Back to Journals » Clinical Ophthalmology » Volume 16
Comparative Study of Safety Outcomes Following Nucleus Disassembly with and without the miLOOP Lens Fragmentation Device During Cataract Surgery
Authors Hu EH, Buie T, Jensen RJ, Wu D , Pamnani RD
Received 11 April 2022
Accepted for publication 15 July 2022
Published 2 August 2022 Volume 2022:16 Pages 2391—2401
DOI https://doi.org/10.2147/OPTH.S370290
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Edward H Hu,1 Therese Buie,2 Rishma J Jensen,2 David Wu,3 Ravinder D Pamnani4
1Wolfe Eye Clinic, Marshalltown, IA, USA; 2Illinois Eye Center, Peoria, IL, USA; 3University of Illinois at Chicago, Chicago, IL, USA; 4Byers Center for Biodesign, Stanford University, Stanford, CA, USA
Correspondence: Edward H Hu, Wolfe Eye Clinic, 309 East Church Street, Marshalltown, IA, USA, Tel +641 754-6200, Email [email protected]
Purpose: To determine the effect of a microinterventional lens prefragmentation wire loop device (miLOOP®; Carl Zeiss Meditec AG, Oberkochen, Germany), on adverse events (AEs), cumulative dispersed energy (CDE), and vision outcomes when used before phacoemulsification of high-grade mature cataracts.
Setting: Three ambulatory surgical centers in the Peoria, IL region.
Design: Retrospective comparative consecutive case series; single-surgeon.
Methods: Patient outcomes were compared before and after introduction of miLOOP-assisted lens fragmentation prior to phacoemulsification during cataract surgeries performed 2016‒2020. The primary outcome was intraoperative AE rate/type. Secondary outcomes included ultrasound cumulative dispersed energy (CDE) administered during phacoemulsification, postoperative AEs, and best-corrected visual acuity (BCVA).
Results: Data from 765 subjects (mean age 72.9 years; 1025 eyes) comprised 524 conventional lens disassembly (Control) eyes and 501 Device eyes. One hundred percent of the cataracts in both groups were advanced WHO Grade 3+ nuclei. Significantly fewer intraoperative AEs occurred in the Device group versus Controls (2.2% and 6.3% of eyes, respectively; p=0.0011). Postoperative AE rates were comparable between groups (Controls=2.9%, Device=3.5%). Mean CDE from ultrasound was significantly reduced by 21% when the microfilament loop device was used for nuclear disassembly (9.6± 5.2 CDE units) versus Controls (11.6± 6.4 CDE units; p< 0.0001). Median postoperative BCVA was 20/25 Snellen (0.091 logMAR) in both groups. More than 70% of both Control and Device eyes had postoperative BCVA better than 20/30 Snellen.
Conclusion: Microinterventional lens fragmentation was associated with lower ultrasound energy use and improved intraoperative safety than traditional unassisted surgery of advanced high-grade cataracts, while maintaining similarly acceptable postoperative complication rates and BCVA functional outcomes.
Keywords: lens disassembly, phacoemulsification cataract surgery, cumulative dispersed energy, safety, microinterventional surgery, adverse events
Plain Language Summary
Advanced dense cataracts are challenging for a surgeon to remove and require more applied energy using common phacoemulsification techniques, which can damage ocular tissues. Pre-dissecting dense cataracts using a retractable microfilament wire loop device reduces subsequent ultrasound energy requirements and is associated with fewer intraoperative complications, while maintaining excellent postoperative visual acuity outcomes that are expected from conventional phacoemulsification cataract surgery.
