Back to Archived Journals » Open Access Animal Physiology » Volume 6
Comparative respiratory physiology: the fundamental mechanisms and the functional designs of the gas exchangers
Authors Maina JN
Received 23 July 2014
Accepted for publication 11 September 2014
Published 10 December 2014 Volume 2014:6 Pages 53—66
DOI https://doi.org/10.2147/OAAP.S53213
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Peter Koulen
John N Maina
Department of Zoology, University of Johannesburg, Johannesburg, South Africa
Abstract: Acquisition of molecular oxygen (O2) from the external fluid media (water and air) and the discharge of carbon dioxide (CO2) into the same milieu is the primary role of respiration. The functional designs of gas exchangers have been considerably determined by the laws of physics which govern the properties and the flux of gases and the physicochemical properties of the respiratory fluid media (water or air and blood). Although the morphologies of gas exchangers differ greatly, certain shared structural and functional features exist. For example, in all cases, the transfer of O2 and CO2 across the water/air–blood (tissue) barriers occurs entirely by passive diffusion along concentration gradients. In the multicellular organisms, gas exchangers have developed either by evagination or invagination. The arrangement, shape, and geometries of the airways and the blood vessels determine the transport and exposure of the respiratory media and, consequently, gas exchange. The thickness of the water/air–blood (tissue) barrier, the respiratory surface area, and volume of pulmonary capillary blood are the foremost structural parameters which determine the diffusing capacity of a gas exchanger for O2. In fish, stratified design of the gills and internal subdivision of the lungs increase the respiratory surface area: the same adaptive property is realized by different means. A surface active phospholipid substance (surfactant) lines the respiratory surface. Adaptive specializations of gas exchangers have developed to meet individual survival needs.
Keywords: gas exchanger, oxygen, respiration, carbon dioxide, diffusing capacity
Introduction
There is no such a thing as ideal gas exchange system. The system that has evolved in each species depends to an impressive extent on the environmental conditions, on body build and size, on animal’s patterns of movement and on its energy consumption.1
The main task of a gas exchanger is to procure molecular oxygen (O2) from the external fluid medium (water/air) and to eliminate carbon dioxide (CO2) from the cells/body back into the same. O2 is a vital resource that is procured from outside at cost. For example, in the human being, ~12,000 L of air pass through the lung every 24 hours.2 Respiratory efficiency is a measure of the performance of a gas exchanger: it registers the ratio of gas transfer against that of the energy involved in procuring it. The cost of breathing water per unit O2 uptake has been reported to range from 0.5% to 70% of the overall O2 consumption (VO2).3–6 While most organisms/animals will live for weeks without food and days without water, the majority constantly need O2 to remain alive. Compared to activities like feeding, thermoregulation, locomotion, and reproduction, which can be adjusted, postponed, or abandoned altogether without lasting ill effect, respiration is a constant activity. The importance of O2 for life comes into sharp personal focus when you realize that, at this very moment that you are reading this line, you are breathing, and, if you stopped doing so, permanent damage to certain of your body tissues, foremost the brain and the heart, would occur in 3 to 6 minutes, and you would certainly be dead in ~7 minutes’ time. Different scholars have remarked on the importance of O2 for life. Krogh termed respiration “the call for oxygen”;7 relating metabolic needs to energy production, Kleiber designated O2 as “the fire of life”;8 Laitman et al asserted that “the acquisition and processing of O2 and its by-products is the primary mission of any air-breathing vertebrate”;9 Knust et al stated that “gas exchange is the main purpose of the lung”;10 Lane described O2 as “the molecule that made the World”;11 and, most recently, Canfield termed O2 “the signature feature of Earth”.12
Since Hippocrates (460–377 BC) espoused that the main purpose of breathing is to “cool the heart”, and Antoine Lavoisier (1743–1794 AD) and Joseph Priestley (1733–1804 AD) later determined that animals actually breathed to acquire O2, remarkable advances have been made in determining the development, the structure, and the function of gas exchangers. In this concise account, the fundamental tenets of comparative respiratory physiology are outlined. Excellent treatises, such as those by Steen,13 Slonim and Hamilton,14 Davenport,15 Dejours,4 Schmidt-Nielsen,16 Cameron,17 Hlastala and Berger,18 Prange,19 Maina,20,21 and Levy et al,22 should be consulted for substantive details.
Storage of O2 and CO2 in the body
Respiration comprises complex and highly integrated biomechanical, physiological, and behavioral processes. The transfer of O2 occurs through a cascade of tissue barriers and compartments by diffusion down a partial pressure gradient, which drops to about zero at the mitochondrial level. Because they are the eventual O2 “sinks”, the mitochondria drive the flow of O2 from the external milieu to the cells. It is undoubtedly because of its toxicity23,24 that O2 is not stored in the body in significant amounts. For example, for a human being weighing 70 kg, only ~1.6 L of O2 exists in the body,25,26,27 with ~370 cm3 of it present in the alveoli, ~280 cm3 in the arterial blood, ~600 cm3 in the capillary and venous blood, ~60 cm3 dissolved in the body tissues, and ~240 cm3 chemically bound to myoglobin. Since the greater part of the O2 store is bound to hemoglobin (Hb), only a small quantity can be released without undesirable decrease of the arterial partial pressure of oxygen (PO2) (PaO2). For example, when Hb is 50% saturated with O2, the PaO2 is only 26 mmHg (3.5 kPa). Breathing pure O2 causes a large increase in the total O2 stores to 4.25 L, as the functional residual capacity congests with O2. The major component of the O2 store then shifts to the lung, from where ~80% of it can be utilized without a drop in Hb saturation: the PaO2 remains at ~100 mmHg (~13 kPa). This explains why preoxygenation is so effective in providing a vital store of O2 during transient periods of apnea. It is because only ~500 cm3 of O2 are acquired per minute (at rest) from the ~6 to 7 L of ventilated air, in addition to the meager ~1.6 L of the total pool in the body, that there is instant increase of irreversible tissue damage after cessation of breathing. This is exacerbated by the fact that not all stored O2 is available for use: severe hypoxemia occurs before even half of the O2 which is stored in the Hb and myoglobin is released. Because O2 store in the body is insignificant, the alveolar PaO2 responds quickly to changes in the concentration of O2 in the pulmonary circulation.
Respiration and pH regulation
The body’s stores of CO2 in solution and in the form of bicarbonate ions (HCO3−1) far exceed those of O2. In a 70 kg person, the total CO2 store is 120 L.27 During apnea, the arterial partial pressure of oxygen (PCO2) (PACO2) increases by ~1 kPa during the first minute. The initial increase in the PACO2 then decreases at a rate of 0.4 kPa · min−1 as the alveolar PCO2 level increases and CO2 elimination by diffusion through the airways increases.
