Back to Journals » Cancer Management and Research » Volume 10
Comparative effectiveness of different chemotherapy regimens of advanced-stage Hodgkin lymphoma in adults: a network meta-analysis
Authors Zhang T , Yao Y, Feng F , Zhao W, Tian J, Zhou C, Wang X, Dong S, Li J, Qi L , Sun C
Received 6 July 2018
Accepted for publication 1 September 2018
Published 22 November 2018 Volume 2018:10 Pages 6017—6028
DOI https://doi.org/10.2147/CMAR.S179356
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Tingting Zhang,1 Yan Yao,2 Fubin Feng,3 Wenge Zhao,2 Jinhui Tian,4 Chao Zhou,3 Xue Wang,5 Shengjie Dong,6 Jia Li,2 Lingyu Qi,7 Changgang Sun3,8
1College of Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan, Shandong Province, People’s Republic of China; 2Clinical Medical College, Weifang Medical University, Weifang, Shandong Province, People’s Republic of China; 3Department of Oncology, Weifang Traditional Chinese Hospital, Weifang, Shandong Province, People’s Republic of China; 4Evidence-Based Medicine Center, School of Basic Medical Sciences, Lanzhou University, Lanzhou, Gansu Province, People’s Republic of China; 5Clinical Medical Colleges, Qingdao University, Shinan District, Qingdao, Shandong Province, People’s Republic of China; 6Department of the Joint and Bone Surgery, Yantaishan Hospital, Yantai, Shandong Province, People’s Republic of China; 7College of First Clinical Medicine, Shandong University of Traditional Chinese Medicine, Ji’nan, People’s Republic of China; 8Department of Oncology, Affiliated Hospital of Weifang Medical University, Kuiwen District, Weifang, Shandong Province, People’s Republic of China
Background: Combined chemotherapy is the cornerstone treatment for patients with advanced Hodgkin lymphoma (HL). The objective of our study was to perform a network meta-analysis of the efficacy of different chemotherapy regimens in adults with advanced-stage HL.
Materials and methods: We searched for relevant randomized controlled trials (RCTs) in titles/abstracts in PubMed, Embase, and the Cochrane Library. The search was last updated on April 3, 2018. RCTs that assessed the effectiveness of one of the following treatments were included: doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD); four cycles of increased dose of bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPPescalated) followed by two or four cycles of standard dose of BEACOPP (4× BEACOPPescalated + 2 or 4× BEACOPPbaseline); brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine (A+AVD); doxorubicin, vinblastine, mechlorethamine, vincristine, bleomycin, etoposide, and prednisone combined with radiation therapy (Stanford V); mechlorethamine (cyclophosphamide), vincristine, procarbazine, and prednisone (M[C]OPP); sequential or alternating chemotherapy regimens with ABVD as the footstone (eg, COPP/ABVD or mechlorethamine, vincristine, procarbazine, and prednisone [MOPP]/ABVD); eight cycles of BEACOPPescalated; hybrid MOPP/ABV; and M[C]EC (M[C]OPP with epidoxorubicin, bleomycin, vinblastine [EBV], and lomustine, doxorubicin, and vindesine [CAD]).
Results: Overall, we screened 3,564 citations and deemed 18 reports of 16 trials eligible and included them in our network meta-analysis. A total of 11,928 participants were randomly assigned to one of the 12 combinations of chemotherapy regimens, of which 11,476 participants were analyzed. For the overall survival (OS), no differences were observed within any interventions when the ABVD regimen was used as the reference treatment. Similarly, relative to A+AVD, 8× BEACOPPescalated and 6× BEACOPPescalated also showed no differences (HR =1.07, 95% credible interval (CrI): 0.58–1.95; HR =0.62, 95% CrI: 0.16–1.83; and HR =0.71, 95% CrI: 0.30–1.72, respectively). In terms of complete remission (CR), enough evidence exists to support a maximum clinical treatment effect for 6× BEACOPPescalated (OR =1.88, 95% CrI: 1.20–2.96; and OR =3.43, 95% CrI: 1.87–6.24).
