Back to Journals » Therapeutics and Clinical Risk Management » Volume 17
Comparative Results Between “Epi-Off” Accelerated and “Epi-Off” Standard Corneal Collagen Crosslinking-UVA in Progressive Keratoconus – 7 Years of Follow-Up
Authors Nicula CA , Rednik AM , Nicula AP, Bulboaca AE , Nicula D , Horvath KU
Received 24 May 2021
Accepted for publication 14 August 2021
Published 7 September 2021 Volume 2021:17 Pages 975—988
DOI https://doi.org/10.2147/TCRM.S321410
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Garry Walsh
Cristina Ariadna Nicula,1,2 Anca Maria Rednik,3 Ariadna Patricia Nicula,4 Adriana Elena Bulboaca,5 Dorin Nicula,2 Karin Ursula Horvath6
1Department of Ophthalmology, Medicine and Pharmacy University “Iuliu Hațieganu”, Cluj-Napoca, Romania; 2Oculens Clinic, Cluj-Napoca, Romania; 3Eye County Hospital, Department of Ophthalmology, Cluj-Napoca, Romania; 4Eye County Hospital, Department of Ophthalmology, Arad, Romania; 5Department of Pathophysiology, Medicine and Pharmacy University “Iuliu Hațieganu”, Cluj Napoca, Romania; 6Department of Ophthalmology, Medicine and Pharmacy Science and Technology University “George Emil Palade”, Târgu Mureș, Romania
Correspondence: Cristina Ariadna Nicula
Department of Ophthalmology, Medicine and Pharmacy University “Iuliu Hațieganu”, Cluj-Napoca, Romania
Tel +011 40-722849575
Email [email protected]
Purpose: The purpose of the present study was to assess the long-term efficiency and safety of the “epi-off” accelerated CXL (9 mW/cm2 for 10 minutes) in comparison to the standard “epi-off” CXL (3 mW/cm2 for 30 minutes) in terms of topographical and keratometric parameters, refractive data and visual outcomes at 7 years of follow-up, in progressive keratoconus.
Material and Method: A retrospective and comparative study was performed. A total of 183 eyes from 183 patients with documented progressive keratoconus were included in the study. The patients were divided in two groups: 93 eyes from 93 patients underwent “epi-off” standard cross-linking technique (3 mW/cm2 for 30 minutes) (S-CXL group) and 90 eyes from 90 patients underwent accelerated “epi-off” corneal CXL technique (9 mW/cm2 for 10 minutes) (A-CXL group).
Results: Improvements in uncorrected distance visual acuity (UDVA) were statistically significant compared to baseline values in both groups at each time-point visit (p=0.0421 at 1 year, p=0.0411 at 7 years for A-CXL and p=0.0375 at 1 year, p=0.0389 at 7 years for S-CXL). At 7 years there was a statistically significant increase in CDVA (p=0.039 in the A-CXL group and p=0.0343 in the S-CXL group at 7 years). Statistically significant reduction was noticed in Ksteep (p=0.0411 in A-CXL group and p=0.0224 in S-CXL group), Kflat (p=0.0198 in A-CXL group and p=0.008 in S-CXL group), K mean (p=0.0106 in A-CXL group and p=0.0193 in S-CXL group) and Kmax (p=0.0413 in A-CXL group and p=0.054 in S-CXL group) at 7 years, compared to baseline values, in both groups, but without any statistically difference between the two procedures, at all time-point visits (p> 0.05).
Conclusion: The long-term outcomes of “epi-off” accelerated corneal collagen crosslinking-UVA (9 mW/cm2 for 10 minutes) are similar to standard “epi-off” corneal collagen crosslinking procedure in the treatment of progressive keratoconus.