CO2 is the most important acid end product of metabolism. Since one of the important roles of the respiratory system is to eliminate CO2, the lung plays a key role in the regulation of the concentration of hydrogen ions (H+) in blood and other body fluids. In addition, the kidneys perform an important role in maintaining acid–base homeostasis. By retaining or excreting H+ or HCO3− ions as necessary, the kidneys influence arterial pH. Renal HCO3−1 ion retention or excretion depends not on HCO3−1 ion concentration or pH, but rather on the PCO2. The cells of the distal convoluted tubule of the kidney, which are rich in the enzyme carbonic anhydrase, regulate HCO3−1 ion concentration, which is high in both metabolic acidosis and uncompensated respiratory acidosis. In the latter case, HCO3−1 ions are retained by the kidney, while, in the former, they are not. The lungs remove ~13,000 mEq · day−1 of carbonic acid, while the kidney excretes >100 mEq · day−1 of sulfates, phosphates, and other fixed acids. An average person produces ~200 cm3 · min−1 of CO2, while the lungs eliminate 300 L of it daily.
The CO2 stores in the body change continuously. During hyper- or hypoventilation with air, the rate of change is approximately one-eighth as fast as that of O2. The unloading of O2 and loading of CO2 in the systemic blood capillaries are mutually beneficial processes: a reciprocal relationship exists so that increased PCO2 aids in the unloading of O2 and decreased PO2 aids in loading CO2 in the tissues. The reverse occurs in the lung, where reduction of PCO2 increases the affinity of Hb for O2 and increased PO2 reduces the affinity of Hb for CO2. The two effects, the Haldane effect on CO2 loading and the Bohr effect on O2 loading, emanate from the unique physicochemical properties of Hb. They result in efficient exchange of respiratory gases in states of high metabolic activity, in which VO2 and CO2 production are increased. The Henderson–Hasselbalch equation is a special form of the law of mass action which states that the rate of a chemical reaction is proportional to the product of the molar concentrations of the reactants. It is expressed as:
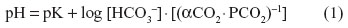
where pK is the apparent acid dissociation constant of CO2 and αCO2 is the physical solubility of CO2. The pK for the HCO3−1/CO2 system in blood is 6.1 at 37°C.
Past changes in the levels of O2 and CO2 in the atmosphere
Life on Earth has developed under the immutable laws of physics and chemistry. In the ~3.5 billion (109) years of existence of life on Earth,12 the atmospheric conditions have changed greatly. Survival has, however, been achieved at inordinate cost. Of all the animal species that have ever appeared on earth, ~99.99% are now extinct.28 Those animals which have succumbed can be considered to have been failed experiments. The fluctuations of the levels of O2 and CO2 in the biosphere have determined the ways and means by which O2 was acquired and CO2 removed. During the late Paleozoic, over a period of ~120 million years, the level of O2 rose to a high of 35% (compared to the present level of 21%) and then precipitously fell to a hypoxic low of 15% in the Triassic.29,30 These shifts were duplicated in water. Among others, Dudley31 supposed that these changes resulted in major events such as mass extinctions. The highest levels of CO2 occurred in the Ordovician and Silurian.32 Due mainly to tectonic activity, the CO2 level had dropped to that of the present (0.036%) by the Carboniferous, rising afterward by a factor of 3 by the end of the Permian.30
The structure and function of the inaugural gas exchangers were largely produced by natural selection under environmental conditions that were totally different from those of today. It is therefore uncertain why some respiratory adaptations were adopted, some conserved, and others discarded. In biology, the so-called Bauplans (German word for “building plans”, “blue prints”, or “frozen cores”)33,34 are conserved (“hardwired”) morphological features/designs. The conserved homeobox genes are known to be responsible for the development of the basic body plans.35,36 The tripartite design of the blood–gas barrier37 and the surfactant lining, a phospholipid lining of the respiratory surface,38,39 are good examples of conserved structures of gas exchangers.
Physicochemical properties of the respiratory fluid
Water and air are the two naturally occurring respirable fluid media. In three-dimensional space, they constitute the biosphere. In the biological range of temperature, water exists in two forms, liquid and gas (water vapor), while air exists in the form of a gas. The physicochemical properties of water and air have consequentially influenced the development, the structure, and the function of animals, and especially that of the gas exchangers.40 Of the three natural states of matter, ie, solid, liquid, and gas, only fluids (liquids and gases) are atomically/molecularly configured to contain or “dissolve” the respiratory gases and thereby facilitate the transport of the gases to the respiratory site. To meet their needs of O2, animals have evolved and adapted to utilize either water or air, or, in very rare cases, to make use of both. The structural and functional requirements for exploiting water and air as sources of O2 and sinks of CO2 are fundamentally different: gas exchangers which work in water perform dismally in air. Liquid breathing (ventilation) was first studied in the early 1960s by Kylstra et al.41 It is now used effectively in the clinical management and treatment of certain pulmonary diseases and conditions,42,43 wherein perfluorocarbons, saturated organofluorides that are biologically inert, are commonly used. Perfluorocarbons have low surface tension, high vapor pressure, high solubility of O2 and CO2, and, although they are nearly twice as dense as water, a kinematic viscosity that is equivalent to that of water. Acquisition of O2 in liquid-ventilated lungs substantiates the fact that a partial pressure gradient and not the nature of the fluid medium from which O2 is extracted is the key driver of gas transfer by diffusion.
While the physicochemical properties of water must be precisely known in order to determine how PO2 and PCO2 and their concentration changes when the two gases are removed or added to it, air (atmosphere) is a relatively simple medium to contend with: only a few properties, such as temperature and pressure, are needed to determine the changes of pO2 and pCO2 and their concentrations when respiratory gases are consumed or generated.
In saturated water, at 20°C, 1 mL of O2 is contained in 200 g of water while 1 mL O2 is present in 5 mL air (7 g). The rate of diffusion of O2 in water (2.5×10−5 cm2 · sec−1) is lower by a factor of 105 compared to that in air (1.98×10−1 cm2 · sec−1), and the capacitance coefficient (increase of concentration per unit increase in partial pressure) of O2 in water is only 1.82 nmol · min−1 · torr−1, compared to the much higher values in air of 54.74 nmol·min−1 · torr−1.4 Because air is “richer” in O2, to maintain a PO2 of 13.3 kPa in the alveolar air, at a respiratory quotient of 1, an air breather transfers only 17 mL of air · min−1 · mL O2−1, compared to an aquatic animal, which must transfer 480 mL of water · min−1 · mL O2−1 in order to maintain an equivalent PO2 in the gill water.44,45 The rate of ventilation of an aquatic animal at 20°C is 28 times that of an air breather. At that temperature, the solubility of CO2 is ~28 times higher than the solubility of O2 in water: the PCO2 in the blood of a fish is  lower that of an air breather. Animals which accomplished air-breathing greatly reduced the ventilatory rate. In doing so, they coped with a large increase of PACO2. To avert respiratory acidosis, renal mechanisms which increased HCO3− ion blood concentration developed to stabilize the OH−/H+ ratio.
lower that of an air breather. Animals which accomplished air-breathing greatly reduced the ventilatory rate. In doing so, they coped with a large increase of PACO2. To avert respiratory acidosis, renal mechanisms which increased HCO3− ion blood concentration developed to stabilize the OH−/H+ ratio.