Conclusion: When compared across the 12 combined chemotherapy regimens, six cycles of BEACOPPescalated may be the optimal treatment for patients with advanced-stage HL.
Keywords: advanced-stage Hodgkin lymphoma, combined chemotherapy, overall survival, network meta-analysis, randomized controlled trial
Introduction
In the European Union, the incidence of Hodgkin lymphoma (HL) is ~2.2/100,000 per year,1 most often affecting young adults aged 20–40 years.2 HL is a malignant tumor of the lymph nodes and lymphatic system, which has the nature of the post-germinal B-cell origin of the malignant Hodgkin and Reed–Sternberg cells.2 Over the last few decades, significant progress has been made in the management of patients with this disease, and it has become treatable even in those with advanced-stage HL.3,4 Chemotherapy is the cornerstone treatment for patients with advanced HL.2 The development of combination chemotherapy not only changed the prognosis but also prolonged the survival time of patients with advanced-stage HL.5 The therapeutic goal of advanced-stage HL is to reduce long-term complications and improve quality of life based on improving or maintaining the existing efficacy.
At present, doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) remains the standard approach of treatment for these patients.6,7 ABVD regimens have a longer 5-year overall survival (OS) rate and are less myelotoxic than mechlorethamine, vincristine, procarbazine, and prednisone (MOPP) or when alternated with MOPP.8 Robust clinical evidence shows that compared to ABVD, treatment with four cycles of increased dose of bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPPescalated) followed by four cycles of baseline-dose BEACOPP (4× BEACOPPescalated + 4× BEACOPPbaseline) had a better initial tumor control. However, with regard to the 7-year OS rate, there was no significant difference between the two groups.9 A previous Phase 1 trial demonstrated that the brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine (A+AVD) had a better acceptance and efficacy in patients with advanced treatment-naive HL.10 In addition, current evidence favoring A+AVD rather than ABVD was demonstrated in ECHELON-1, a randomized Phase 3 trial involving patients with advanced-stage HL.11 It is difficult to compare the efficacy of all tested regimens in the same trial, even though many clinical trials have been used to compare therapeutic regimens. In a previous network meta-analysis, six cycles of BEACOPPescalated were thought to be the optimal choice for patients with advanced-stage HL.12 Until now, there is no direct comparison between BEACOPP and A+AVD.
Thus, we adopted a network meta-analysis method in order to investigate this crucial problem further. In addition to collecting the data of different clinical trials, network meta-analysis was also used to combine direct and indirect evidence, rank these regimens, and elect the optimal regimen.13–15 The objective of our study was to perform direct and indirect comparisons of the efficacy of different chemotherapy regimens in adults with advanced-stage HL using a network meta-analysis of randomized controlled trials (RCTs).
Materials and methods
Search strategy
The network meta-analysis was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) extension to network meta-analysis.16 In this network meta-analysis, we searched for relevant RCTs in titles/abstracts in PubMed, Embase, and the Cochrane Library. We placed a restriction on language, and only articles in English were included. No publication date or publication status restrictions were imposed. We used the following medical subject headings: “Hodgkin disease”, “brentuximab vedotin”, “procarbazine,” “bleomycin”, “dacarbazine”, “mechlorethamine”, “doxorubicin”, “cyclophosphamide”, “prednisone”, “etoposide”, “vinblastine”, “vincristine”, and “RCTs” combined with lists of free text words for searching. The search was last updated on April 3, 2018. Additionally, we also manually searched for additional eligible trials in the reference lists of retrieved publications and relevant meta-analysis. Complete search strategies are shown in Tables S1–S3.