Keywords: progression, keratoconus, accelerated cross-linking, standard cross-linking
Introduction
Keratoconus (KCN) is a bilateral1,2 and asymmetric3,4 corneal ectatic dystrophy, appearing in the second decade of life. It is defined by the thinning of the cornea, inducing an irregular astigmatism, corneal protrusion, biomechanical weakening and decreased visual acuity.5 Oftentimes, the onset is at puberty with a fast and severe progression. The corneal collagen cross-linking UVA (CXL) procedure was described for the first time in 1999 by Spoerl and Seiler6 and was introduced in order to stop the progression of keratoconus. The procedure combines the action of riboflavin (photosensitizing agent) with ultraviolet A light (UVA) in order to increase the strength and rigidity of the cornea.7 Several studies revealed the long-term outcomes regarding the efficacy and safety of the “epi-off” standard CXL (Dresden protocol) using 30 min of 3 mW/cm2 irradiation of UVA light.8–14 Different types of riboflavin substances were used since the CXL procedure was introduced in practice. A notable progress regarding riboflavin was the use of dextran-free substance as a consequence of the hyperoncotic effect of dextran which was associated with intraoperative decrease of corneal thickness and risk of stromal corneal wound-related complications.15 The use of hydroxypropyl methylcellulose rather than dextran has been demonstrated to prevent the corneal thickness reduction.16 Furthermore, the iontophoresis-assisted riboflavin utilized in the iontophoresis CXL was introduced in order to minimize the imbibition time.17 Since 2020, the SafeCross® riboflavin solution 0.25% has been used for thin corneas (under 400 μm) and simultaneously combined the technique of CXL and trans-epithelial Excimer laser central corneal aberrometric remodeling.18 A recent preliminary study revealed the efficacy and safety of this solution in addition to the potential prevention of endothelium-toxic damage.19
Up to now, the CXL procedure is the only method to stop KCN progression. In order to reduce the time of the procedure, to improve convenience and comfort of the patients, the accelerated “epi-off” CXL technique performed in a pulsed or a continuous manner was introduced.20,21 This novel protocol was acquired from the Bunsen–Roscoe law of reciprocity of photochemistry.22 In this regard, the accelerated procedure uses higher irradiation energy (9 mW/cm2) in a shorter time (10 minutes) with a cumulative irradiation dose of 5.4 J/cm2 and brief time for the instillation of riboflavin drops.23 Another type of A-CXL procedure using 15 mW pulsed-light CXL was shown to have efficacy and safety in the treatment of progressive KCN.24 Pulsing of the UV light during CXL theoretically restarts the photodynamic reaction, therefore achieving an additional oxygen concentration, permitting more singlet oxygen release for CXL.25 Lately, a new combined procedure of selective transepithelial ablation with simultaneous accelerated CXL for corneal regularization of KCN (the STARE-X protocol) was introduced.26 The procedure was demonstrated to have efficacy in stopping the progression of KCN, enhancing corneal regularity in a safer manner and improving visual outcomes and corneal aberrations.26
There are few studies demonstrating the efficacy and safety of “epi-off” accelerated CXL during a period of more than 2 years of follow-up.27–32 Furthermore, there are only some comparative studies with 12 months or greater follow-up33,34 especially in comparison to standard CXL.34–36 Thus, there is a lack of clinical studies data on long-term outcomes of accelerated “epi-off” CXL (9 mW/cm2, 10 min) versus standard “epi-off” CXL.
The purpose of the present study was to assess the long-term efficiency and safety of the “epi-off” accelerated CXL (9 mW/cm2 for 10 minutes) in comparison to the standard “epi-off” CXL (3 mW/cm2 for 30 minutes) in terms of topographical and keratometric parameters, refractive data and visual outcomes at 7 years of follow-up, in progressive keratoconus.
Materials and Methods
A retrospective, comparative, single-center study was conducted at the Oculens Clinic from Cluj-Napoca, Romania, was approved by the ethical committee of the clinic (No. 2/2021) and adhered to the principles proposed by the Declaration of Helsinki. Due to the retrospective nature of the study the ethical committee of the clinic ruled that no consent for participation was necessary. A total of 183 eyes from 183 patients with documented progressive KCN were included in the study. The patients were divided in 2 groups: the accelerated “epi-off” crosslinking group (9 mW/cm2 for 10 minutes) (A-CXL) included 90 eyes from 90 patients, and the standard “epi-off” crosslinking (3 mW/cm2 for 30 minutes) (S-CXL) group included 93 eyes from 93 patients. The patients were consecutively included in the study. The fellow eye was not included in the study, being diagnosed with fruste keratoconus or advanced keratoconus, thus not meeting the inclusion criteria. The patients underwent surgery between January 2012 and January 2014.
The inclusion criteria were: clinical KCN in patients older than 18 years, documented KCN progression and a corneal thickness more than 400 μm. A worsening in the last three to six months of follow-up was considered as progression criteria: myopia and astigmatism changes >3 D, a mean change of central K value >1.5 D in three consecutive corneal topographic measurements, increase in the maximum keratometry (Kmax) in topography of more than 1 D or a mean decrease in central corneal thickness >5% in three consecutive tomographic measurements.37 The “Belin ABCD” grading system incorporated in the Oculus Pentacam topographer (Pentacam® HR Premium, Oculus Optikgerate GmbH, Wetzlar, Germany) was used to establish the stage of KCN, taking into account the anterior (“A”) and posterior radius of curvature (“B”) from a 3.0 mm zone centered on the thinnest point, “C” for the corneal thickness at the thinnest point and “D” for best corrected distance visual acuity.38,39 The exclusion criteria were: cases of KCN with corneal thickness under 400 μm, stromal scarring or acute hydrops, Vogt striae, history of herpetic keratitis, dry eye syndrome, cornea guttata, autoimmune diseases, breast feeding or pregnancy. Before the procedure all the patients underwent a complete ocular exam consisting of the uncorrected distance visual acuity (UDVA) and best corrected distance visual acuity (CDVA), refractometry (manifest and cycloplegic) (Topcon auto refracto-kerato-meter, KR 8900), keratometry (Pentacam® HR Premium, Oculus Optikgerate GmbH, Wetzlar, Germany), slit lamp exam (Slit Lamp BX 900, Haag-Streit AG), eye fundus examination, intraocular pressure measured by applanation tonometry, corneal topography and tomography (Pentacam® HR Premium, Oculus Optikgerate GmbH, Wetzlar, Germany), endothelial cell counting (Konan SP-9000, Hyogo, Japan) and corneal thickness (Pentacam® HR Premium, Oculus Optikgerate GmbH, Wetzlar, Germany). For scientific purposes, the visual acuity was measured as logarithm of minimum angle of resolution. Patients were asked to interrupt the wear of contact lenses 2 weeks before the ocular assessment or surgery. All patients signed the specific informed consent before “epi-off” accelerated or standard CXL procedure after a discussion regarding the steps of the procedure, effect of the technique upon the cornea and possible postoperative complications.