Compared to water, air is a more favorable respiratory medium. Larger quantities of O2 are transferred by diffusion at lower energy expenditure. Water breathers have adapted to the constraints imposed by a relatively more O2-deficient respiratory medium. The interactions of the respiratory gases with water and air differ greatly. Because of the high solubility of CO2 in water, the molar concentration of the free gas is about equal to that in air, while the concentration of O2 in water is only ~5% of that in air. Instead of concentration, the reduced diffusion coefficient limits the rate of CO2 transfer in water, while both lower diffusion coefficient and lower concentration impede the transfer of O2 in the same medium. The most important factors that determine the movement of O2 and CO2 across the blood–gas barrier are: 1) the molecular properties of the respiratory gases; 2) the solubility of the respiratory gases in the respiratory fluid media; and 3) transfers of the respiratory gases in the respiratory fluid media and the water/air–blood (tissue) barrier. Since, except for living in different media, there have not been any other physical impediments during the evolution of the gills and the lungs, water gills, air gills, water lungs, and air lungs should have evolved to the same degree. However, this is not the case because of the low solubility of O2 in water, high viscosity of water, and low vapor pressure of O2 in air. Water lungs and air gills have, however, evolved, but only rarely, particularly in the simplest of the animal forms, eg, in the respiratory pleopods of terrestrial isopods46 and in the aquatic pneumonate gastropods, in which air-breathing has retrogressed back to water-breathing. It is because of the considerable physicochemical differences in the properties of water and air that direct conversion of the gills to lungs has not been possible: a transitory gas exchanger which functions equally well in the two media would have to form.
Many accounts which deal with adaptations of organisms to dissolved respiratory gas levels in water largely address the availability of O2 rather than the concentration of CO2. However, due to the high CO2/O2 solubility ratio, if initially normoxic water was made anoxic by aerobic metabolism only, the PCO2 would only increase by ~0.9 kPa.45 Due to the constantly high PO2 in air, diffusion across the blood–gas barrier is efficient, and the PAO2 approaches that in the external fluid medium. While O2 extraction in the water breathers is high, eg, 90% in sponges; 60%–90% in the crab, Calappa granulata; 33%–70% in the octopus; 85% in the eel;47,48 and, on average, 85% in fishes,49 FIO2 rarely exceeds 25% in air breathers.
The physical laws of flow of gases
Respiratory gases interact with the biological tissues in specific ways which are determined by the laws of physics (which determine the flow of gases) and the materials properties of the tissue barrier. Conductance is the reciprocal of resistance, and vice versa. In absolute and relative terms, O2 and CO2 diffuse in water and air differently, largely according to Dalton’s and Henry’s laws. The principle of independent action of gases is the basis of Dalton’s law, which states that the total pressure of a gas mixture (P) is equal to the sum of the fractional partial pressures (FX) of all the gases in the mixture. Stated differently, the partial pressure of an individual gas (PX) in a mixture is the pressure that the gas would exert if it occupied the total volume of the mixture in absence of the other components, thus:

In gas saturated with water vapor at 37°C, where the vapor pressure is 47 mmHg (~6 kPa):
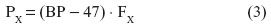
where BP is the barometric pressure.
In solution, the partial pressure of a gas in solution is its partial pressure in a gas mixture that is in equilibrium with the solution. According to Henry’s law, the concentration of a gas dissolved in a liquid is proportional to its partial pressure, thus:

where CX is the concentration of dissolved gas, βX the solubility coefficient, and PX the partial pressure of a gas.
The rate of the transfer of a gas across a tissue barrier depends on its solubility in the aqueous phase of the membrane. Constrained diffusion hinders O2 transfer long before CO2 transfer is affected: hypoxemia occurs before hypercapnic acidosis happens. Except in patients with severely compromised exchange who require O2 therapy, outward diffusion of CO2 is never a clinical problem. The Krogh’s diffusion constants for O2 and CO2 are much higher in air than in water; O2 capacitance (increase in solubility per unit pressure) is much higher in air than in water, while CO2 has about the same capacitance in both media (air and water). It is because of these differences that respiration in air breathers differs greatly from that of the water breathers. Endothermic homeothermy and extremely highly aerobic activities, such as flight, could only have been achieved after transition from water- to air-breathing. Air breathers “breathe” much less than water breathers and, for a given O2 tension, they acquire equivalent amounts of O2 because the O2 concentration is much higher in air than in water. Because the CO2 capacitances of air and water are similar, PCO2 is much higher in the air breather than in the water breather. Given that the pH in an air and a water breather are similar, it follows that blood HCO3− ion concentration is much higher in the former than in the latter group. With the viscosity and density being, respectively, 60 and 800 times lower for air compared to water, the rheological properties of these media greatly impact on their convective transport in and out of the gas exchanger. The work of breathing is much less for an air breather than for a water breather.
The transfer of respiratory media by convective (bulk = mass) flow requires energy. The bifurcation, the shapes, and the sizes of the airways and the blood vessels are optimized to save energy. The law which governs the resistance met by a fluid flowing through rigid, straight tubes, such as the airways (Figure 1) and blood vessels, is analogous to Ohm’s law for the flow of electricity that relates resistance (R) and flow of electric current (C) to electromotive force (EMF), thus:

For fluid flow, resistance (R) correlates the driving pressure between two points – the inlet (Pi) and the outlet (Po) – and the flow (V), thus:

For laminar flow of a Newtonian fluid in a rigid cylindrical tube, Poiseuille’s equation describes the process as follows:
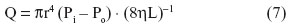
where Q is volume rate of flow (the volume of fluid flowing past a given point per unit time); r is the internal radius of the tube; L is the length of the tube; Pi – Po is the difference of pressure between the inflow (i) and outflow pressures (o); η is the viscosity; and π is the constant of proportionality.
Poiseuille’s equation shows that the flow rate Q is directly proportional to the viscosity of the fluid, and that Q depends on the fourth power of the radius of the tube. In practical terms, all other conditions remaining constant, reducing the radius by one-half reduces flow by a factor of 16 (24)!: the flow rate or, alternatively, the pressure required to maintain a given flow is greatly affected by only a small change in the radius. Since Poiseuille’s equation is valid only for streamlined flow of an incompressible fluid with constant viscosity, it cannot be precisely applied to blood, which is, rheologically, a heterogeneous suspension medium. Blood contains cells, some of which (eg, erythrocytes) have a diameter that is about equal to that of the blood capillaries. The apparent viscosity of blood varies as a function of the hematocrit. Its viscosity is 2.5 times that of plasma. In severe anemia, blood viscosity is low, while it increases greatly in, eg, polycythemia vera. For the gas exchangers, the size and the geometry of the airways and the blood vessels (especially the arteries) pattern each other very closely.50
In the past, it was believed, even by physiologists as eminent as Christian Bohr51 and JS Haldane (see Haldane52), that gas exchange across tissue barriers occurred by an active process, ie, O2 was “secreted” into the blood capillaries. This process was thought to happen particularly during exercise – when O2 needs are higher – and under hypoxia. With more accurate experimental techniques and instrumentation and better understanding of respiratory physiology, it has since been established that O2 transfer across tissue barriers occurs by diffusion.53 The water/air–blood barrier essentially functions as a passive “player” in the transfer of O2 and CO2 through it. It is only in rare and highly specialized organs, such as the swim bladder and the choroid rete of the eye of teleosts, that O2 is known to be secreted against a concentration gradient. The respiratory roles of such organs is, however, questionable. After combining with the Hb, O2 exerts negligible back pressure. That way, a partial pressure gradient and, consequently, O2 flow from the external milieu are maintained.