Introduction
Cataract surgery is the most commonly performed surgical procedure in the United States.1 Phacoemulsification remains the gold standard for routine cataract removal despite its introduction more than 50 years ago.2,3 Rates of adverse events (AEs) in cataract surgery are very low. In a study of Medicare beneficiaries, representing >80% of all cataract surgery in the US,4 only 0.5% of the people who underwent cataract surgery experienced a severe AE through postoperative 1-year.5 Despite cataract surgery’s excellent safety profile, patients with dense brunescent cataracts are at significantly increased risk of an intraoperative or postoperative complication by nearly threefold.6
Dense brunescent lenses are a sign of advanced cataracts that have grown in size and become increasingly hard.3 Surgery on these mature cataracts tends to be more difficult because they are associated with loose zonules, low endothelial cell count, shallow anterior chamber, pseudoexfoliation, inadequate pupillary dilatation, and an increased risk of intraoperative complications.3,7,8 Dense brunescent lenses are endemic in resource-constrained geographic regions, where patients are more likely to delay cataract surgery due to factors such as poor infrastructure, inadequate human resources, insufficient awareness, cost considerations, or inability to travel to healthcare facilities.9
Addressing advanced cataracts typically requires modification to the surgical preparation or technique, including the use of additional instrumentation for successful removal. While there are a wide range of different approaches to dense cataract nuclear disassembly, such as divide and conquer, in situ fracture, stop and chop, and phaco chop,10 nearly all techniques result in increased phacoemulsification time and intensity required to fragment and emulsify the solidified nucleus. Higher levels of ultrasonic phacoemulsification energy inherently expose a greater risk of trauma to the cornea and capsular structures.3 Higher rates of corneal endothelial cell damage have been associated with a firmer nucleus and increased levels of ultrasonic energy,11 including an increased risk of severe endothelial cell loss in patients with hard cataract undergoing phacoemulsification.12 Longer phaco times also reduce the throughput in a cataract surgery practice, reducing overall resource utilization efficiency.13 Techniques that streamline lens disassembly and reduce cumulative dispersed energy (CDE) requirements during phacoemulsification cataract surgery may reduce the incidence and severity of ultrasound-associated ocular AEs.
Typical nucleus disassembly techniques generate centrifugal (inside→outside) forces to create the fracture plane, which risks physically stressing the capsular complex and causing iatrogenic complications, especially in eyes with pre-existing zonulopathy.14 A recently developed microinterventional technique to fragment dense lenses employs a nickel–titanium (nitinol) filament loop that ensnares and mechanically divides the lens nucleus following anterior capsulotomy.15 This device (miLOOP®; Carl Zeiss Meditac AG, Oberkochen, Germany) exerts physical cutting force on the lens in an outside→inside direction as the loop diameter is reduced, thereby reducing the likelihood of inadvertent capsular trauma. The microinterventional lens fragmentation technique does not introduce any additional risk of complications compared to conventional cataract surgery,16 provides more consistent refractive outcomes,17 and reduces total phaco time and irrigation time.18 The current study retrospectively compared AE incidence, operative CDE levels, and postoperative visual acuity in a continuous series of more than 1000 eyes from 755 consecutive subjects who underwent phacoemulsification of high-grade advanced cataracts, with approximately half of procedures employing device-assisted lens disassembly before beginning ultrasound fragmentation.
Methods
Study Design and Ethical Oversight
Record reviews were performed for consecutive patients who underwent cataract surgery by a single surgeon (EHH) between January 2016 and September 2020 at three locations in the Peoria, IL region. Of 755 total subjects, 410 underwent surgery before the introduction of the microloop lens fragmentation device, and 345 underwent surgery that involved device-assisted lens disassembly. Study sites were 1) the Order of Saint Francis Center for Health Ambulatory Surgical Center (CFH), Peoria, IL; 2) UnityPoint Proctor Hospital (Proctor), Peoria, IL; and 3) the Order of Saint Francis St. Luke’s Hospital (SLH), Kewanee, IL. Each study site provided IRB approval and oversight; informed consent requirements were waived due to retrospective nature of the study that involved only de-identified patient data with minimal risk of privacy compromise. Study performance conformed to the tenets of the Declaration of Helsinki and the US Health Insurance Portability and Accountability Act (HIPPA) of 1996.