Before the surgery, all patients received a drop of 2% pilocarpine solution in the inferior conjunctival fornix in order to prevent a possible injury to the lens or the retina. After that a topical analgesic (alkaline solution), 3–4 drops, was instilled 15–20 minutes prior to the procedure. The “epi-off” CXL procedures were performed in the operating room [40 Mazzotta 2021–58]. After the mechanical corneal epithelial removal with a spatula, on an optical zone of 9.0 mm diameter in the center of the cornea, riboflavin 0.1%–dextran 20% solution was instilled every 2 minutes for 20 minutes in the “epi-off” accelerated procedure and for 30 minutes in the “epi-off” standard technique. Afterwards, the eyes were exposed to ultraviolet rays (UVA) 370 nm under a power of 9 mW/cm2 for 10 minutes in the “epi-off” accelerated procedure and under a power of 3 mW/ cm2 for 30 minutes in the “epi-off” standard technique. During irradiation, riboflavin 0.1%–dextran 20% solution was instilled every 2 minutes. Finally, the eyes were washed with saline solution, and a drop of antibiotic and steroids (Tobradex, Alcon Novartis, Dallas Worth, USA) was instilled. At the end of the procedure, a bandage contact lens was placed on the cornea for three days, until the cornea was totally healed. After the procedure, the patients received Tobradex (Alcon Novartis, Dallas Worth, USA) 5 times/day for one month, and a tapered dose and artificial tears for 6 months. After the procedure, all patients from both groups were examined at 24 hours, 3 days, 1, 6, 12 months and each year for 7 years. UDVA, CDVA, keratometric measurements (Ksteep, Kflat, Kmax) at Oculus Pentacam, ocular refraction exam (spherical equivalent), slit lamp examination, corneal tomography (topographic/tomographic indices, including the topographical cylinder) were measured at all time points. At one month after CXL procedure, anterior segment ocular coherence tomography (AS-OCT) analysis (Triton, Topcon, Japan) was performed in order to establish the penetration of the treatment, documenting the presence of the demarcation line. During the follow-up period, the efficacy of the CXL procedures in both groups was assessed by the Kmax values in addition to the topographic/tomographic parameters (Belin/Ambrosio Enhanced Ectasia Display [BAD_D], Ambrosio relational thickness [ART], thinnest point of the cornea [TP], index vertical asymmetry [IVA], index of surface variance [ISV], index of height asymmetry [IHA], index of height decentration [IHD], root mean square values [RMS] total) and the Belin ABCD progression display.41 Progression after CXL procedure was considered if two of the following criteria were present: increase of “A” value, increase of “B” value or decrease of minimum corneal thickness evaluated with the ABCD progression display from Oculus Pentacam.42
Statistical Analysis
Statistical analysis was performed using the PRISM 6.0 GraphPad software (GraphPad Holdings, LLC, California, USA). The data were described as mean±SD. All data passed the normality test. The comparison between the baseline parameters at each time point in both groups was done using an unpaired t-test. The statistical differences between baseline and post-CXL parameters in both groups were established with a paired t-test. For the entire research, a p value of <0.05 was considered statistically significant.
Results
A total of 183 eyes from 183 patients were included in the study, divided in 2 groups, as mentioned above. In Table 1 the baseline characteristics are presented, including age, gender, KCN stages, the presence of atopy, Ksteep, Kflat, Kmax and spherical equivalent.
 |
Table 1 Baseline Characteristics of the Patients |
Corneal Curvature Change
Ksteep decreased by mean 1 D and 1.64 D, respectively, in A-CXL group and S-CXL group at 7 years of follow-up. Kflat decreased by mean 0.88 D and 1.37 D, respectively, in A-CXL group and S-CXL group at 7 years follow-up. In Figures 1 and 2 the trend of change in Ksteep and Kflat, respectively, over time, in both groups is shown. In comparison to the baseline values, the decrease of Ksteep was statistically significant at all time-point visits in both groups (Table 2).
 |
Table 2 P values Comparing Each Time Point with Baseline |
 |
Figure 1 Evolution of Ksteep in both groups. |
 |
Figure 2 Evolution of Kflat in both groups. |
 |
Figure 3 Evolution of Kavg in both groups. |
There was no statistically significant difference between the two groups at each time-point visit (p>0.05) (Table 3).
 |
Table 3 Ksteep, Kflat, Kavg and Kmax at Each Time-Point Visit |
In comparison with the baseline values, the decrease of Kflat was statistically significant at all time-point visits in both groups (Table 2). There was no statistically significant difference between the two groups at each time-point visit (p>0.05) (Table 3).