According to Graham’s law, the rate of diffusion of a gas is directly proportional to the velocity of its molecules, which is, in turn, inversely proportional to the square root of its density. Whether in a gas or a liquid medium, larger gas molecules diffuse more slowly. On the basis of molecular weight only, O2 (molecular weight =32) diffuses slightly faster than CO2 (molecular weight =44). In the lung, the diffusion of O2 and CO2 occurs between a gaseous environment and an extracellular fluid film which covers the respiratory surface: O2 concentrates in the fluid and then diffuses across the blood–gas barrier. The fluid lining was unequivocally demonstrated by Weibel and Gil.54 Through a delicate process that entails regulation of hydrostatic and osmotic forces across the blood capillary wall, the pulmonary surface is kept moist but is not flooded.55,56 In the human lung, ~15 cm3 of the extracellular lung fluid is preserved, despite the fact that the oncotic pressure is normally higher than lung microvascular hydrostatic pressure. Maina20 argued that since a dry surface (lung) cannot exchange respiratory gases, the so-called air breathers have not strictly evolved. Loosely, an air breather is defined as an organism/animal that acquires O2 from air and discharges CO2 into the same. Like for many instances in biology, some animals, such as the minute aerial arthropods, and probably the book lungs of some spiders exchange respiratory gases through a dry cuticle.7 For the reason that its (CO2) concentration in the aqueous layer that covers the lung is high, a high concentration gradient of CO2 forms between the surface and deeper layers of the fluid and the tissues. This explains why CO2 diffuses faster between alveolar gas and the capillary blood than O2, although CO2 diffuses less rapidly in the alveolar space.
Where the surface-to-volume ratio is large and the distances small, diffusion suffices in transferring O2 and removing CO2 across the tissue barrier (Figure 2). Constraints on diffusion have had great impact on the size and shape of microorganisms. Only the unicellular organisms, some simple multicellular organisms, and embryos rely entirely on diffusion across the surface for their O2 needs. Since volume increases as the cube of the radius, while surface area increases as the square of the radius, surface-to-volume ratio decreases with body size. A spherical body confers the lowest surface-to-volume ratio. According to Harvey,57 the maximum radius (r) of a spherical organism which can wholly rely on diffusion for gas exchange can be determined as follows:
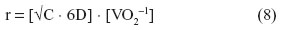
where D is the Krogh’s diffusion constant, C the partial pressure of O2, and VO2 the O2 consumption.
Harvey57 determined that the radius of a spherical organism which utilizes O2 at a rate such that the PO2 is zero at the center, the PO2 at the surface of the sphere is 0.21 atmosphere, the Krogh’s diffusion constant for tissue is 0.01 cm2 · min−1 · kPa−1, and VO2 is 0.02 cm3 · min−1 (a realistic value for a protozoan) is 0.25 mm. Krogh7 estimated that a spherical organism with a radius of 1 cm and a VO2 of 100 cm3 of O2 · kg−1 · h−1 (approximately one-half of that of a resting human being) would require an external O2 pressure of 25 atmospheres or ~19,000 mmHg (~2.5 · 103 kPa) to supply O2 up to its center by diffusion. As both structural complexity and, with it, the distances from the surface to the deeper sites of an organism/animal increased, convective movement of respiratory fluid media (water, air, and blood) became obligatory: perfusion became crucial after ventilation could no longer satisfy the O2 needs (Figure 2).
According to Fick’s law of diffusion, the diffusive conductance (the volumetric rate of gas transfer by diffusion [Q]) of a gas (eg, O2) between two compartments, A and B, is directly proportional to the Krogh’s permeation constant (Kt), the respiratory surface area (S), and the ΔPO2 (PO2 [A] – PO2 [B]) between the compartments but is inversely proportional to the thickness of the tissue barrier (τ) (Figure 3), ie:
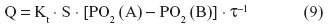
Krogh’s constant of O2 is the product of the diffusion coefficient (d), which is determined by the materials properties of the tissue barrier and its solubility (β). At 38°C, Kt is ~25 times greater for CO2 than O2 (mainly due to the differences in their solubilities). Since d and β are affected in different directions (with d increasing and β decreasing), temperature normally has little effect on Kt.
The distance from the terminal respiratory units to Hb which is contained in the red blood cells comprises the so-called air–Hb diffusion pathway, which comprises the following: 1) a very thin surfactant lining; 2) an aqueous hypophase; 3) the tissue barrier, which comprises an epithelial cell, a basement membrane, and an endothelial cell; 4) a plasma layer; 5) the membrane of the erythrocyte; and 6) the cytoplasm of the erythrocyte, through which O2 molecules move randomly before they are chemically bound to the Hb (Figure 4).
  | Figure 4 A stereogram and a transmission electron micrograph showing the oxygen–hemoglobin pathway. |
The physiological pulmonary diffusing capacity of O2 (DLO2P) is the measure of the lung’s conductance of the gas per unit time per unit partial pressure gradient. It is calculated as the ratio of VO2 to the mean alveolar gas tension (PaO2) and the mean pulmonary capillary gas tension (PCO2), thus:
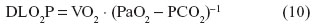
Morphometric estimations of surface areas (S) and thicknesses of the barriers (τ) and their integration with the relevant Krogh’s permeation constant58 allow the anatomical diffusing capacities (DAs) of the various components of the air–Hb pathway, eg, the tissue barrier (Dto2) and the plasma layer (Dpo2), to be estimated (Figure 4) as follows:

The diffusing capacities correlate directly with surface area and inversely with the thickness of the barrier (Figure 3). The diffusing capacity of the erythrocytes (DeO2) is calculated as the product of the O2 uptake coefficient (ΘO2) and the volume of the pulmonary capillary blood volume (Vc):

The total anatomical diffusing capacity of the lung for O2 (DLO2A) is calculated as the reciprocal of the sum of reciprocals of the conductances through the blood–gas (tissue) barrier, the plasma layer, and the erythrocyte, thus:
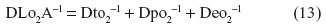
Designs of gas exchangers
In his now-classic book, Thompson argued that biological “structure arises by direct physical forces, with molecular forces acting on very small structures and mechanical ones on the larger ones”.59 Since then, this view has been moderated by, among others, Bonner and Weibel, who, respectively, opined that “the physical forces are not the sole determinants of form or morphology”60 and that “biological form is founded on the genome”.61 If the all-pervasive, immutable laws of physics uncompromisingly compelled morphological design, only one kind/type of organism and gas exchanger would still exist today. Conceivably, this would be the inaugural unicell with its outer membrane. Gas exchangers would not have advanced to the levels of complexity of today. Through natural selection acting on the genotype, factors such as environment, lifestyle, body size, behavior, respiratory medium utilized, and phylogenetic level of development have, to various extents, regulated the development of the different gas exchangers to meet specific metabolic needs.