Selection criteria
Two authors (Zhang T and Yao Y) independently assessed the studies for eligibility. Eligible patients were aged ≥18 years with newly diagnosed and previously untreated advanced-stage (stage III or IV) HL. To be included, the studies had to be RCTs that assessed the effectiveness and safety of one of the following treatments: ABVD, four cycles of BEACOPPescalated followed by two or four cycles of BEACOPPbaseline (4× BEACOPPescalated+2 or 4× BEACOPPbaseline), A+AVD, doxorubicin, vinblastine, mechlorethamine, vincristine, bleomycin, etoposide, and prednisone combined with radiation therapy (Stanford V), mechlorethamine (cyclophosphamide), vincristine, procarbazine, and prednisone (M[C]OPP), sequential or alternating chemotherapy regimens with ABVD as the footstone (eg, COPP/ABVD or MOPP/ABVD), eight cycles of BEACOPPescalated, hybrid MOPP/ABV, M[C]EC (M[C]OPP with epidoxorubicin, bleomycin, vinblastine [EBV], and lomustine, doxorubicin, and vindesine [CAD]), eight cycles of BEACOPPbaseline, six cycles of BEACOPPescalated, and eight cycles of baseline-dose BEACOPP given in 14-day intervals (8× BEACOPP14). The primary outcomes were complete remission (CR) and OS. The literature in which the data related to survival could not be obtained or those that failed to provide the original text were excluded. If several publications were based on the same trial, only the newest and/or the most informative study was included in our network meta-analysis.
Data extraction
Two authors (Yao Y and Feng FB) independently extracted the basic characteristics of studies that met the inclusion criteria based on a prespecified data sheet. All authors tested the data extraction sheet before formally extracting the data. The following information was extracted: the first author, the year of publication, study design, sample size, intervention details (such as name, frequency, and dose), patient characteristics (such as median age, clinical stage, performance status, international prognostic score, histologic subtype, and median follow-up), outcome results, and safety data. The extracted data were those used in the intention-to-treat analysis. When any discrepancies arose, the abovementioned two authors reached consensus via discussions. After the data were extracted, these two authors cross-checked the data for accuracy against the studies.
Quality assessments
According to the Cochrane Handbook for Systematic Reviews of Interventions, we evaluated the risk of bias of individual eligible trials.17 We carried out the following seven domain-based evaluations: sequence generation, allocation concealment, masking of participants and personnel, masking of outcome assessors, incomplete outcome data, reporting bias, and other biases. The following criteria were adopted to judge each domain: low risk of bias, unclear risk of bias, and high risk of bias.
Statistical methods
The estimates of the network meta-analysis are represented as the HR with the corresponding 95% credible intervals (CrIs) when the effect size is time-to-event outcomes, and as the OR with associated 95% CrIs when there is a dichotomous outcome. With regard to HR and 95% CI, we extracted the data from the publication of the RCT. If HR and 95% CI were not directly reported in the studies, we used Engauge Digitizer 4.1 (http://digitizer.sourcenet/) to extract the survival information from the Kaplan–Meier curve and also to estimate the HR.18 Whenever possible, to the best of our abilities, we tried to use the longest follow-up data that were available. If only the percentage of patients with CR was reported in the publication, it was required to be converted to a decimal.
We performed our analysis using the linear regression model within the Bayesian framework. The WinBUGS, version 1.4.3 (MRC Biostatistics Unit, Cambridge, UK), based on Markov chain Monte Carlo method, was used for data analysis. Three initial values were randomly selected to run the Markov chain simultaneously. For the HR of OS, the model runs 60,000 iterations in total, and the number of iterations per chain is 20,000. We installed 5,000 iterations for each chain, which is regarded as the “burn-in” period. For the OR of CR, each chain runs 10,000 iterations, of which the first 3,000 are the “burn-in” period. A fixed-effect model or random-effect model is used, based on the Deviance Information Criteria (DIC) value. Ultimately, the optimum model is used for the primary analysis. The pooled estimated value is presented as the median and 95% CrI (2.5 and 97.5%, respectively) of the distribution of the final calculated data. Furthermore, we evaluated the ranking of each intervention regimen by plotting the surface under the cumulative ranking (SUCRA). The larger the SUCRA value, the higher the rank of the corresponding treatment regimen among the networks.19 When a loop existed in three arms, we used a node-splitting approach to evaluate inconsistencies among direct evidence and indirect evidence.