Kaverage (Kavg) decreased statistically significantly at 7 years' follow-up comparative to baseline values by a mean of 0.91 in the A-CXL group and 1.74 in S-CXL group (Tables 2 and 3).
Kmax decreased by mean 2.42 in A-CXL group and 2.23 in S-CXL group. The trend of change in Kmax over the follow-up period is shown in Figure 4. The decrease of Kmax was statistically significant in comparison to the baseline value at all time points in both groups (Table 2), but there were no statistically significant differences between A-CXL group and S-CXL group at each time-point visit (p>0.05) (Table 3).
 |
Figure 4 Evolution of Kmax in both groups. |
During the follow-up period, keratometric progression was present in 7 eyes (3.83%): 4 eyes from A-CXL group and 3 eyes from S-CXL group. All patients developed allergic conjunctivitis with consecutive rubbing of the eye. In all these cases, we observed a progression of Kmax after 2–3 years, with a loss in CDVA of 1–2 lines. No re-treatment was performed in any of the cases.
In the A-CXL group, the cylinder decreased statistically significantly from baseline −4.34±2.12 D to −3.7±2.12 D at 7 years (p=0.0311). In the S-CXL group the cylinder decreased from baseline −3.72±2.16 D to −2.98±2.4 D at 7 years (p=0.0277) (Table 4). There was no statistically significant difference between the two groups at each time-point visit (p>0.05).
 |
Table 4 The Evolution of Cylinder and Spherical Equivalent from Baseline to 7 Year Follow-Up Time Point in Both Groups |
In the A-CXL group, the mean SE decreased statistically significantly from −5.55±3.88 D to −4.77±3.44 D at 7 years (p=0.015). In the S-CXL group, the SE decreased statistically significantly from −4.95±3.65 D to −4.21±3.34 D at 7 years (p=0.0197). Regarding the SE, we did not notice any statistically significant difference between the two groups at each time-point visit (p>0.05).
The evolution of cylinder and SE is shown in Table 4.
Corneal Topographical Indices Change
Topographical indices such as thinnest corneal point (TP), corneal volume (CV), index vertical asymmetry (IVA), index of surface variance (ISV), index of height asymmetry (IHA), index of height decentration (IHD), Belin/Ambrosio enhanced ectasia display (BAD_D) and Ambrosio relational thickness (ART Max) were statistically significantly decreased compared to baseline values at all visits, in both groups (Table 5). There was no statistically significant difference between the two groups at each time-point visit (p>0.05). The changes in total high ocular aberration (HOA) and root mean square values (RMS) decreased statistically significantly at 7 years, comparative to baseline values, in both groups (p=0.0309 in A-CXL group and p=0.02 in S-CXL group). No statistically significant difference was present between the two groups (p>0.05 at each time point) (Table 5).
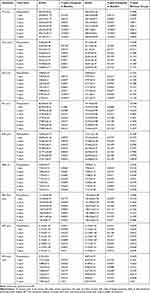 |
Table 5 Topographical and Tomographical Parameters Evolution from Baseline to the Final Visit |
Visual Acuity Change
Improvements in UDVA were statistically significant compared to baseline values in both groups at each time-point visit (p=0.0421 at 1 year, p=0.0389 at 7 years) (Figure 5).
 |
Figure 5 Evolution of UDVA in both groups. |
Improvements in CDVA were statistically significant compared to baseline values in both groups at each time-point visit (p=0.045 at 1 year, 0.0343 at 7 years for S-CXL) (Figure 6).
 |
Figure 6 Evolution of CDVA in both groups. |
The improvements in CDVA were not statistically significantly different between the two groups (p=0.644 at 1 year, p=0.589 at 2 years, p=0.3371 at 3 years, p=0.6621 at 4 years, p=0.0711 at 5 years, p=0.674 at 6 years, p=0.0575 at 7 years).
Corneal Thickness Change
There was no statistically significant difference in the postoperative central corneal thickness at 7 years of follow-up in the two groups (p=0.12).
Demarcation Line
The demarcation line was indentified at a mean depth of 203±12.03 μm in the A-CXL group and 211±13.2 μm in S-CXL group.
There were no cases of corneal melting, persistent epithelial defect, corneal decompensation or other adverse events. Transitory corneal haze was present in the majority of cases (75.3%) but disappeared after 6 months after both procedures of CXL.