In vertebrates, the thickness of the water/air–blood barrier decreases in order from fish gills to amphibian, to reptilian, to mammalian, and avian lungs.62–65 Regarding the respiratory surface area, in the air breathers, the values increase from amphibians, to reptiles, to mammals, to birds.62,64,66,67 In the gills, the respiratory surface area is increased by a hierarchical organization, wherein a few gill arches (normally four pairs in the teleosts) give rise to hundreds of fill filaments which, in turn, give rise to thousands of secondary lamellae (Figure 5A–C); in the mammalian (Figure 5D–F) and avian (Figure 5G–I) lungs, large surface area is achieved by internal subdivision. In the avian lungs, which are firmly affixed to the vertebrae and the ribs66 and which have thus been rendered practically rigid (noncompliant),68 the gas exchange tissue is extremely intensely subdivided (Figure 5G–I), giving rise to air capillaries, the terminal respiratory units, which are ~10 to 20 μm in diameter.69,70
In the mammalian lung, the numbers of terminal respiratory units, and hence the respiratory surface area, are increased by the process of branching morphogenesis, which is controlled by certain molecular factors.71–73 While a sphere of a volume of 1 cm3 has a surface area of 4.8 cm2, in the lung of the minute shrew, Sorex minutus, a surface area of 2,100 cm2 is granted by the number of alveoli which are contained in 1 cm3 of the parenchyma.74 The extreme compartmentalization of the exchange tissue of the avian lung (Figure 5G–I) explains why, in spite of birds having relatively smaller lung volumes (compared to mammals of equivalent body mass), the respiratory surface area in a bird lung is relatively larger.64,66,75 In disease and pathological conditions, surface area is reduced in cases like those of the collapse of sections of the lung (atelectasis), break-up (failure) of the interalveolar septa resulting in abnormally large terminal air spaces (emphysema), and thickening of the blood–gas barrier in cases of, for example, fibrosis and edema. Changes in the thickness of the plasma layer and that of the intracytoplasmic distance across which O2 molecules travel before binding to Hb molecules occur in cases of anemia. Impairments of the O2–Hb pathway lead to what is termed “physical block to diffusion”.
Compromise design of the gas exchangers
In addition to gas exchange, respiratory organs perform other vital functions. For example, in addition to serving water-breathing organs, gills perform roles such as osmotic and ionic regulation, acid–base regulation, and excretion of nitrogenous wastes, eg, ammonia and urea.76 Lungs modify and regulate chemical synthesis of important pharmacological agents such as biogenic amines (eg, serotonin, histamine, and norepinephrine), peptides (eg, bradykinin and angiotensin I and II), lipids (eg, dipalmitoyl lecithin, which is the main component of the surfactant), and prostaglandins.77 For them to perform the various functions, different structural properties, which, in most cases, are at variance with each other, are needed. For example, the large respiratory surface area of the gills which promote gas exchange may aggravate water and ion flux, establishing inordinate gradients between the fish’s extracellular fluids and the aquatic milieu. Regarding the skin (integument), respiration, water conservation, thermal regulation, and ion and pH regulation call for different structural requirements. The sporadic attenuation of the blood–gas barrier (Figure 4) optimizes gas exchange while maintaining its mechanical integrity.78,79 Herein, the author contends that respiratory processes and strategies have been set up by multiple factors, resulting in compromise (trade-off) designs.
It is the author’s contention that there are no rules in respiration, but only necessities. Gas exchangers have developed on a need-to-have basis. Since animals occupy different habitats and lead different lifestyles, the structure of a gas exchanger in any one animal taxon cannot be predicted in a simple and direct way. For example, unlike the brain, which has reached the pinnacle of development in the human being, the bronchoalveolar lung of the human being is far from being the most efficient of the evolved gas exchangers.20,66 There are no tissues or cells that are unique to gas exchangers, as, eg, a neuron is to nervous tissue, an osteocyte to bone, and a podocyte to the kidney. Although often claimed to be archetypical to the lung, the type II (granular) pneumocyte which secretes the surfactant is not particular to the organ. Surfactant-like phospholipids are produced in many tissues and organs, including the stomach, the intestines, the swim bladder, the gas mantle of an air-breathing snail (Helix aspersa), the prostate gland, the female reproductive tract, the lacrimal gland, the mesothelial cells of the pleura, the pericardium, the peritoneum, and the Eustachian tube epithelium.38,80–82
Evagination and invagination of gas exchangers
In multicellular animals, where a plain cell membrane doesn’t suffice (Figures 2 and 6A), gas exchangers develop by evagination (out-pouching = out-pocketing) or by invagination (Figure 6). The gills form by evagination (Figure 6B), while the lungs form by invagination (in-tucking = diverticulation = cavitation) (Figure 6C and D). Depending on needs and circumstances, bimodal breathers, animals which utilize water and air as sources of O2, possess both invaginated and evaginated gas exchangers (Figure 6C).