A subject database was constructed containing procedure date, patient sex, patient age, operative eye, cataract density/severity (grade number), type of cataract (nuclear sclerotic, cortical, posterior subcapsular), preoperative best-corrected visual acuity (BCVA), ocular comorbidities, phacoemulsification time, utilization of the microinterventional miLOOP® lens fragmentation device, intraoperative complications, postoperative BCVA, and postoperative complications. Eyes were grouped according to lens fragmentation device usage into either Control (unassisted traditional phaco) or Device (miLOOP-assisted phaco) groups.
Preoperative examinations were conducted at the Illinois Eye Center, Peoria IL. At CFH and Proctor, preoperative biometry was obtained using the Lenstar LS900 Ver. 1.1.0 with EyeSuite Biometry Ver. 2.4.0 (Haag-Streit AG, Kõniz, Switzerland), while at SLH the eyes were evaluated using the IOLMaster 500 (Carl Zeiss Meditec AG). For the surgical procedures, Lumera microscopes (Carl Zeiss Meditec AG) were used at CFH and Proctor, while at SLH the LuxOR microscope (Alcon AG, Geneva, Switzerland) was employed. The phacoemulsification system used at all facilities was the Centurion Vision System (Alcon).
Selection Criteria
Operative cases were selected for inclusion in the analysis based on the lens density/cataract severity using the WHO Cataract Grading System.19 Dense cataracts with a minimum grade of 3+ nuclear sclerosis were included in the analysis. Eyes with ocular comorbidities, other ocular conditions, or other medical comorbidities were included in the study on an all-comer basis. If a patient’s right and left eye were operated on during the study period, and both met the cataract severity selection criteria, then both eyes were included in the analysis population.
Lens Fragmentation Technique
For the traditional phacoemulsification Control group, nuclear disassembly was performed primarily by vertical chopping and occasionally by divide-and-conquer techniques. Dense lenses were fragmented with vertical chop technique using a Rosen Chopper. Divide-and-conquer technique was utilized if initial nuclear chop was difficult to complete by the surgeon. Following the initial division of the lens, subsequent vertical chopping of large quadrants and fragments was then conducted.
Eyes in the Device group received loop-assisted lens disassembly prior to beginning phacoemulsification. The miLOOP is a single-use handheld device designed to fragment cataractous lenses after anterior capsulotomy. The device consists of a handle and a microfilament loop composed of nitinol alloy with a wire diameter of 300 μm and a user-controlled loop radius ranging from 1.5 mm to a fully expanded 10.5 mm (Figure 1). To perform lens fragmentation, after capsulorrhexis and hydrodissection were performed through a clear cornea incision, the microfilament loop was inserted in the fully contracted state into the anterior chamber through the corneal incision. Once inside the eye, the tip of loop was placed on the anterior lens surface and positioned behind the edge of the capsulorrhexis. The loop was then expanded in the coronal plane immediately posterior to the anterior capsule surface until it reaches the capsular equatorial fornix. The loop was then rotated clockwise, sweeping the loop along the posterior hydrodissection plane against the internal posterior lens capsular surface until it encapsulates the nucleus in the sagittal plane (Figure 1C). Upon loop retraction, the nitinol microfilament transected the nucleus. When sufficient zonular strength allowed, the loop was re-expanded, and the nucleus was rotated 90° along the coronal plane to repeat the transection and divide the nucleus into four quadrants. Loop-dissected lens quadrants were subsequently fragmented further with phacoemulsification and aspirated per standard techniques. Patients were prescribed a standard postoperative regimen including 1 week of topical antibiotics (ofloxacin) and 4 weeks of topical steroid (prednisolone) and non-steroidal anti-inflammatory (ketorolac) drops. Patients were asked to return 2‒3 weeks after surgery for a postoperative safety and vision follow-up examination.
