23-gauge (23G) vitrectomy system in 2005. Small-gauge transconjunctival microincision vitrectomy surgery (MIVS) using 23G and 25G instrument systems was reported to reduce surgically induced sclerotomy trauma, healing time, intraocular inflammation, astigmatism, and operative time.4–7 Subsequently, Oshima et al8 introduced the novel 27-gauge (27G) vitrectomy system in 2010. Recently, several authors including us have reported on the safety and availability of 27G MIVS, and the application of the 27G system has been expanded to several vitreoretinal diseases such as epiretinal membrane (ERM), proliferative retinopathy, retinal detachment, and proliferative vitreoretinopathy.9–12
Perpendicular wounds have been reported to leak more intra- and postoperatively, while oblique, angled, or beveled wounds have been reported to show decreased wound leakage.13,14 Recently, Khan et al15 have reported that eyes with straight incisions had lower intraocular pressure (IOP) only in the very early postoperative period compared with the angled-incision group. However, the results of optical coherence tomography (OCT)-based analysis of the difference between straight and angled incisions in 27G vitrectomy have not been reported. The aim of this study was to compare clinical outcomes, including IOP, rate of hypotony, and scleral wound closure, using OCT after straight vs angled trocar entry in eyes undergoing 27G MIVS for ERM.
Patients and methods
We retrospectively studied 73 eyes of 68 patients with ERM treated with 27G transconjunctival sutureless vitrectomy (TSV) at St Marianna University School of Medicine Hospital between August 2015 and October 2016. This study was approved by the Institutional Review Committee of St Marianna University School of Medicine, and written informed consent for participation was obtained from all patients. This study was conducted in accordance with the Declaration of Helsinki.
Eyes with vitreomacular diseases such as ERM and idiopathic macular hole were randomly assigned to the straight- or angled-incision group in an alternating fashion before surgery. If a patient had bilateral vitreomacular disease, the first eye operated on was randomly assigned. Only eyes with ERM were analyzed in this study.
All patients underwent 27G MIVS using the Alcon Constellation Vision System (Alcon Laboratories, Inc., Fort Worth, TX, USA) including a three-port trocar cannula system (Total Plus Pak). This instrument continuously monitors the infusion rate and intraocular fluid dynamics, allowing true IOP control during the entire surgical procedure. The surgical parameters were 5,000–7,500 cuts per minute and a vacuum of 0–650 mmHg. During surgery, IOP was controlled to 20 mmHg. For straight incisions, the trocar entered perpendicular to the sclera. Angled incisions were constructed in a conventional tunnel-like manner with the trocar first engaging the sclera at an approximately 30° angle before entry. Both the ERM and internal limiting membrane (ILM) were peeled using 27G ILM forceps in all cases with Brilliant Blue G dye utilized to assist in the peeling process. The conjunctiva was displaced from the intended sclerotomy site, and the trocar was placed 3.5–4.0 mm posterior to the limbus in both groups. The wound locations were superotemporal, superonasal, and inferotemporal as infusion sites in all eyes. If a significant cataract was present, combined cataract surgery was performed before the scleral incision for vitrectomy. All cataract surgeries were performed through a clear corneal 2.4 mm incision. All surgeries used the wide-viewing system and a magnified contact lens for macular work as necessary. At the end of the surgery, the cannulas were removed and moderate pressure was applied to the sclerotomy sites with a cotton-tipped applicator. Topical antibiotic ointment was applied, and the eye was patched and shielded after surgery. All surgeries were performed by the same right-handed surgeon (HT).
To compare the clinical outcomes including IOP and wound closure, the patients were divided into two groups based on the incision procedure (straight or angled). The best-corrected visual acuity (BCVA), IOP, rate of hypotony, and scleral wound closure were compared. We defined “hypotony” as an IOP of ≤6 mmHg or less and “normal pressure” as an IOP of >6 mmHg. To confirm would closure, we captured the 360° around the sclerotomies using CASIA SS-1000 OCT scanner (Tomey, Nagoya, Japan) so as not to overlook the sclerotomies. The BCVA was examined before and 1, 3, and 6 months after surgery, IOP was examined before and 1, 2, 3, and 10 days after surgery, and wound closure was evaluated 1, 2, 3, and 10 days postoperatively.