For the analysis mentioned above, we used Stata software version 13.0 (Stata Corporation, College Station, TX, USA). In addition, for assessment of bias, we used Review Manager, version 5.3 (The Nordic Cochrane Center: the Cochrane Collaboration, Copenhagen, Norway).
Results
Literature search results
Overall, 3,564 citations were identified by the search of PubMed, Embase, and Cochrane Library. After reviewing citation titles and abstracts, we excluded obviously irrelevant citations. A total of 51 potentially appropriate studies were retrieved in full text. After further reviewing the full texts, we excluded 33 studies for the following reasons: non-eligible interventions (n=5), duplicate reports (n=5), one study (n=9), no data on outcomes (n=12), or not an RCT (n=2). Finally, we deemed 18 reports8,9,11,20–34 of 16 trials eligible and included them in our network meta-analysis. The PRISMA flowchart demonstrating the literature search process is shown in Figure 1.
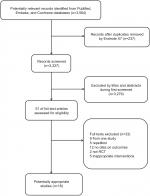  | Figure 1 Flowchart for search results and selection details. Abbreviation: RCT, randomized controlled trial. |
Characteristics of eligible studies
In total, 11,928 participants were randomly assigned to one of the 12 combination chemotherapy regimens and were included in the network meta-analysis, of which 11,476 patients were analyzed. The detailed characteristics of 12 combination chemotherapy regimens are shown in Table S4. Across trials, the publication year of eligible studies ranged from 1992 to 2018, and the median follow-up ranged from 2.05 to 9.25 years. All trials were prospective RCTs by cooperative groups. Of these trials, five studies were three-arm trials while the remaining were two-arm trials. With regard to clinical characteristics, the median age was from 28 to 70 years. In terms of B symptoms, only eight trials reported this index; the minimum percentage was 56.9, and the maximum percentage was 88. Definition of advanced-stages varies between investigators and trials, but about 78% of the participants were in stage III or IV. Table 1 summarizes the main characteristics of eligible trials.
The assessment of the risk of bias
As for the risk of bias, detailed information is shown in Figure 2. For the majority of studies, it is difficult to assess the risk of bias mainly due to the lack of detailed reports. Therefore, most studies were judged to be unclear risk of bias. In terms of study quality, only five trials reported specific random sequence generation and allocation concealment. The majority of studies were based on intention-to-treat analysis.9,11,21–34 There were four trials judged as low risk of bias for blinding of participants and personnel. However, for blinding of outcome assessment, there were four trials judged as having a high risk of bias.
  | Figure 2 Risk of bias graph presented as percentage across all included studies. |
Network meta-analysis
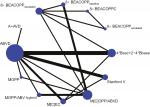
Networks of eligible comparisons for OS are shown in Figure 3. The network of CR is the same as that of OS. There were a total of 26 possible pair-wise comparisons between the 12 treatments, and out of those ABVD was the most commonly compared chemotherapy regimen. A+AVD, MOPP, MOPP/ABV hybrid, M[C]EC, M[C]OPP/ABVD, Stanford V, and 4× BEACOPPescalated+2 or 4× BEACOPPbaseline regimens were compared directly with ABVD. In addition to the ABVD regimen, M[C]OPP/ABVD was directly compared with five chemotherapy regimens.
There are six closed loops by three interventions in the network plot as shown in Figure 3. In each closed loop, the inconsistency between direct and indirect evidence was assessed using the inconsistency factor (IF) and 95% CI. Except for the three-arm trials, there remained a closed loop in ABVD–M[C]OPP/ABVD–MOPP/ABV hybrid. All the existing closed loops were consistent, since their 95% CIs included 0, indicating that there was no difference between direct and indirect estimates. IFs were 0.55 (95% CI: 0–1.24) for ABVD–M[C]OPP/ABVD–MOPP/ABV hybrid.