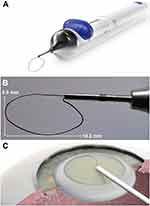 |
Figure 1 The miLOOP® lens fragmentation device (Meditac AG, Oberkochen, Germany). (A) The unit is single-use and consists of a metal filament loop made of 300 µm-thick nickel–titanium alloy (nitinol) wire that is used to bisect the nucleus. The loop can be extended or contracted using a slider button on the device handle. (B) The maximally expanded nitinol filament loop is 10.5 mm by 5.5 mm and shaped to match the contours of the lens surface. At maximal contraction, the loop diameter is 1.5 mm. (C) Device loop expansion in the coronal hydrodissection plane prior to nuclear encirclement, endocapsular rotation, and loop contraction that bisects the cataractous lens. Panel C was reproduced from: Ianchulev T, Chang DF, Koo E et al. Microinterventional endocapsular nucleus disassembly: novel technique and results of first-in-human randomised controlled study. Br J Ophthalmol. 2019;103:176–180. doi:10.1136/bjophthalmol-2017-311766.16 © Author(s) (or their employer(s)) 2019. Creative Commons CC BY-NC (https://creativecommons.org/licenses/by-nc/4.0/legalcode). |
Outcomes
The primary outcome was the difference in the rate of intraoperative AEs in eyes that underwent cataract surgery with conventional lens fragmentation versus microinterventional device-assisted prefragmentation. Secondary outcomes included postoperative AE incidence, operative CDE levels, and postoperative BCVA.
Statistical Analysis
Data are presented as mean±SD or as n (%) of group, as indicated. Continuous variables were compared using Student’s t-test for normally distributed data and the Mann–Whitney U-test for nonparametric data. Proportions of categorical variables were compared using Fisher’s exact test using 2×2 contingency tables. Two-tailed P-values <0.05 were considered indicative of statistically significant differences. Mean BCVA values were converted from Snellen to Decimal format before performing statistical comparisons, with “Counts Fingers/Sees Hand Waving” notations assigned a Decimal value of 0.025 (Snellen 20/800) for numerical inclusion. Data were analyzed using Prism v.5.03 statistical and graphing software (GraphPad Software Inc., San Diego, CA).
Results
Baseline Characteristics
During the study period, 755 of all consecutive cataract surgery patients at the three study sites met the cataract severity study eligibility criterion. This comprised 410 subjects (54.3% of total; 55.6% female, mean age 72.5±9.1 years) in the Control group who underwent traditional phacoemulsification without miLOOP-assisted lens prefragmentation and 345 subjects (45.7% of total; 49.3% female; mean age 73.3±9.9 years) in the Device group who received miLOOP-assisted lens disassembly before phacoemulsification (Table 1). In total, 1025 eyes were enrolled, and the number of eyes in the Control group (n=524; 51.1%) were nearly identical to that of the Device group (n=501; 48.9%). All Control eyes were operated on between January 5, 2016 and January 31, 2018, and all Device eyes were operated between February 7, 2018, when the miLOOP device was first adopted by the Primary Investigator (EHH) for routine use with advanced (grade 3+) cataracts, and September 28, 2020. Bilateral eyes of 270 patients were included in the analysis, representing 52.7% (540/1025 total eyes), with the remaining 485 unilateral eyes each from a unique patient. None of the bilateral patients had their eyes split between the two groups; bilaterally operated patients had both eyes in either the Control group (n=114 subjects) or the Device group (n=156 subjects).
 |
Table 1 Baseline Characteristics |
Both groups had a high proportion of advanced cataracts, and 100% of study eyes met the inclusion requirement of WHO Grade 3+ cataracts (Table 1). Average cataract severity scores were similarly 3.94±0.59 units in Control eyes and 3.98±0.57 units in miLOOP-assisted eyes (P=0.2068). Median preoperative BCVA measurements were slightly better in Device eyes (20/70 Snellen) than Control eyes (20/100; P=0.0001); however, a greater proportion of eyes had advanced vision compromise and were classified as “Counts Fingers/Sees Hand Waving or worse” in the Device group than Controls (15.8% versus 10.3%, respectively; P=0.0118) (Table 1).