All statistical analyses were performed using IBM SPSS Statistics version 21.0 (IBM Corp., Armonk, NY, USA). Mann–Whitney U-test and Wilcoxon signed-rank test were used for comparisons of the logarithm of minimal angle of resolution (logMAR) BCVA and IOP between the two groups. Wound closure rates were analyzed using chi-squared test. P-values of <0.05 were considered to represent a statistically significant difference.
Results
The baseline patient characteristics are shown in Table 1. Thirty-five of 73 eyes underwent 27G vitrectomy with a straight incision and 38 eyes with an angled incision. The age, preoperative BCVA, preoperative IOP, axial length, and rate of cataract surgery did not differ significantly between the two groups. The changes in BCVA are shown in Figure 1. The mean logMAR BCVA at baseline and 1, 3, and 6 months after surgery was 0.107±0.231, 0.022±0.130, 0.003±0.105, and 0.0001±0.102, respectively, in the straight-incision group and 0.079±0.178, 0.112±0.288, 0.095±0.222, and 0.090±0.225, respectively, in the angled-incision group. There were no significant differences in logMAR BCVA between the two groups at any time point.
  | Table 1 Baseline patient characteristics |
The time-course changes in IOP in both groups are shown in Figure 2. The mean IOP at baseline and 1, 2, 3, and 10 days after surgery was 14.9±3.6, 11.5±5.9, 12.1±6.3, 11.9±4.2, and 15.0±3.6 mmHg, respectively, in the straight group and 14.8±3.6, 13.4±7.4, 12.9±7.0, 12.6±3.9, and 14.2±4.3 mmHg, respectively, in the angled group. There were no significant differences in IOP between the two groups at any time point. However, the mean IOP in the straight group at 1, 2, and 3 days after surgery was significantly lower than that at baseline (P=0.010, P=0.002, P=0.030, respectively). On the other hand, no statistically significant difference was found in terms of the changes in IOP in the angled-incision group at any time point compared with baseline. Seven eyes in the straight group and three eyes in the angled group developed postoperative hypotony, defined as an IOP of <6 mmHg. There was thus no significant difference in the rate of hypotony between the two groups. In all eyes, hypotony was detected only on postoperative day 1, and it resolved in all by postoperative day 2.
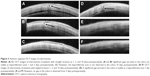
All sclerotomies were evaluated using anterior segment OCT 1, 2, 3, and 10 days after surgery (Figure 3). The rate of wound closure in both groups is shown in Table 2. Among the sclerotomies, there were no significant differences in the rate of wound closure between the two groups at any time point. All sclerotomies were closed by postoperative day 10.
  | Table 2 Rate of wound closure of each port |
Discussion
To the best of our knowledge, this is the first clinical study reporting on OCT-based analysis of the difference between a straight and an angled incision in 27G vitrectomy. Overall, rates of hypotony and postoperative complications were equally low. Eyes in the straight group, however, had comparatively lower IOP on postoperative days 1, 2, and 3.
The results of the present study are consistent with previous reports demonstrating that the use of the 27G system is safe and effective, with good anatomic outcomes.16,17 In the present study, surgical success was achieved in all cases and none required conversion to 25G instrumentation. The IOP and sclerotomies were followed up for a minimum of 10 days in 73 eyes. Postoperative complications were limited and managed effectively with conservative measures. No eye required subsequent surgery for ocular hypertension, hypotony, or vitreous hemorrhage. No cases of postoperative endophthalmitis, sclerotomy-related retinal tears, or choroidal detachment were encountered in the follow-up period.
In this study, postoperative BCVA was not significantly different at any time point examined in the two groups. Regarding the relationship between wound construction strategies and postoperative visual acuity, our study showed that postoperative BCVA scores did not differ significantly at any point after vitrectomy.