Sixteen trials involving 12 intervention arms were reported for the OS. According to the size of DIC, we chose to use a fixed-effect model (DIC =21.724) to perform data analysis involving OS, instead of a random-effect model (DIC =25.614). For OS, no differences were observed within any interventions when ABVD regimen was used as the referent agent (Table 2). Similarly, relative to A+AVD, 8× BEACOPPescalated, 6× BEACOPPescalated, and 8× BEACOPP14 also showed no differences (HR =1.07, 95% CrI: 0.58–1.95; HR =0.62, 95% CrI: 0.16–1.83; and HR =0.71, 95% CrI: 0.30–1.72, respectively). Additionally, compared to 6× BEACOPPescalated, there was no difference in 8× BEACOPP14 (HR =1.15, 95% CrI: 0.63–2.06). We also performed a fixed-effect network meta-analysis for CR. A total of 16 trials including 11,928 participants were included in the assessment of CR. Current evidence suggests that for 4 of the 12 interventions, CR was improved when compared to ABVD regimen. However, MOPP and MEC regimens were inferior to ABVD (OR =0.39, 95% CrI: 0.23–0.67 and OR =0.59, 95% CrI: 0.39–0.90, respectively). For 8× BEACOPPescalated (OR =1.88, 95% CrI: 1.20–2.96), 6× BEACOPPescalated (OR =3.43, 95% CrI: 1.87–6.24), and 8× BEACOPP14 (OR =2.52, 95% CrI: 1.41–4.51) enough evidence exists to support a maximum clinical treatment effect. Furthermore, 8× BEACOPP14 did not differ from 6× BEACOPPescalated (OR =0.74, 95% CrI: 0.48–1.12). More detailed information on OS and CR is summarized in Table 2. In addition, we also added a forest plot indicating HRs (Figure S1) and ORs (Figure S2) comparative to ABVD.
As is shown in Figure 4, we ranked all the interventions according to the size of SUCRA value. The SUCRA value of 12 combined chemotherapy regimens for OS and CR is shown in Table S5. There is strong evidence indicating that 6× BEACOPPescalated (SUCRA =86.5) had the highest probability of being the optimal treatment for OS, followed by 8× BEACOPP14 (SUCRA =86.0) and A+AVD regimen (SUCRA =68.5). Despite high SUCRA value, two-by-two comparisons of the three regimens show no statistically significant survival advantage over the others. In contrast, M[C]OPP/ABVD regimen had the minimum probability (SUCRA =10.6). With regard to CR, 6× BEACOPPescalated (SUCRA =99.3) was significantly beneficial in improving CR, followed by 8× BEACOPP14 (SUCRA =91.0) and 8× BEACOPPescalated regimens (SUCRA =82.3). Among all the intervention rankings, the MOPP regimen was the worst (SUCRA =1.2).
Discussion
As far as the likelihood of cure is concerned, the introduction of combined chemotherapy has made HL become one of the more favorable malignancies. At present, for the majority of patients with advanced-stage HL, ABVD regimen is the first line of treatment because of its better efficacy and fewer adverse effects than the MOPP regimen.8 However, ~20% of the advanced-stage patients are not completely in remission. To further improve the efficacy of combined chemotherapy for advanced HL, the BEACOPP regimen was introduced by the German HL Research Group.35 In addition, A+AVD regimen was introduced so that it could reduce the fatal lung toxicity of bleomycin.11
We draw the following conclusions from the results of our network meta-analysis: neither six cycles of BEACOPPescalated nor the A+AVD regimen significantly prolonged OS. Although a previous network meta-analysis12 indicated that when compared to ABVD regimen, six cycles of BEACOPPescalated might be the optimal treatment and significantly prolonged OS. However, 6× BEACOPPescalated and 8× BEACOPP14 could be significantly beneficial for the improvement of CR. This network meta-analysis provides the most comprehensive unified hierarchies of evidence for currently available combination chemotherapy regimen for adults who have advanced-stage HL, thus overcoming the lack of comparative data in RCTs.