Of baseline ocular comorbidities, Device eyes had numerically higher proportions of age-related macular degeneration versus Control eyes (7.2% versus 5.2%, respectively), macular disease (4.2% versus 3.2%), retinal disease (2.8% versus 1.5%), and background glaucoma (5.4% versus 4.4%), while a larger proportion of Control eyes than Device eyes had pre-existing vitreous conditions (5.5% versus 2.6%); however, none of these differences reached statistical significance (Table 1). More Control eyes (13.7%) than Device eyes (10.0%) were from subjects with type 2-diabetes, though this difference was not significant and did not correlate with the low and similar incidence of diabetic retinopathy in Control eyes (1.3%) and Device eyes (1.4%). Significantly more Device eyes displayed anterior basement membrane dystrophy than Controls (1.0% versus 0.0%; P=0.0249), and numerically more Device eyes (5.4%) were from subjects with diagnosed or suspected glaucoma than Control eyes (4.4%; P>0.05).
Intraoperative Ultrasound Energy Use
The mean ultrasound energy was significantly reduced by 21% in the Device group that underwent nuclear sectioning with the fragmentation device before starting phacoemulsification (9.6±5.2 CDE units) versus Control eyes that underwent traditional unassisted phaco (11.6±6.4 CDE units) (P<0.0001).
Intraoperative Complications
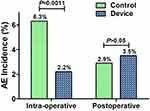
The rate of eyes experiencing any intraoperative AE or complication was significantly higher in Control eyes that underwent phacoemulsification without the lens fragmentation device than in Device eyes where cataracts were pre-sectioned using the miLOOP device (6.3% versus 2.2%, respectively; p=0.0011) (Table 2 and Figure 2). Posterior capsule rupture occurred in 2.5% of Control eyes versus 1.2% of eyes that underwent device-assisted lens prefragmentation (P>0.05). Anterior vitreous loss with unplanned vitrectomy was required in 1.0% of Control eyes versus 0.6% of Device eyes (P>0.05). A sulcus IOL was placed in 0.4% of Control eyes compared with 0.2% of Device eyes (P>0.05). Extracapsular cataract extraction was needed in 0.6% of Control eyes versus 0.0% of Device eyes (P>0.05).
 |
Table 2 Intraoperative Adverse Events |
Perioperative Corneal Changes
In the immediate postoperative period after phacoemulsification surgery, corneal edema of variable magnitude can occur, and likelihood increases with high-density cataracts.20 We noted a similar rate of corneal edema (Grade 3+) between Control and Device eyes (5.5% and 5.0% of eyes, respectively).
Postoperative AEs
The incidence of postoperative AEs with onset or observation occurring between postoperative discharge and follow-up was similar in Control (2.9%) and Device eyes (3.5%; P>0.05) (Table 3 and Figure 2). Lens fragment retention occurred in 1.8% of Control eyes and 0.8% of Device eyes (P>0.05), IOP was elevated (≥40mmHg requiring intervention) in 0.0% of Control eyes and 0.7% of Device eyes (P>0.05), and hypotony (≤5mmHg) was observed in 0.0% of Controls and 0.2% of Device eyes (P>0.05). The only postoperative AE with a significantly different incidence between Control and Loop eyes was corneal abrasion treated with a bandage contact lens, occurring in 0.0% of Controls and 1.1% of Device eyes (P=0.0218).
 |
Table 3 Postoperative Adverse Events |
Visual Acuity
Mean baseline BCVA values were worse in Controls (0.302±0.214 Decimal Units; 20/93 Snellen) than Device eyes (0.214±0.175 Decimal Units; 20/66 Snellen; P=0.0001) although the Device group had a significantly larger proportion of eyes with baseline BCVA worse than 20/400 (15.8% versus 10.3% in Controls; P=0.0118) (Table 1 and Figure 3). Postoperative BCVA was similar in Control eyes and Device eyes (Figure 3). Mean postoperative BCVA was 0.710±0.263 Decimal Units (20/28 Snellen; 0.146 logMAR) in Controls and 0.695±0.285 Decimal Units (20/27 Snellen; 0.161 logMAR) in Device eyes (P>0.05). Median postoperative BCVA values were similar at 20/25 Snellen in both Control and Device groups. The proportion of eyes with postoperative BCVA better than 20/30 Snellen was similar at 73.4% of Control eyes and 71.0% of Device eyes.