Certain advantages and disadvantages exist in the externalized (evaginated) and internalized (invaginated) gas exchangers. When removed from water to air, fish largely die of anoxia – paradoxically, not from lack of O2, but because the secondary lamellae (Figure 5A–C) adhere to each other under surface tension forces, drastically reducing the respiratory surface area. Other complications which soon arise are desiccation and excessive increase of CO2 in the blood, as CO2 is less soluble in air. While hypoxia was the main driver for transition from water to land, desiccation was the foremost threat,83,84 and called for development of invaginated gas exchangers (lungs). Hypothetically, if the human lung was evaginated, ie, it projected out into air, even in a moderately desiccating environment, water loss would occur at a rate ~1,000 times greater than normally occurs. The person would die in less than 3 minutes, ie, sooner than would occur from asphyxia! The lungs, being invaginated, can only be ventilated in and out (ie, bidirectionally = tidally). They therefore fail to exploit the high PO2 in the atmosphere. During inhalation, only a small fraction of fresh air (tidal volume) is introduced into the gas exchanger and much less of it reaches the respiratory surface. On the other hand, invagination allows creation of stable, well-regulated respiratory sites and conditions in the gas exchanger. For example, in the alveoli, the PO2 is lower, while the PCO2 is higher, than in the atmosphere. Such microenvironments cannot form in evaginated gas exchangers. In the vertebrate air breathers, the high alveolar PCO2 in the terminal air spaces is important for maintenance of the HCO3- ion-mediated buffering system that is involved in the regulation of pH. Interestingly, although invaginated, as is the mammalian lung, the separation of the lung (the gas exchanger) from the mechanical ventilator (the air sacs) in the avian respiratory system66 allows the avian lungs to be ventilated continuously and unidirectionally (similarly to gills) in a caudocranial direction.85
Surface tension arises at a gas–liquid interface because the forces between the molecules of the liquid are stronger than those between the liquid and the gas. In the mammalian and the avian lungs, surface tension forces are generated at the fluid-lined surface. Like in a soap bubble, the tension in the wall has the tendency of obliterating the air space. According to Laplace’s equation, the degree of the inward-directed collapsing pressure (P) or the pressure needed to keep the bubble open is proportional to the surface tension (T) and inversely proportional to the radius (r), ie:

If the surface force acts on both sides, like it does for a soap bubble, a coefficient of 4 is substituted for that of 2. Under normal conditions, the alveolar surface tension at equilibrium is ~25 mN · m−1 (0.025 kPa). In order to oppose the forces created by the reduced alveolar radius, this must decrease to near zero at the end of expiration.86
The stability of the alveoli is maintained by presence of a connective tissue continuum, which mechanically supports the air spaces,87,88 and by the lowering of surface forces by the surfactant.89,90 First appearing in the lungs of the lungfishes (Dipnoi), the surfactant has been conserved for at least the last ~300 million years.28 In addition to stabilizing the terminal air spaces, the surfactant prevents transudation of fluid (edema),39,86,91 is involved in host defense,72 and relaxes the airway smooth muscles.92
Functional designs of the gas exchangers
Gas exchange efficiency is considerably dependent on the arrangement of the airways and the blood vessels. They determine how the respiratory fluid media, air and blood, are delivered and exposed to each other. Where the respiratory media (water and air) flow in opposite directions (eg, in the fish gills), the design is termed “counter-current” (Figure 7); where the media run orthogonally (perpendicularly), eg, in the parabronchus of the avian lung, it is termed “cross-current”; and, when the gas exchanger is ventilated with a medium which is fairly uniform in PO2 (eg, in the mammalian lung), the design is termed “uniform-pool”.93,94 Respiratory efficiency decreases from the counter-current, the cross-current, to the ventilated-pool designs. From this comparison, the design of the mammalian lung (including that of the human) is the least efficient.
The thickness of the tissue barrier in the frog’s skin, which ranges from 25 to 100 μm,95,96 is very thick. It offers considerable resistance to gas exchange by diffusion. The respiratory function of the skin is, however, augmented by other respiratory sites such as the buccal cavity and the lung. The urodele salamanders (Plethodontidae), which subsist in cold, well-oxygenated water, rely entirely on cutaneous respiration for their O2 needs: they lack a lung.93,97 Without the highly efficient counter-current gas exchange system, fish could not possibly survive in water, a medium which is relatively low in O2 concentration. In the counter-current system, venous blood which is low in PO2 meets incoming water with high PO2 (Figure 7). Experimental reversal of the direction of the flow of water over the gills would create a “con-current” system, in which the gas exchange media flow in the same direction. In such a case, the O2 uptake drops to well below 10%.98 In the cross-current design of the avian lung, reversing the flow of air in the parabronchial lumen does not affect gas exchange efficiency: only the sequence of the arterialization of the blood capillaries changes.94
Conclusion
Life evolved under the universal laws of physics and chemistry. It is therefore axiomatic that these laws, together with the physicochemical properties of the respiratory fluid media, consequentially influenced the functional designs of gas exchangers. Although differences exist in their external morphologies, shared features also exist. The omnipresent laws that govern the behavior, movement, transfer, and interactions of the respiratory gases with respiratory media and the materials properties of the tissue barrier may have contributed to the development of the conserved (hardwired) features. For example, in the various vertebrate lungs (Figure 8), or indeed in all the gas exchangers, transfer of respiratory gases across the blood–gas barrier occurs solely by diffusion. Factors such as lifestyle and the environment occupied have adaptively refined the structural and functional properties in order to meet specific energetic demands. For example, bats have retained, but highly honed, the mammalian (bronchoalveolar) lung.64,99–102 The adage that “necessity is the mother of invention” is as much relevant to natural selection as it is in the advances in human engineering. In what can be termed “evolutionary erosion”, when need has justified it, gas exchangers have been deconstructed and earlier and simpler designs readopted. There are no rules in the designs of gas exchangers, but only necessities. Exceptions exist for every case.103,104
  | Figure 8 The different morphologies of lungs. |
Acknowledgment
The author is grateful to the National Research Foundation (NRF) of South Africa for supporting the preparation of this work.
Disclosure