According to the results mentioned above, with regard to survival outcomes, most of the chemotherapy regimens did not significantly differ. A clinical trial of BEACOPP in patients with advanced-stage HL demonstrated that it could significantly and stably improve long-term freedom from treatment failure and OS in terms of a 10-year follow-up.34 Additionally, brentuximab vedotin is an antibody–drug conjugate composed of an anti-CD30 chimeric monoclonal antibody, covalently linked.36 In our study, we included an A+AVD regimen, because in a previous clinical trial, whether progression-free survival or secondary efficacy, this regimen was significantly superior to the ABVD regimen.11 The replacement of bleomycin, based on the ABVD regimen, not only increases the efficacy but also reduces the risk of fatal pulmonary toxicity.37,38 In spite of this, without considering the safety, six cycles of BEACOPPescalated are still more effective than the A+AVD regimen, based on the SUCRA results, which could improve CR for advanced-stage HL.
Regarding the consistency of our study, we verified the inconsistency of the existing closed loop. We found that there was no difference in consistency, which indicated that direct comparison evidence corresponded with indirect comparison evidence. Additionally, we also evaluated the quality of included trials and noted that the majority of trials were open-labeled with no-blinding. In 10 of the 16 trials, sequence generation was judged as unclear, and in another 11 of the 16 trials, allocation concealment was judged as unclear. The unclear sequence generation and allocation concealment may give rise to bias. Nevertheless, we used an objective evaluation method, and the patient characteristics were equally distributed among each intervention group, so we thought the effect of these unclear factors was of little significance.
The dominant advantage of our study is the evaluation of available, published RCTs and the use of a network meta-analysis, which allowed for a comprehensive evaluation of the effect of different combined chemotherapy regimens. Moreover, the most up-to-date data were from 2018, and the published years of eligible studies were from 1992 to 2018. This network meta-analysis provides insight into the best combined chemotherapy for advanced-stage HL. Without doubt, there are several limitations that need to be acknowledged. First, in eight studies, digitized HRs were not provided directly in the survival curves, and so we had to extract HRs for OS from the survival curves. In this case, there was a trend toward a relatively large error. Second, despite some eligible studies having reported adverse events, because of the inconsistency of adverse events reported in the majority of studies, we could not analyze the safety data. When physicians make a decision about a chemotherapy regimen for initial treatment not only the efficacy of the medication but also the toxic effects of the treatment should be taken into consideration. Therefore, it is unfortunate that the safety data could not be merged to draw conclusions. Finally, published information rather than individual patient data was used to merge and analyze. Perhaps individual patient data could become a more detailed estimate of OS.
Conclusion
When compared across the 12 combined chemotherapy regimens, six cycles of BEACOPPescalated may be the optimal treatment for patients with advanced-stage HL. We believe that our study can provide high-level clinical decisions for clinicians and patients. However, we hope that more RCTs composed of any one of the mentioned chemotherapy regimens for patients with advanced-stage HL will be performed to develop more efficacious chemotherapy regimens.
Acknowledgments
The authors would like to acknowledge all the members of Department of Oncology of Weifang Traditional Chinese Hospital. This study was supported by grants from the National Natural Science Foundation of China (No. 81473513; No. 81673799).