Discussion
Microinterventional lens fragmentation employs the single-use nitinol filament miLOOP device to efficiently disassemble very dense cataractous lenses into smaller and more manageable sections before ultrasound fragmentation by phacoemulsification.15,18 The initial fragmentation step of a very dense lens can be challenging, particularly in patients with ocular comorbidities and zonular weakness.10,11,14 While the rate of AEs in phacoemulsification cataract surgery is quite low, in this large, single-surgeon, non-randomized cohort, the lens fragmentation device was associated with a statistically significant reduction in the frequency of overall intraoperative AEs and complications, Serious intraoperative AEs such as posterior capsule rupture and unplanned anterior vitrectomy are more common with very dense cataracts. Posterior capsule rent, for example, occurs in 0.45‒3.6% of cataract surgeries performed by experienced surgeons,20 and the incidence of posterior capsule rupture or rent in the current study was similar and within this range in both study groups. The rates of all observed intraoperative AEs and complications were numerically lower in eyes that received loop-assisted lens disassembly before phacoemulsification, although rates of these individual events did not statistically differ between groups. Notably, four Control eyes required conversion to unplanned extracapsular cataract extraction compared to none of the eyes that underwent lens disassembly using the miLOOP device. Device-assisted lens prefragmentation of dense mature cataracts (mean WHO grade 3.5) has previously been associated with 35% reduction of CDE requirements in subsequent phacoemulsification in 101 subjects randomized to undergo miLOOP-assisted prefragmentation or not before phacoemulsification.16 Corneal endothelial cell density and central corneal thickness at postoperative 1 month in that study were similar between miLOOP-assisted phacoemulsification versus control eyes that received unassisted phacoemulsification.16 The comparatively lower subject number (53 miLOOP-assisted eyes and 48 Control eyes) in that study may have limited statistical power to detect differences in AE incidence.16 The current study evaluated a similar population of advanced cataracts. We speculate that the significantly lower incidence of Device eyes experiencing any intraoperative AE compared to Controls was associated, at least in part, with the 21% reduction in ultrasound energy required to phacoemulsify the microloop-prefragmented dense cataracts.
In the immediate postoperative period after phacoemulsification surgery, corneal edema of variable magnitude can occur, and likelihood increases with high-density cataracts.21 We noted a similar rate of clinically relevant corneal edema (Grade 3+) between Control and Device eyes (5.5% and 5.0% of eyes, respectively).
Postoperative complication rates were low and similar in both the traditional and device-assisted cataract surgery groups. Risk of increased lens fragment retention is increased in challenging cataract cases and in the setting of extreme myopia.22 Retained lens fragments were detected in 1.8% of Control and 0.8% of Device eyes. Cystoid macular edema was observed in a single Control eye and two Device eyes, identified by clinical complaint and verified by OCT. No cases of endophthalmitis were documented. Cataract surgery complications have a negative impact on patient outcomes, require additional time to treat, and may necessitate additional postoperative clinic visits.23 In a previous report, lens disassembly using the miLOOP device was accomplished in less than 3 minutes, and there was no difference in overall operating time in eyes that underwent prefragmentation versus those that did not.16 Total ultrasound energy use was also significantly reduced in that study, as we observed in the current study. The low and similar overall rate of postoperative AEs in Control and Device eyes supports the utility and safety of using the microfilament loop device as an adjunctive technique for pre-sectioning cataracts to reduce subsequent phaco CDE requirements. Thus, nuclear prefragmentation might mitigate CDE-related injury, and could be particularly useful for disassembling higher-risk dense brunescent cataracts.