Discussion
Several long-term studies have established the efficacy and safety of the S-CXL UVA procedure in the treatment of KCN.8,13,14,43 Moreover, Elmassry et al44 in a long-term study showed that the CXL procedure is an effective method to prevent the progression of corneal ectasia, regardless of the type of ectasia. The idea of A-CXL was introduced by Schumacher et al45 in an experimental animal model, demonstrating the same efficacy in terms of corneal stiffening between the Dresden protocol (S-CXL) and A-CXL. By introducing the “epi-off” A-CXL, the procedure duration was shortened and the irradiation energy rose. There are comparative studies demonstrating similar results between the S-CXL and A-CXL.33,46–48
Our study revealed a statistically significant reduction of Kmax (2.23 in S-CXL and 2.42 in A-CXL), Kflat (1.37 in S-CXL and 0.88 in A-CXL), Ksteep (1.64 in S-CXL and 1 in A-CXL) and Kmean (1.74 in S-CXL and 0.91 in A-CXL) in comparison to the baseline values at 7 years of follow-up in both groups, without any statistically significant difference between the two groups (p>0.05 at each time point). Similar results were demonstrated by Ting et al49 reporting a significant decrease of Kmax (−1.68), Ksteep (−0.64) and Kmean (−0.50) at 24 months of follow-up. In a comparative study between A-CXL (18 mW/cm2) and S-CXL, Hashemi et al50 demonstrated that the decrease in Kmax values was not statistically significantly different between the two groups. Furthermore, Sarac et al48 and Vounotripidis et al51 revealed important improvement of Kmax from −0.3 to –1.4 D after A-CXL. Moreover, Males et al47 revealed a statistically significant decrease in Ksteep values after both A-CXL (51.19±6.01 to 49.95±4.90) and S-CXL (48.50±2.92 to 47.89±3.62), with no difference between the groups at 30 months after treatment. Similarly, in a prospective, interventional study, Mazzotta et al24 compared the outcomes of “epi-off” pulsed A-CXL at 30 mW/cm2 UVA exposure for 8 min versus continuous-light A-CXL at 30 mW/cm2 UVA exposure for 4 min. Their conclusion was that the “epi-off” pulsed A-CXL offered better results in terms of keratometric values, topographical indices and stromal penetration by optimizing oxygen availability during the procedure. Moreover, Elbaz et al52 showed that after A-CXL (9 mW/cm2, 10 min) stable keratometric values were achieved. Furthermore, Alnawaiseh et al53 revealed stable CDVA and decrease of keratometric values after the same procedure after 21.7 months of follow-up. In a comparative study of high-fluence A-CXL (7 mW/cm2 UVA irradiation) versus S-CXL, Kanellopoulos et al54 demonstrated a statistically significant decrease in Ksteep and SE and an increase of CDVA in both procedures. Conflicting studies46,51 showed a non-significant improvement of Kmean following A-CXL after 3 years of follow-up. Cummings et al34 revealed a statistically significant corneal flattening after A-CXL in comparison to the S-CXL at 1 year follow-up. Recent findings by Chan et al55 revealed that A-CXL gives better keratometry flattening, being more efficient in advanced KCN compared to mild or moderate stages. Chow et al35 demonstrated that S-CXL (18 mW/cm2) gave a better corneal flattening effect compared to the A-CXL procedure.
Our findings showed that the SE decreased statistically significantly from baseline up to 7-year follow-up in both groups (p<0.05), but with no statistically significant difference between the two procedures at each time point (p>0.05). Similar results were reported by Males et al,47 noting that modification in SE values were better in the A-CXL group compared to the S-CXL, but not statistically significant, indicating that both CXL procedures were efficient.
The present study revealed a reduction in the cylinder value of 0.34 D at 1 year and 0.74 D at 7 years in the A-CXL group and of 0.08 D at 1 year and 0.64 D at 7 years in the S-CXL group. Similarly, Ting et al49 showed that the astigmatism decreased from baseline, but it was not statistically significant at 2 years' follow-up after A-CXL (p>0.05).
Our findings revealed a statistically significant CDVA improvement at 7 years compared to baseline in both groups (p<0.05). Similarly, several studies showed no statistically significant difference in the improvement of CDVA between A-CXL and S-CXL.47,48,51 Moreover, Ng et al33 revealed significant visual improvement after S-CXL compared with the A-CXL procedure. Similar results were reported by Ting et al49 concluding that the final CDVA was associated with lower baseline CDVA (p=0.002) and greater Kmax (p=0.018) at baseline. Moreover, Hashemi et al11 and Wittig-Silva et al10 achieved an increase of the CDVA of 0.12 logMAR at 5-year postoperative and 0.09 logMAR at 3-year postoperative after S-CXL procedure. Moreover, there are studies56,57 which reported better vision improvement after CXL when the preoperative CDVA was under 0.3 logMAR value. Kanellopoulos et al54 showed an improvement in CDVA in both groups of high-fluence A-CXL (7 mW/cm2) and S-CXL group at 6 months of follow-up.
Our study revealed a statistically significant reduction in the topographical/tomographical parameters such as TP, corneal volume, IHA, ISV, ISA, IHD, BAD_D and ART Max from baseline values in both groups, without any statistically significant difference between the two groups at each time point (p<0.05). Greenstein et al56 noted that HOAs, total coma, 3rd-order coma, trefoil and spherical aberration decreased statistically significantly at 1 year after CXL compared with the control group (p=0.01). The same author demonstrated that modifications in HOAs were not statistically associated with an improvement in visual outcomes.56 Furthermore, Shetty et al36 demonstrated improvement in topographic parameters in A-CXL and S-CXL after 12 months of follow-up. Our findings showed that RMS total was not statistically significantly different between the two groups at all time points. Kang et al58 demonstrated that, after A-CXL with high UVA energy dose (7.2 J/cm2) particularly, total root mean square and higher order aberrations improved at 12 to 24 months after CXL.