The author reports no conflicts of interest in this work.
References
Jürgens KD, Gros G. [Phylogeny of gas exchange systems]. Anasthesiol Intensivmed Notfallmed Schmerzther. 2002;37:185–198. German. | |
Burri PH. Morphology and respiratory function of the alveolar unit. Int Arch AllergyAppl Immunol. 1985;76 Suppl 1:2–12. | |
Alexander RMcN. Functional Design in Fishes. London: Hutchison; 1967. | |
Dejours P. Respiration in Water and Air: Adaptations, Regulation, Evolution. Amsterdam: Elsevier; 1988. | |
Schumann D, Piiper J. [Oxygen requirement in fish respiration according to measurements on the anesthetized tench (Tinca tinca)]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;288:15–26. German. | |
Wood SC, Lenfant C. Oxygen transport and oxygen delivery. In: Wood SC, Lenfant C, editors. Evolution of Respiratory Processes: A Comparative Approach: Lung Biology in Health and Disease. Vol 13. New York: Marcel Dekker; 1979:193–223. | |
Krogh A. The Comparative Physiology of Respiratory Mechanisms. Philadelphia, PA: University of Pennsylvania Press; 1941. | |
Kleiber M. Respiratory exchange and metabolic rate. In: Fenn WO, Rahn H, editors. Handbook of Physiology, Section 3, Respiration. Vol II. Washington, DC: American Physiological Society; 1965:927–938. | |
Laitman JT, Reidenberg JS, Marquez S, Gannon PJ. What the nose knows: new understandings of Neanderthal upper respiratory tract specializations. Proc Natl Acad Sci U S A. 1996;93:10543–10545. | |
Knust J, Ochs M, Gundersen HJ, Nyengaard JR. Stereological estimates of alveolar number and size and capillary length and surface area in mice lungs. Anat Rec (Hoboken). 2009;292:113–122. | |
Lane N. Oxygen: The Molecule That Made the World. Oxford: Oxford University Press; 2002. | |
Canfield DE. Oxygen: A Four Billion Year History. Princeton, NJ: Princeton University Press; 2014. | |
Steen JB. Comparative Physiology of Respiratory Mechanisms. London: Academic Press; 1971. | |
Slonim NB, Hamilton LH. Respiratory Physiology. 2nd ed. St Louis, MO: The CV Mosby Company; 1971. | |
Davenport HW. The ABC of Acid-Base Chemistry. 6th ed. Chicago, IL: The University of Chicago Press; 1974. | |
Schmidt-Nielsen K. Animal Physiology: Adaptation and Environment. 4th ed. Cambridge: Cambridge University Press; 1990. | |
Cameron JN. The Respiratory Physiology of Animals. Oxford: Oxford University Press; 1989. | |
Hlastala MP, Berger AJ. Physiology of Respiration. Oxford: Oxford University Press; 1996. | |
Prange HD. Respiratory Physiology: Understanding Gas Exchange. New York, NY: Chapman and Hall; 1996. | |
Maina JN. The Gas Exchangers: Structure, Function, and Evolution of the Respiratory Processes. Heidelberg: Springer-Verlag; 1998. | |
Maina JN. Bioengineering Aspects in the Design of Gas Exchangers: Comparative Evolutionary, Morphological, Functional, and Molecular Perspectives. Berlin: Springer-Verlag; 2011. | |
Levy MN, Koeppen BM, Starton BA. Berne and Levy Principles of Physiology. 4th ed. Philadelphia, PA: Elsevier Mosby; 2006. | |
Harrison J, Frazier MR, Henry JR, Kaiser A, Klok CJ, Rascón B. Responses of terrestrial insects to hypoxia or hyperoxia. Respir Physiol Neurobiol. 2006;154:4–17. | |
Maltepe E, Saugstad OD. Oxygen in health and disease: regulation of oxygen homeostasis – clinical implications. Pediatr Res. 2009;65: 261–268. | |
Farhi L. Gas stores of the body. In: Fenn WO, Rahn H, editors. Handbook of Physiology, Section 3: Respiration. Vol I. Washington, DC: American Physiological Society; 1964:873–924. | |
Farhi L, Rahn H. Gas stores of the body and the unsteady state. J Appl Physiol. 1955;7:472–484. | |
Pinnock C, Lin T, Smith T, editors. Fundamentals of Anaethesia. Greenwich: Greenwich Medical Media; 2009. | |
Pough FH, Heiser JB, McFarland WN. Vertebrate Life. 3rd ed. New York: Macmillan Publishing Company; 1989. | |
Berner RA, Vandenbrooks JM, Ward PD. Evolution. Oxygen and evolution. Science. 2007;316:557–558. | |
Graham JB, Dudley R, Aguilar NM, Gans C. Implications of the late Palaeozoic oxygen pulse for physiology and evolution. Nature. 1995;375:117–120. | |
Dudley R. Atmospheric oxygen, giant Paleozoic insects and the evolution of aerial locomotor performance. J Exp Biol. 1998;201:1043–1050. | |
Holland HD. The Chemical Evolution of the Atmosphere and Oceans. Princeton, NJ: Princeton University Press; 1984. | |
Wagner GP. The origin of morphological characters and the biological basis of homology. Evolution. 1989;43:1157–1171. | |
Wagner GP. Complexity matters. Science. 1998;279:1158–1159. | |
Balavoine G, Telford MJ. Identification of planarian homeobox sequences indicates the antiquity of most Hox/homeotic gene subclasses. Proc Natl Acad Sci U S A. 1995;92:7227–7231. | |
Walldorf U, Fleig R, Gehring WJ. Comparison of homeobox-containing genes of the honeybee and Drosophila. Proc Natl Acad Sci U S A. 1989;86:9971–9975. | |
Maina JN, West JB. Thin but strong! The bioengineering dilemma in the structural and functional design of the blood-gas barrier. Physiol Rev. 2005;85:811–844. | |
Daniels CB, Orgeig S. The comparative biology of pulmonary surfactant: past, present and future. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:9–36. | |
Orgeig S, Daniels CB, Johnston SD, Sullivan LC. The pattern of surfactant cholesterol during vertebrate evolution and development: does ontogeny recapitulate phylogeny? Reprod Fertil Dev. 2003;15: 55–73. | |
Bliss DE. From sea to tree: saga of a land crab. Am Zool. 1979;19: 385–410. | |
Kylstra JA, Tissing MO, van der Maen. Of mice as fish. Trans Am Soc Artif Intern Organs. 1962;8:378–383. | |
Tawfic QA, Kausalya R. Liquid ventilation. Oman Med J. 2011; 26(1):4–9. | |
Wolf GK, Sheeran P, Heitz D, Thompson JE, Arnold JH. Gas exchange and lung mechanics during high frequency ventilation in the perflubron-treated lung. Pediatr Crit Care Med. 2008;9:641–646. | |
Howell BJ. Acid-base balance in the transition from water-breathing to air-breathing. In: Farhi LE, Rahn H, editors. Studies in Pulmonary Physiology, Mechanics, Chemistry, and Circulation. TX: USAF School of Aerospace Medicine, Aerospace Medical Division (AFSC), Brooks Air Force Base; 1969:270–275. | |
Rahn H. Aquatic gas exchange: theory. Respir Physiol. 1966;1:1–12. | |
Hoese B. Struktur und Entwicklung der Lungen der Tylidae (Crustacea, Isopoda, Oniscoidea) [Structure and development of the lungs of Tylidae (Crustacea, Isopoda, Oniscoidea)]. Zool Jahrb Abt Anat Ontogenie Tiere. 1983;109:487–501. German. | |
Van Dam L. On the Utilization of Oxygen and Regulation of Breathing in Some AQUATIC animals [dissertation]. Groningen: University of Groeningen; 1938. | |
Van Dam L. On the respiration in scallops (Lamellibranchiata). Biol Bull. 1954;107:192–202. | |
Steen JB, Kruysse A. The respiratory function of teleostean gills. Comp Biochem Physiol. 1964;12:127–142. | |
Maina JN, van Gils P. Morphometric characterization of the airway and vascular systems of the lung of the domestic pig, Sus scrofa: comparison of the airway, arterial and venous systems. Comp Biochem Physiol A Mol Integr Physiol. 2001;130:781–798. | |
Bohr C. Über die spezifische Tätigkeit der Lungen bei der respiratorischen Gasaufnahme und ihr Verhalten zu der durch die Alveolarwand stattfindenden Gasdiffusion. Skand Arch Physiol. 1909;22(2):221–280. | |