Vision outcomes are a critical outcome measure of any cataract surgery investigation. It was encouraging that both mean and median postoperative BCVA measurements were similarly very good in both study groups (>70% of all subjects had postoperative 20/30 BCVA or better). Thus, adoption of the lens prefragmentation device before phacoemulsification appears to be a safe way to approach dense mature cataract disassembly during cataract surgery.
While strengths of this study include the consecutive case series design including a large number of eyes, limitations include the lack of randomization inherent to a retrospective assessment, only including a single surgeon, and relying on a semi-quantitative cataract scoring system instead of densitometric measurements. Future studies exploring the effects of cataract disassembly before phaco would benefit from a randomized controlled design with surgeons of varying experience levels and lens fragmentation styles, to confirm our observed reduction in intraoperative complications and to explore possible effects of using the device on operational efficiency and total procedural time. A larger cohort size suitable to perform pre-planned subgroup analyses with respect to subject age, gender, and additional comorbidities could make study findings generalizable to more diverse patient populations.24 The current study findings support prior observations that device-assisted lens prefragmentation is a safe method to facilitate the treatment of advanced dense cataracts, and the associated reduction in ultrasound energy required for phacoemulsification of the pre-segmented nucleus might have protective benefits for delicate intraocular structures. The prefragmentation device can be operated with one hand, has an uncomplicated learning curve, and might improve operational efficiency and patient outcomes.
In summary, microinterventional lens prefragmentation of advanced high-grade cataracts using the miLOOP device before conventional phacoemulsification is associated with lower ultrasound energy use and improved intraoperative safety than unassisted surgery. Cataract surgery assisted by the miLOOP system results in similarly acceptable postoperative complication rates and good BCVA functional outcomes as traditional phacoemulsification alone.
Prior Presentation
These data were presented, in part, at the American Academy of Ophthalmology Annual Meeting in Las Vegas, NV, USA, November 2020.
Data Sharing Statement
All datasets used in the construction of this manuscript are available from the corresponding author, upon reasonable request.
Acknowledgment
The authors thank Matthew Silverman PhD (Biomedical Publishing Solutions, Wakulla Springs, FL) for expert statistical and medical editing assistance.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
This study was funded by Carl Zeiss Meditec Cataract Technology, Inc., Reno, NV. The sponsor had no role in the design, conduct, or interpretation of this research.
Disclosure
EHH reports grants from Zeiss, during the conduct of the study, and has received consulting fees from Carl Zeiss Meditec Cataract Technology, Inc. RDP reports personal fees from Carl Zeiss Meditec AG, during the conduct of the study; personal fees from Iantrek, Inc., outside the submitted work; and has received consulting fees from Carl Zeiss Meditec Cataract Technology, Inc. The authors report no other potential conflicts of interest in relation to this work, financial or otherwise.
References
1. Schein OD, Cassard SD, Tielsch JM, Gower EW. Cataract surgery among Medicare beneficiaries. Ophthalmic Epidemiol. 2012;19(5):
2. Kelman CD. Phaco-emulsification and aspiration. A new technique of cataract removal. A preliminary report. Am J Ophthalmol. 1967;64(1):
3. Lam D, Rao SK, Ratra V, et al. Cataract. Nat Rev Dis Primers. 2015;11(1):15014. doi:10.1038/nrdp.2015.144