Our findings revealed that the demarcation line, as an efficacy parameter, was localized at 203±12.03 μm in the A-CXL group and at 211±13.2 μm in the S-CXL group. Kymionis et al59 noted that the location of the demarcation line using the AS-OCT was at 300.67±41.56 μm (range, 240 to 385 μm) and using confocal microscopy was at 306.22±51.54 μm (range, 245 to 417 μm) after high-intensity (18 mW/cm2) UVA irradiation for a 5-minute collagen CXL. Furthermore, Shetty et al36 measured the depth of the demarcation line after four protocols of CXL. They demonstrated that a deeper demarcation line was present in the 3 mW/cm2 and 9 mW/cm2 groups and an incomplete one was noted in the higher-intensity CXL groups. Moreover, recently Mazzotta et al40 revealed that the demarcation line at the 1st postoperative month was at a depth of 332.6 ± 23.6 μm in the overall study cohorts after A-CXL procedure.
The present paper showed a progression of the KCN after CXL in 3.84% of cases. Vinciquera et al42 reported 7.4% of rate failure after S-CXL at up to 13 years of follow-up, using a combined progression system that includes anterior and posterior curvature and with thickness map together. Thirteen eyes (8.33%) of 8 patients had a Kmax progression of 1 D within the 2nd–3rd year follow-up visits, returning to baseline value after 30±6 months. All patients with such progression were affected with severe allergic papillary conjunctivitis. No re-treatment was performed in the entire 5-year follow-up period.40
A limitation of the present study is that it does not compare the biomechanical parameters between the two groups, although it has a long-term follow-up period and includes a large sample of patients. This study meant to show the stability of keratometric parameters, visual outcomes and topographical/tomographical indices after A-CXL in comparison with the S-CXL procedure at 7 years of follow-up. To our knowledge, this is the first study conducted in Romania, with such a long period of follow-up.
Conclusions
The long-term outcome of “epi-off” accelerated corneal collagen crosslinking-UVA (9 mW/cm2 for 10 minutes) is similar to standard “epi-off” corneal collagen crosslinking procedure at 7 years follow-up, being an efficient and safe procedure in the treatment of progressive keratoconus.
Author Contributions
Cristina Ariadna Nicula and Dorin Nicula carried out the surgical procedures and were involved in writing the manuscript and the design. Adriana Elena Bulboacă, Karin Ursula Horvath and Anca Maria Rednik were responsible for methodology, data collection and review process. Ariadna Patricia Nicula and Anca Maria Rednik carried out the statistical analysis and contributed to the design of the paper. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, aged to the submitted journal, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there are no conflicts of interest regarding the publication of this article.
References
1. Zadnik K, Barr JT, Gordon MO, Edrington TB; CLEK study Group. Biomicroscopic signs and disease severity in keratoconus. Cornea. 1996;15:139–146.
2. Kennedy RH, Bourne WM, Gyer JA. A 48-year clinical and epidemiologic study of keratoconus. A J Ophthalmol. 1986;101:267–273.
3. Zadnik K, Steger May K, Fink BA, et al. Between-eye asymmetry in keratoconus. Cornea. 2002;21:671–679.
4. Chopra I, Jain AK. Between-eye asymmetry in keratoconus in an Indian population. Clin Exp Optom. 2005;88:146–152.
5. Rabinowits YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319.
6. Spoerl E, Seiler T. Techniques for stiffening the cornea. J Refract Surg. 1999;15(6):711–713.
7. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620–627.
8. Caporossi A, Mazzotta C, Baiocchi S, Caporossi T. Long-term results of riboflavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy: the Siena eye cross study. Am J Ophthalmol. 2010;149:585–593.
9. Mazzotta C, Traversi C, Baiocchi S, et al. Corneal collagen cross-linking with riboflavin and ultraviolet A light for pediatric keratoconus: ten-year results. Cornea. 2018;37:560–566.
10. Wittig-Silva C, Chan E, Islam FM, Wu T, Whiting M, Snibson GR. A randomized, controlled trial of corneal collagen crosslinking in progressive keratoconus: three-year results. Ophthalmology. 2014;121:812–821.
11. Hashemi H, Seyedian MA, Miraftab M, Fotouhi A, Asgari S. Corneal collagen cross-linking with riboflavin and ultraviolet A irradiation for keratoconus: long-term results. Ophthalmology. 2013;120:1515–1520.
12. Hersh PS, Stulting RD, Muller D, et al. United States multicenter clinical trial of corneal collagen cross-linking for keratoconus treatment. Ophthalmology. 2017;124(9):1259–1270.
13. Raiskup F, Theuring A, Pillunat LE, et al. Corneal collagen crosslinking with riboflavin and ultraviolet- a light in progressive keratoconus: ten-year results. J Cataract Refract Surg. 2015;41:41–46.
14. Nicula C, Pop R, Rednik AM, Nicula D. 10-year results of standard cross-linking in patients with progressive keratoconus in Romania. J Ophthalmol. 2019;2019:8285649.