Haldane JS. Respiration. New Haven, CT: Yale University Press; 1922. | |
Krogh A. On the mechanism of the gas-exchange in the lungs. Skand Arch Physiol. 1910;23:248–278. | |
Weibel ER, Gil J. Electron microscopic demonstration of an extracellular duplex lining layer of the alveoli. Respir Physiol. 1968;4:42–57. | |
Fishman AP. Pulmonary edema. The water-exchanging function of the lung. Circulation. 1972;46:390–408. | |
Levine OR, Mellins TB, Fishman AP. Quantitative assessment of pulmonary edema. Circ Res. 1965;17:414–426. | |
Harvey EN. The oxygen consumption of luminous bacteria. J Gen Physiol. 1928;11:469–475. | |
Weibel ER. Morphometric estimation of pulmonary diffusion capacity. I. Model and method. Respir Physiol. 1970–1971;11:54–75. | |
Thompson D’AW. On Growth and Form. Canto edition 1992. Oxford: Oxford University Press; 1942. | |
Bonner JT, editor. Addendum. In: On Growth and Form. Cambridge: Cambridge University Press; 1992:xxi–xxii. | |
Weibel ER. Symmorphosis: On Form and Function in Shaping Life. Harvard, MA: Harvard University Press; 2000. | |
Gehr P, Mwangi DK, Ammann A, Maloiy GM, Taylor CR, Weibel ER. Design of the mammalian respiratory system. V. Scaling morphometric pulmonary diffusing capacity to body mass: wild and domestic animals. Respir Physiol. 1981;44:61–86. | |
Hughes GM, Morgan M. The structure of fish gills in relation to their respiratory function. Biological Reviews. 1973;48:419–475. | |
Maina JN, King AS, Settle G. An allometric study of the pulmonary morphometric parameters in birds, with mammalian comparisons. Phil Trans R Soc Lond B Biol Sci. 1989;326:1–57. | |
Meban C. Thicknesses of the air-blood barriers in vertebrate lungs. J Anat. 1980;131:299–307. | |
Maina JN. The Lung Air-Sac System of Birds: Development, Structure, and Function. Berlin: Springer-Verlag; 2005. | |
Perry SF. Reptilian lungs: functional anatomy and evolution. Adv Anat Embryol Cell Biol. 1983;79:1–81. | |
Jones JH, Effmann EL, Schmidt-Nielsen K. Lung volume changes during respiration in ducks. Respir Physiol. 1985;59:15–25. | |
Duncker HR. Structure of the avian respiratory tract. Respir Physiol. 1974;22:1–19. | |
Maina JN, Nathaniel C. A qualitative and quantitative study of the lung of an ostrich, Struthio camelus. J Exp Biol. 2001;204:2313–2330. | |
De Langhe SP, Reynolds SD. Wnt signaling in lung morphogenesis. Organogenesis. 2008;4:100–108. | |
Metzger RJ, Krasnow MA. Genetic control of branching morphogenesis. Science. 1999;284:1635–1639. | |
Warburton D, Bellusci S, De Langhe S, et al. Molecular mechanisms of early lung specification and branching morphogenesis. Pediatr Res. 2005;57:26R–37R. | |
Gehr P, Sehovic S, Burri PH, Claasen H, Weibel ER. The lung of shrews: morphometric estimation of diffusion capacity. Respir Physiol. 1980;40:33–47. | |
Maina JN. Morphometrics of the avian lung. In: King AS, McLelland J, editors. Form and Function in Birds. Vol 4. London: Academic Press; 1989:307–368. | |
Evans DH, Piermarini PM, Choe KP. The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev. 2005;85: 97–177. | |
Bakhle YS. The pharmacokinetic function of lung, In: Junod AF, de Haller R, editors. Lung Metabolism. New York, NY: Academic Press, Inc.; 1975:293–299. | |
Maina JN, King AS. The thickness of avian blood-gas barrier: qualitative and quantitative observations. J Anat. 1982;134:553–562. | |
Weibel ER, Knight BW. A morphometric study on the thickness of the pulmonary air-blood barrier. J Cell Biol. 1964;21:367–384. | |
Akiyama J, Hoffman A, Brown C, et al. Tissue distribution of surfactant proteins A and D in the mouse. J Histochem Cytochem. 2002;50: 993–996. | |
Bourbon JR, Chailley-Heu B. Surfactant proteins in the digestive tract, mesentery, and other organs: evolutionary significance. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:151–161. | |
Oberley RE, Goss KL, Ault KA, Crouch EC, Snyder JM. Surfactant protein D is present in the human female reproductive tract and inhibits Chlamydia trachomatis infection. Mol Hum Reprod. 2004;10: 861–870. | |
Randall DJ, Burggren WW, Farrell AP, Haswell MS. The Evolution of Air Breathing in Vertebrates. Cambridge: Cambridge University Press; 1981. | |
Schmalhausen II. The Origin of Terrestrial Vertebrates. London: Academic Press; 1968. | |
Fedde MR. Structure and gas flow pattern in the avian lung. Poult Sci. 1980;59:2642–2653. | |
Alonso C, Waring A, Zasadzinski JA. Keeping lung surfactant where it belongs: protein regulation of two-dimensional viscosity. Biophys J. 2005;89:266–273. | |
Bachofen H, Wilson TA. Micromechanics of the acinus and alveolar cells. In: Crystal RG, West JB, Barnes PJ, Cherniak NS, Weibel ER, editors. The Lung: Scientific Foundations. New York: Raven Publishers; 1997:1159–1167. | |
Weibel ER. The Pathway for Oxygen: Structure and Function in the Mammalian Respiratory System. Harvard, MA: Harvard University Press; 1984. | |
Bernhard W, Schmiedl A, Koster G, et al. Developmental changes in rat surfactant lipidomics in the context of species variability. Pediatr Pulmonol. 2007;42:794–804. | |
Serrano AG, Pérez-Gil J. Protein-lipid interactions and surface activity in the pulmonary surfactant system. Chem Phys Lipids. 2006;141: 105–118. | |
Bernhard W, Haslam PL, Floros J. From birds to humans: new concepts on airways relative to alveolar surfactant. Am J Respir Cell Mol Biol. 2004;30:6–11. | |
Koetzler R, Saifeddine M, Yu Z, Schürch S, Hollenberg MD, Green FH. Surfactant as an airway smooth muscle relaxant. Am J Respir Cell Mol Biol. 2006;34:609–615. | |
Piiper J, Gatz RN, Crawford EC. Gas transport characteristics in an exclusively skin breathing salamander, Desmognathus fuscus (Plethodontidae). In: Hughes GM, editor. Respiration of Amphibious Vertebrates. London: Academic Press; 1976:339–356. | |
Scheid P, Piiper J. Cross-current gas exchange in avian lungs: effects of reversed parabronchial air flow in ducks. Respir Physiol. 1972;16: 304–312. | |
Czopek J. Vascularization of respiratory surfaces in some caudata. Copeia. 1962:576–587. | |
Czopek J. Quantitative studies of the morphology of respiratory surfaces in amphibians. Acta Anat (Basel). 1965;62:296–323. | |
Gatz RN, Crawford EC Jr, Piiper J. Respiratory properties of the blood of lungless and gill-less salamander, Desmognathus fuscus. Respir Physiol. 1974;20:33–41. | |
Hughes GM. Some features of gas transfer in fish. Bull Inst Math Appl. 1978;14:39–43. | |
Maina JN. A scanning and transmission electron microscopic study of the bat lung. J Zool. 1985;205:19–27. | |
Maina JN, King AS. The structural functional correlation in the design of the bat lung. A morphometric study. J Exp Biol. 1984;111:43–63. | |
Maina JN, King AS, King DZ. A morphometric analysis of the lung of a species of bat. Respir Physiol. 1982;50:1–11. | |
Dhar PK, Giuliani A. Laws of biology: why so few? Syst Synth Biol. 2010;4:7–13. | |
Maina JN, Thomas SP, Dallas DM. A morphometric study of bats of different size: correlations between structure and function of the chiropteran lung. Phil Trans R Soc Lond B Biol Sci. 1991;333:31–50. | |
Rahn H. Gas transport from from the external environment to the cell. In: de Reuck AVS, Porter R, editors. Development of the Lung. A CIBA Foundation Symposium. London: Churchill; 1967:3–23. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.