Author contributions
TZ and CS were involved in the concept and design of the study. TZ drafted the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper, gave approval of the final version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Globocan.iarc.fr [webpage on the Internet]. GLOBOCAN 2018: estimated cancer incidence, mortality and prevalence worldwide in 2018. Available from: http://gco.iarc.fr/today/online-analysis-table. Accessed October 17, 2018. | ||
Eichenauer DA, Aleman BMP, André M, et al. ESMO Guidelines Committee. Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv19–iv29. | ||
Canellos GP, Rosenberg SA, Friedberg JW, Lister TA, Devita VT. Treatment of Hodgkin lymphoma: a 50-year perspective. J Clin Oncol. 2014;32(3):163–168. | ||
Hoppe RT, Advani RH, Ai WZ, et al. Hodgkin Lymphoma Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(5):608–638. | ||
Engert A. ABVD or BEACOPP for Advanced Hodgkin Lymphoma. J Clin Oncol. 2016;34(11):1167–1169. | ||
Bonadonna G, Bonfante V, Viviani S, Di Russo A, Villani F, Valagussa P. ABVD plus subtotal nodal versus involved-field radiotherapy in early-stage Hodgkin’s disease: long-term results. J Clin Oncol. 2004;22(14):2835–2841. | ||
Longo DL. Treatment of advanced Hodgkin lymphoma: the more things change, the more they stay the same. J Clin Oncol. 2013;31(6):660–662. | ||
Canellos GP, Anderson JR, Propert KJ, et al. Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992;327(21):1478–1484. | ||
Viviani S, Zinzani PL, Rambaldi A, et al; Michelangelo Foundation; Gruppo Italiano di Terapie Innovative nei Linfomi; Intergruppo Italiano Linfomi. ABVD versus BEACOPP for Hodgkin’s lymphoma when high-dose salvage is planned. N Engl J Med. 2011;365(3):203–212. | ||
Younes A, Connors JM, Park SI, et al. Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin’s lymphoma: a phase 1, open-label, dose-escalation study. Lancet Oncol. 2013;14(13):1348–1356. | ||
Connors JM, Jurczak W, Straus DJ, et al; ECHELON-1 Study Group. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. N Engl J Med. 2018;378(4):331–344. | ||
Skoetz N, Trelle S, Rancea M, et al. Effect of initial treatment strategy on survival of patients with advanced-stage Hodgkin’s lymphoma: a systematic review and network meta-analysis. Lancet Oncol. 2013;14(10):943–952. | ||
Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Stat Methods Med Res. 2008;17(3):279–301. | ||
Caldwell DM, Ades AE, Higgins JPT. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331(7521):897–900. | ||
Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23(20):3105–3124. | ||
Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. | ||
Higgins JP, Altman DG, Gotzsche PC, et al; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. | ||
Zhou ZR, Zhang TS, Li B, Mao Z, Zeng XT, Liu SX. [Extracting and transforming of appropriate data of meta-analysis in survival curve] Shěngcún qǔ xiàn zhōng meta fēnxī shìyí shùjù de tíqú yǔ zhuǎnhuàn. Chin J Evid Based Cardiovasc Med. 2014;6:243–247. Chinese. | ||
Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–171. | ||
Ballova V, Rüffer JU, Haverkamp H, et al. A prospectively randomized trial carried out by the German Hodgkin Study Group (GHSG) for elderly patients with advanced Hodgkin’s disease comparing BEACOPP baseline and COPP-ABVD (study HD9elderly). Ann Oncol. 2005;16(1):124–131. | ||
Glick JH, Young ML, Harrington D, et al. MOPP/ABV hybrid chemotherapy for advanced Hodgkin’s disease significantly improves failure-free and overall survival: the 8-year results of the intergroup trial. J Clin Oncol. 1998;16(1):19–26. | ||
Hoskin PJ, Lowry L, Horwich A, et al. Randomized comparison of the stanford V regimen and ABVD in the treatment of advanced Hodgkin’s Lymphoma: United Kingdom National Cancer Research Institute Lymphoma Group Study ISRCTN 64141244. J Clin Oncol. 2009;27(32):5390–5396. | ||