Postoperative hypotony is reported in 0%–25% of 25G sutureless vitrectomy cases.11,18–24 Hypotony is usually transient and in most cases resolves with conservative measures. It was reported that the adoption of angled incisions decreased wound-related complications such as hypotony and choroidal detachment in 25G sutureless vitrectomy.25–27 Since the introduction of sutureless vitrectomy, several groups have focused on the incidence of postoperative hypotony after 27G vitrectomy, reporting risks that range from 0% to 9.4%.10,11,15,28 On postoperative day 1, IOP was 11.5±5.9 mmHg in the straight-incision group and 13.4±7.4 mmHg in the angled-incision group in the present study. The IOP in the straight group was lower than at baseline. However, no statistically significant difference was found in either group. The rate of hypotony in this series was 13.7%. When compared with prior series, this rate is higher than that reported for 27G systems but within range of that reported for 25G systems. At the end of surgery, all cannulas were pulled out over the light pipe. With this maneuver, the illumination probe is used to displace the vitreous trapped inside the cannula into the vitreous cavity. Removing the cannula over the light pipe results in a greater frequency of sclerotomy leakage in patients undergoing TSV.29 Some studies have stated that a partial air fill lowers the risk of hypotony in 23G30 and 25G16,26 vitrectomy. Air-gas exchange is proposed to help seal the sclerotomies from the interior due to different surface tensions between air-gas and fluid.8,27,31 In the present study, we did not use gas tamponade and removed the cannula over the light pipe, which may explain the difference in the postoperative IOP between those studies and ours.
It was previously reported that the adoption of angled incisions with 25G systems helps to reduce initial concerns regarding postoperative wound-related complications such as hypotony, endophthalmitis, and choroidal detachment.16 In our series, two distinct wound construction techniques were used, which allows for comparison. There were seven cases of hypotony in the straight-incision group and three in the angled-incision group. None of the differences in the IOP between the two groups were significant at any follow-up visit, and the IOP tended to increase afterward, reaching >10 mmHg in all eyes 10 days postoperatively. This tendency for IOP to increase with time and healing is compatible with the findings of previous studies.10,15,28 Because IOP decreased significantly after surgery in the straight group, we speculated that even if angled incisions were unclosed, functional closure may have been achieved. Acar et al32 reported a significant decrease in IOP at both 2 hours and 1 day postoperatively after 25G vitrectomy with straight incisions. At 2 hours postoperatively, they observed hypotony in 26% of eyes. In their series, the hypotony rate decreased with time and was 17% on the first postoperative day. In the present study, IOP might have been significantly higher in the angled group than in the straight group 2 hours after 27G TSV. We evaluated early postoperative hypotony to determine whether it might lead to complications but did not observe any choroidal detachment or folds and endophthalmitis due to hypotony.
In a previous study, we demonstrated that the mean time required for scleral wound closure after vitrectomy using the DORC 27G system was 7.7±4.7 weeks.11 However, in the present study, scleral wound closure was seen by postoperative day 10 in all eyes. Prior studies noted that excess pivoting of angled incisions may result in a transient wound gap or misalignment, resulting in wound incompetence.33 Extensive intraocular instrument manipulation can lead to wound leakage by enlarging the sclerotomy incision, possibly causing wound gap and leakage.34 We speculated that incisions in the straight group would seal more easily than those in the angled group. However, no significant difference in the mean time of scleral wound closure was noted between the two groups at any time point. Although we assumed that the shorter incision length would decrease the time required for wound healing, there was no significant difference between the two groups. We hypothesize that because of the small gauge size, less time was required for scleral wound closure and ERM tended to require less trocar manipulation and rotation to work on a smaller confined area on the retina, and therefore, no significant difference between the two groups occurred. The absence of wound-related complications in our patients suggests that both wound construction techniques may be used successfully with 27G instrumentation, allowing surgeons a choice based on the clinical scenario and personal preference.
Study limitations
There were potential limitations in this study. The number of cases was small, reflecting experience from a single institution. In addition, the insertion angle of the trocar was not measured during scleral penetration. Thus, there may have been some variations in the angle. To minimize such variations, all sclerotomies were performed by a single surgeon. Importantly, only eyes with idiopathic ERM which were fluid filled at the end of surgery were included, and therefore, the results may not be directly applicable to other surgical indications. Finally, because of the relatively short follow-up duration in the current study, no long-term complications could be addressed.