4. French DD, Margo CE, Behrens JJ, Greenberg PB. Rates of routine cataract surgery among Medicare beneficiaries. JAMA Ophthalmol. 2017;135(2):163–165. doi:10.1001/jamaophthalmol.2016.5174
5. Stein JD, Grossman DS, Mundy KM, Sugar A, Sloan FA. Severe adverse events after cataract surgery among Medicare beneficiaries. Ophthalmology. 2011;118(9):
6. Narendran N, Jaycock P, Johnston RL, et al. The Cataract National Dataset electronic multicentre audit of 55,567 operations: risk stratification for posterior capsule rupture and vitreous loss. Eye. 2009;23(1):
7. Ergun ŞB, Kocamış Sİ, Çakmak HB, Çağıl N. The evaluation of the risk factors for capsular complications in phacoemulsification. Int Ophthalmol. 2018;38(5):1851–1861. doi:10.1007/s10792-017-0667-3
8. Jaggernath J, Gogate P, Moodley V, Naidoo KS. Comparison of cataract surgery techniques: safety, efficacy, and cost-effectiveness. Eur J Ophthalmol. 2014;24(4):
9. Mailu EW, Virendrakumar B, Bechange S, Jolley E, Schmidt E. Factors associated with the uptake of cataract surgery and interventions to improve uptake in low- and middle-income countries: a systematic review. PLoS One. 2020;15(7):e0235699. doi:10.1371/journal.pone.0235699
10. Crispim J, Jung LS, Paz PL, Allemann N, Schor P. The surgical challenges dense brunescent cataracts present. Expert Rev Ophthalmol. 2015;10(1):
11. Hayashi K, Hayashi H, Nakao F, Hayashi F. Risk factors for corneal endothelial injury during phacoemulsification. J Cataract Refract Surg. 1996;22(8):
12. Bourne RR, Minassian DC, Dart JK, Rosen P, Kaushal S, Wingate N. Effect of cataract surgery on the corneal endothelium: modern phacoemulsification compared with extracapsular cataract surgery. Ophthalmology. 2004;111(4):
13. McKay KM, Borkar DS, Moustafa GA, Haviland MJ, Kloek CE; PCIOL Study Group. Clinical factors affecting operating room utilization in cataract surgery: results from the PCIOL study. J Cataract Refract Surg. 2020;46(1):
14. Mansour AM, Antonios RS, Ahmed IIK. Central cortical cleanup and zonular deficiency. Clin Ophthalmol. 2016;10:1919–1923. doi:10.2147/OPTH.S116314
15. Ianchulev T, Chang DF, Koo E, MacDonald S. Microinterventional endocapsular nucleus disassembly for phacoemulsification-free full-thickness fragmentation. J Cataract Refract Surg. 2018;44(8):
16. Ianchulev T, Chang DF, Koo E, et al. Microinterventional endocapsular nucleus disassembly: novel technique and results of first-in-human randomised controlled study. Br J Ophthalmol. 2019;103(2):
17. Roper GJ, Hoffer KJ, Pamnani RD. Effect of microinterventional endocapsular nucleus disassembly using centripetal loop fragmentation on refractive outcomes after cataract surgery. J Cataract Refract Surg. 2020;46(11):
18. Wiley WF, Shamik B, Hercules D L. Comparative study of phacoemulsification parameters with and without nitinol filament nuclear disassembly. J Cataract Refract Surg. 2021;47(8):
19. Thylefors B, Chylack LT Jr, Konyama K, et al.; WHO Cataract Grading Group. A simplified cataract grading system. Ophthalmic Epidemiol. 2002;9(2):
20. Chakrabarti A, Nazm N. Posterior capsular rent: prevention and management. Indian J Ophthalmol. 2017;65(12):1359–1369. doi:10.4103/ijo.IJO_1057_17
21. Sharma N, Singhal D, Nair SP, Sahay P, Sreeshankar SS, Maharana PK. Corneal edema after phacoemulsification. Indian J Ophthalmol. 2017;65(12):
22. Matarazzo F, Phylactou M, Aiello F, Gallo Afflitto G, Yue Sim S, Maurino V. Incidence and complications of retained lens fragment in the anterior chamber after uneventful cataract surgery in a United Kingdom tertiary center. J Cataract Refract Surg. 2021;47(8):
23. Chan E, Mahroo OA, Spalton DJ. Complications of cataract surgery. Clin Exp Optom. 2010;93(6):
24. Lin IH, Lee CY, Chen JT, et al. Predisposing factors for severe complications after cataract surgery: a nationwide population-based Study. J Clin Med. 2021;10(15):3336. doi:10.3390/jcm10153336
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.