Diehl V, Franklin J, Pfreundschuh M, et al; German Hodgkin’s Lymphoma Study Group. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin’s disease. N Engl J Med. 2003;348(24):2386–2395. | ||
Federico M, Luminari S, Iannitto E, et al ; HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. ABVD compared with BEACOPP compared with CEC for the initial treatment of patients with advanced Hodgkin’s lymphoma: results from the HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. J Clin Oncol. 2009;27(5):805–811. | ||
Chisesi T, Bellei M, Luminari S, et al. Long-term follow-up analysis of HD9601 trial comparing ABVD versus Stanford V versus MOPP/EBV/CAD in patients with newly diagnosed advanced-stage Hodgkin’s lymphoma: a study from the Intergruppo Italiano Linfomi. J Clin Oncol. 2011;29(32):4227–4233. | ||
Merli F, Luminari S, Gobbi PG, et al. Long-Term Results of the HD2000 Trial Comparing ABVD Versus BEACOPP Versus COPP-EBV-CAD in Untreated Patients With Advanced Hodgkin Lymphoma: A Study by Fondazione Italiana Linfomi. J Clin Oncol. 2016;34(11):1175–1181. | ||
Duggan DB, Petroni GR, Johnson JL, et al. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin’s disease: report of an intergroup trial. J Clin Oncol. 2003;21(4):607–614. | ||
Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol. 2013;31(6):684–691. | ||
Engert A, Haverkamp H, Kobe C, et al; German Hodgkin Study Group; Swiss Group for Clinical Cancer Research; Arbeitsgemeinschaft Medikamentöse Tumortherapie. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379(9828):1791–1799. | ||
Connors JM, Klimo P, Adams G, et al. Treatment of advanced Hodgkin’s disease with chemotherapy--comparison of MOPP/ABV hybrid regimen with alternating courses of MOPP and ABVD: a report from the National Cancer Institute of Canada clinical trials group. J Clin Oncol. 1997;15(4):1638–1645. | ||
Mounier N, Brice P, Bologna S, et al; Lymphoma Study Association (LYSA). ABVD (8 cycles) versus BEACOPP (4 escalated cycles ≥4 baseline): final results in stage III-IV low-risk Hodgkin lymphoma (IPS 0-2) of the LYSA H34 randomized trial. Ann Oncol. 2014;25(8):1622–1628. | ||
Carde P, Karrasch M, Fortpied C, et al. Eight Cycles of ABVD Versus Four Cycles of BEACOPPescalated Plus Four Cycles of BEACOPPbaseline in Stage III to IV, International Prognostic Score ≥ 3, High-Risk Hodgkin Lymphoma: First Results of the Phase III EORTC 20012 Intergroup Trial. J Clin Oncol. 2016;34(17):2028–2036. | ||
Borchmann P, Haverkamp H, Diehl V, et al. Eight cycles of escalated-dose BEACOPP compared with four cycles of escalated-dose BEACOPP followed by four cycles of baseline-dose BEACOPP with or without radiotherapy in patients with advanced-stage hodgkin’s lymphoma: final analysis of the HD12 trial of the German Hodgkin Study Group. J Clin Oncol. 2011;29(32):4234–4242. | ||
Engert A, Diehl V, Franklin J, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin’s lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27(27):4548–4554. | ||
Diehl V, Sieber M, Rüffer U, et al. BEACOPP: an intensified chemotherapy regimen in advanced Hodgkin’s disease. The German Hodgkin’s Lymphoma Study Group. Ann Oncol. 1997;8(2):143–148. | ||
The European Medicines Agency. Adcetris (brentuximab vedotin). EU summary of product characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002455/WC500135055.pdf. Acsessed August 6, 2018. | ||
Canellos GP, Duggan D, Johnson J, Niedzwiecki D. How important is bleomycin in the adriamycin + bleomycin + vinblastine + dacarbazine regimen? J Clin Oncol. 2004;22(8):1532–1533. | ||
Martin WG, Ristow KM, Habermann TM, Colgan JP, Witzig TE, Ansell SM. Bleomycin pulmonary toxicity has a negative impact on the outcome of patients with Hodgkin’s lymphoma. J Clin Oncol. 2005;23(30):7614–7620. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.