Conclusion
Both angled and straight trocar incisions were clinically well tolerated in this study after 27G pars plana vitrectomy for ERM in fluid-filled eyes. The clinical outcomes of this study suggest that the two types of incisions are comparably safe. Straight incisions are therefore as safe and useful for ERM vitrectomy as angled ones.
Disclosure
The authors report no conflicts of interest in this work.
References
Machemer P, Parel JM, Norton EW. Vitrectomy: a pars plana approach. Technical improvements and further results. Trans Am Acad Ophthalmol Otolaryngol. 1971;76:462–466. | ||
Fujii GY, de Juan E, Humayun MS, et al. A new 25-gauge instrument system for transconjunctival sutureless vitrectomy surgery. Ophthalmology. 2002;109(10):1807–1812. | ||
Eckardt C. Transconjunctival sutureless 23-gauge vitrectomy. Retina. 2005;25(2):208–211. | ||
Okamoto F, Okamoto C, Sakata N, et al. Changes in corneal topography after 25-gauge transconjunctival sutureless vitrectomy versus after 20-gauge standard vitrectomy. Ophthalmology. 2007;114(12):2138–2141. | ||
Inoue Y, Kadonosono K, Yamakawa T, et al. Surgically-induced inflammation with 20-, 23-, and 25-gauge vitrectomy systems: an experimental study. Retina. 2009;29(4):477–480. | ||
Avitabile T, Castiglione F, Bonfiglio V, Castiglione F. Transconjunctival sutureless 25-gauge versus 20-gauge standard vitrectomy: correlation between corneal topography and ultrasound biomicroscopy measurements of sclerotomy sites. Cornea. 2010;29(1):19–25. | ||
Haas A, Seidel G, Steinbrugger I, et al. Twenty-three-gauge and 20-gauge vitrectomy in epiretinal membrane surgery. Retina. 2010;30(1):112–116. | ||
Oshima Y, Wakabayashi T, Sato T, Ohji M, Tano Y. A 27-gauge instrument system for transconjunctival sutureless microincision vitrectomy surgery. Ophthalmology. 2010;117(1):93–102. | ||
Rizzo S, Barca F, Caporossi T, Mariotti C. Twenty-seven-gauge vitrectomy for various vitreoretinal diseases. Retina. 2015;35(6):1273–1278. | ||
Khan MA, Shahlaee A, Toussaint B, et al. Outcomes of 27 gauge microincision vitrectomy surgery for posterior segment disease. Am J Ophthalmol. 2016;161:36–43. | ||
Mitsui K, Kogo J, Takeda H, et al. Comparative study of 27-gauge vs 25-gauge vitrectomy for epiretinal membrane. Eye. 2016;30(4):538–544. | ||
Yoneda K, Morikawa K, Oshima Y, Kinoshita S, Sotozono C; Japan Microincision Vitrectomy Surgery Study Group. Surgical outcomes of 27-gauge vitrectomy for a consecutive series of 163 eyes with various vitreous diseases. Retina. 2017;37(11):2130–2137. | ||
Shimada H, Nakashizuka H, Mori R, Mizutani Y, Hattori T. 25-gauge scleral tunnel transconjunctival vitrectomy. Am J Ophthalmol. 2006;142(5):871–873. | ||
Taban M, Ventura AA, Sharma S, Kaiser PK, Alezandre A, Venture CM. Dynamic evaluation of sutureless vitrectomy wounds: an optical coherence tomography and histopathology study. Ophthalmology. 2008;115(12):2221–2228. | ||
Khan MA, Durrani AK, Hsu J, Regillo CD. 27-Gauge vitrectomy wound integrity: a Randomized pilot study comparing angled versus straight entry in fluid-filled vitrectomized eyes. Retina. 2018;38(4):678–683. | ||
Shimada H, Nakashizuka H, Mori R, Mizutani Y, Hattori T. 25-gauge scleral tunnel transconjunctival vitrectomy. Am J Ophthalmol. 2006;142(5):871–873. | ||
Hsu J, Chen E, Gupta O, Fineman MS, Garg SJ, Regillo CD. Hypotony after 25-gauge vitrectomy using oblique versus direct cannula insertions in fluid-filled eyes. Retina. 2008;28(7):937–940. | ||