15. Mazzotta C, Caragiuli S. Intraoperative corneal thickness measurement by optical coherence tomography in keratoconic patients undergoing corneal collagen cross-linking. Am J Ophthalmol. 2014;157:1156–1162.
16. Mazzotta C, Baiocchi S, Caporossi T, Caragiuli S, Paradiso AL, Caporossi A. Riboflavin 0.1% for the treatment of keratoconus. Expert Opinion Orphan Drugs. 2013;3:235–240.
17. Mastropasqua L, Lanzini M, Curcio C, et al. Structural modifications and tissue response after standard epi-off and iontophoretic corneal cross-linking with different irradiation procedures. Invest Ophthalmol Vis Sci. 2014;55:2526–2533.
18. Kanelloupoulos AJ. Ten-year outcomes of progressive keratoconus management with the Athens protocol (Topography-Guided partial refraction PRK combined with CXL). J Refract Surg. 2019;35:478–483.
19. Mazzotta C, Ferrise M. Chemically-boosted corneal cross-linking for the treatment of keratoconus through a riboflavin 0.25% optimized solution with high superoxide anion release. J Clin Med. 2021;10(6):1324.
20. Krueger PR, Herekar S, Spoerl E. First proposed efficacy study of high versus standard irradiance and fractionated riboflavin/ultraviolet a cross-linking with equivalent energy exposure. Eye Contact Lens. 2014;40(6):353–357.
21. Mazzotta C, Traversi C, Paradiso AL, Latronico ME, Rechichi M. Pulsed light accelerated crosslinking versus continuous accelerated crosslinking: one–year results. J Ophthalmol. 2014;2014:604731.
22. Briendly GS. The Bunsen-Roscoe law for the human eye at very short durations. J Physiol. 1952;118(1):135–139.
23. Perez-Straziota C, Gaster R, Rabinowitz Y. Corneal collagen crosslinking for pediatric keratoconus review. Cornea. 2018;37(6):802–809.
24. Mazzotta C, Baiocchi S, Bagaglia S, Fruschelli M, Meduri A, Rechichi M. Accelerated 15 mW pulsed-light crosslinking to treat progressive keratoconus: two-year clinical results. J Cataract Refract Surg. 2017;43(8):1081–1088.
25. Mazzotta C, Traversi C, Caragiuli S, Rechichi M. Pulsed vs continuous light accelerated corneal collagen cross-linking: in vivo qualitative investigation by confocal microscopy and corneal OCT. Eye (Lond). 2014;28(10):1179–1183.
26. Rechichi M, Mazzotta C, Giovanni W, et al. Selective transepithelial ablation with simultaneous accelerated corneal cross-linking for corneal regularization of keratoconus: the STARE-X protocol. J Cataract Refract Surg. 2021. doi:10.1097/j.jcrs
27. Waszczykowka A, Jurowski P. Two-year accelerated corneal cross-linking outcome in patients with progressive keratoconus. J of Biomedicine and Biotechnology. 2015;2015(3):325157.
28. Bozkurt E, Ozgurhan EB, Ilkay B, et al. Refractive, topographic, and aberrometric results at 2-year follow-up for accelerated corneal cross-link for progressive keratoconus. J Ophthalmol. 2017;2017:5714372.
29. Ghanem CR, Santhiago MR, Berti T, Netto MV, Ghanem VC. Topographic, corneal wavefront, and refractive outcomes 2 years after collagen crosslinking for progressive keratoconus. Cornea. 2014;33(1):43–48.
30. Gore M, Leucci MT, Koay S, et al. Accelerated pulsed high-fluence corneal cross-linking for progressive keratoconus. Am J Ophthalmol. 2021;221:9–16.
31. Kortuem K, Vounotrypidis V, Athanasiou A, et al. Differences in corneal clinical findings after standard and accelerated cross-linking in patients with progressive keratoconus. BMC Ophthalmol. 2017;222:1–8.
32. Wen D, Li Q, Song B, et al. Comparison of standard versus accelerated corneal collagen cross-linking for keratoconus: a meta-analysis. Invest Ophthalmol Vis Sci. 2018;59:3920–3931.
33. Ng AL, Chan TC, Cheng AC. Conventional versus accelerated corneal collagen crosslinking in the treatment of keratoconus. Clin Exp Ophthalmol. 2016;44:8–14.
34. Cummings AB, McQuaid R, Naughton S, Brennan E, Mrochen M. Optimizing corneal crosslinking in the treatment of keratoconus: a comparison of outcomes after standard- and high intensity protocols. Cornea. 2016;35(6):814–822.
35. Chow VW, Chan TC, Yu M, Wong VW, Jhanji V. One-year outcomes of conventional and accelerated collagen crosslinking in progressive keratoconus. Sci Rep. 2015;5:14425.
36. Shetty R, Pahuja NK, Nuijts RM, et al. Current protocols of corneal collagen crosslinking – visual, refractive and tomographic outcomes. Am J Ophthalmol. 2015;160(2):243–249.