Scott IU, Flynn HW, Dev S, et al. Endophthalmitis after 25-gauge and 20-gauge pars plana vitrectomy: incidence and outcomes. Retina. 2008;28(1):138–142. | ||
Teixeira A, Allemann N, Yamada AC, Uno F, Maia A, Bonomo PP. Ultrasound biomicroscopy in recently postoperative 23-gauge transconjunctival vitrectomy sutureless self-sealing sclerotomy. Retina. 2009;29(9):1305–1309. | ||
Sandali O, El Sanharawi M, Lecuen N, et al. 25-, 23-, and 20-gauge vitrectomy in epiretinal membrane surgery: a comparative study of 553 cases. Graefes Arch Clin Exp Ophthalmol. 2011;249(12):1811–1819. | ||
Kim M, Park YS, Lee DH, Koh HJ, Lee SC, Kim SS. Comparison of surgical outcome of 23-gauge and 25-gauge microincision vitrectomy surgery for management of idiopathic epiretinal membrane in pseudophakic eyes. Retina. 2015;35(10):2115–2120. | ||
Chen E. 25-Gauge transconjunctival sutureless vitrectomy. Curr Opin Ophthalmol. 2007;18(3):188–193. | ||
Byeon SH, Chu YK, Lee SC, Koh HJ, Kim SS, Kwon OW. Problems associated with the 25-gauge transconjunctival sutureless vitrectomy system during and after surgery. Ophthalmologica. 2006;220(4):259–265. | ||
Bamonte G, Mura M, Stevie Tan H, Tan HS. Hypotony after 25-gauge vitrectomy. Am J Ophthalmol. 2011;151(1):156–160. | ||
López-Guajardo L, Vleming-Pinilla E, Pareja-Esteban J, Teus-Guezala MA. Ultrasound biomicroscopy study of direct and oblique 25-gauge vitrectomy sclerotomies. Am J Ophthalmol. 2007;143(5):881–883. | ||
Oshima Y, Ohji M, Tano Y. Surgical outcomes of 25-gauge transconjunctival vitrectomy combined with cataract surgery for vitreoretinal diseases. Ann Acad Med Singapore. 2006;35(3):175–180. | ||
Bourgault S, Tourville E. Incidence of postoperative hypotony in 25-gauge vitrectomy: oblique versus straight sclerotomies. Can J Ophthalmol. 2012;47(1):21–23. | ||
Mori R, Naruse S, Shimada H. Comparative study of 27-gauge and 25-gauge vitrectomy performed as day surgery. Int Ophthalmol. 2018;38(4):1575–1582. | ||
Javey G, Rigi M, Barkmeier AJ, Heffez JL, Carvounis PE. Sclerotomy leakage in transconjunctival small-gauge pars plana vitrectomy: Effect of removing the cannula over the light pipe. Retina. 2017;37(6):1079–1083. | ||
Parolini B, Prigione G, Romanelli F, Cereda MG, Sartore M, Pertile G. Postoperative complications and intraocular pressure in 943 consecutive cases of 23-gauge transconjunctival pars plana vitrectomy with 1-year follow-up. Retina. 2010;30(1):107–111. | ||
Yamane S, Kadonosono K, Inoue M, Kobayashi S, Watanabe Y, Arakawa A. Effect of intravitreal gas tamponade for sutureless vitrectomy wounds: three-dimensional corneal and anterior segment optical coherence tomography study. Retina. 2011;31(4):702–706. | ||
Acar N, Kapran Z, Unver YB, Altan T, Ozdogan S. Early postoperative hypotony after 25-gauge sutureless vitrectomy with straight incisions. Retina. 2008;28(4):545–552. | ||
Chen D, Lian Y, Cui L, Lu F, Ke Z, Song Z. Sutureless vitrectomy incision architecture in the immediate postoperative period evaluated in vivo using optical coherence tomography. Ophthalmology. 2010;117(10):2003–2009. | ||
Lakhanpal RR, Humayun MS, de Juan E, et al. Outcomes of 140 consecutive cases of 25-gauge transconjunctival surgery for posterior segment disease. Ophthalmology. 2005;112(5):817–824. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.