37. Vinciguerra P, Albè E, Trazza S, Seiler T, Epstein D. Intraoperative and postoperative effects of corneal collagen cross-linking on progressive keratoconus. Arch Ophthalmol. 2009;127(10):1258–1265.
38. Belin MW, Duncan JK, Ambrósio R
39. Belin MW, Duncan JK. Keratoconus: the ABCD grading system. Klin Monbl Augenheilkd. 2016;233(6):701–707.
40. Mazzotta Raiskup F, Hafezi F, Torres-Netto EA, Balamoun AA, Giannaccare G, Bagaglia S. Long term results of accelerated 9 mW corneal crosslinking for early progressive keratoconus: the Siena eye-cross study 2. Eye and Vision. 2021;8:16.
41. Belin M, Meyer J, Duncan J, Gelman R, Borstrom AR. Assessing progression of keratoconus and cross-linking efficacy: the Belin ABCD progression display. Int J Keratoconus Ectatic Corneal Dis. 2017;6(1):1–10.
42. Vinciquera R, Pagano L, Borgia A, et al. Corneal cross-linking for progressive keratoconus: up to 13 years of follow-up. L Refract Surg. 2020;36(12):838–843.
43. Viswanathan D, Males J. Prospective longitudinal study of corneal collagen crosslinking in progressive keratoconus. Clin Experiment Ophthalmol. 2013;41(6):531–536.
44. Elmassry A, Ahmed OIS, Abdalla MF, Gaballah K. Ten years experience of corneal collagen cross-linking: an observational study of 6120 cases. Eur J Ophthalmol. 2021;31(3):951–958.
45. Schumacher S, Oeftiger L, Mrochen M. Equivalence of biomechanical changes induced by rapid and standard corneal cross-linking, using riboflavin and ultraviolet radiation. Invest Ophthalmol Vis Sci. 2011;52:9048–9052.
46. Wang YM, Chan TC, Yu MCY, Jhanji V. Comparative evaluation of progression rate in keratoconus before and after collagen crosslinking. Br J Ophthalmol. 2018;102:1109–1113.
47. Males JJ, Viswanathan D. Comparative study of long-term outcomes of accelerated and Conventional collagen crosslinking for progressive keratoconus. Eye (Lond). 2018;32:32–38.
48. Sarac O, Caglayan M, Uysal BS, Uzel AG, Tanriverdi B, Cagil N. Accelerated versus standard crosslinking in pediatric keratoconus patients: 24 months follow-up results. Cont Lens Anterior Eye. 2018;S1367-0484(17):30338–7.29.
49. Ting DS, Rana-Rahman R, Chen Y, et al. Effectiveness and safety of accelerated (9mW/cm2) corneal collagen cross-linking for progressive keratoconus: a 24-month follow-up. Eye. 2019;33:812–818.
50. Hashemi H, Fotouhi A, Miraftab M, et al. Short-term comparison of accelerated and standard methods of corneal collagen crosslinking. J Cataract Refract Surg. 2015;41(3):533–540.
51. Vounotripidis E, Athanasiou A, Kortuem K, et al. Long –term data base analysis of conventional and accelerated crosslinked keratoconic mid-European eyes. Graefes Arch Clin Exp Ophthalmol. 2018;256:1165–1172.
52. Elbaz U, Shen C, Lichtinger A, et al. Accelerated (9 mJ/cm2)corneal collagen crosslinking for keratoconus-A 1 year follow-up. Cornea. 2014;33(8):769–773.
53. Alnawaiseh M, Rosentreter A, Bohm MR, Eveslage M, Eter N, Zumhagen L. Accelerated (18 mJ/cm2) corneal collagen crosslinking for progressive keratoconus. Cornea. 2015;34(11):1427–1431.
54. Kanellopoulos AJ. Long-term results of a prospective randomized bilateral eye comparison trial of higher fluence, shorter duration ultraviolet A radiation, and riboflavin collagen cross-linking for progressive keratoconus. Clin Ophthalmol. 2012;6:97–101.
55. Chan TC, Chow VW, Jhanji V, Wong VW. Different topographic response between mild to moderate and advanced keratoconus after accelerated collagen cross-linking. Cornea. 2015;34(8):922–927.
56. Greenstein SA, Hersch PS. Characteristics influencing outcomes of corneal collagen cross-linking for keratoconus and ectasia: implications for patient selection. J Cataract Surg. 2013;39:1133–1140.
57. Toprak I, Yaylali V, Yildirim C. Factors affecting outcomes of corneal collagen cross-linking treatment. Eye (Lond). 2014;28:41–46.
58. Kang Y, Li S, Liu C, et al. Accelerated epithelium-off corneal cross-linking with high ultraviolet energy dose (7.2J/cm2) for progressive keratoconus: 2-year results in a Chinese population. J Refract Surg. 2020;36(11):731–739.
59. Kymionis GD, Tsoulnaras KI, Grentzelos MA, et al. Evaluation of corneal stromal demarcation line depth following standard and a modified-accelerated collagen cross-linking protocol. Am J Ophthalmol. 2014;158(4):671–675.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
