Back to Journals » Journal of Pain Research » Volume 9
Comparative effectiveness of dextrose prolotherapy versus control injections and exercise in the management of osteoarthritis pain: a systematic review and meta-analysis
Authors Hung CY , Hsiao MY, Chang KV, Han DS, Wang TG
Received 2 August 2016
Accepted for publication 9 September 2016
Published 18 October 2016 Volume 2016:9 Pages 847—857
DOI https://doi.org/10.2147/JPR.S118669
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Video abstract presented by Dr Chen-Yu Hung.
Views: 3931
Chen-Yu Hung,1 Ming-Yen Hsiao,2,3 Ke-Vin Chang,1,2 Der-Sheng Han,1,2 Tyng-Guey Wang2,3
1Department of Physical Medicine and Rehabilitation, National Taiwan University Hospital, Bei-Hu Branch, Taipei, Taiwan; 2National Taiwan University College of Medicine, Taipei, Taiwan; 3Department of Physical Medicine and Rehabilitation, National Taiwan University Hospital, Taipei, Taiwan
Background: Increasing evidence has supported the use of dextrose prolotherapy for patients with osteoarthritis. However, the real benefits may be affected by differences in injection protocols, comparative regimens, and evaluation scales.
Methods: PubMed and Scopus were searched from the earliest record until February 2016. One single-arm study and five randomized controlled trials were included, comprising 326 participants. We estimated the effect sizes of pain reduction before and after serial dextrose injections and compared the values between dextrose prolotherapy, comparative regimens, and exercise 6 months after the initial injection.
Results: Regarding the treatment arm using dextrose prolotherapy, the effect sizes compared with baseline were 0.65 (95% confidence interval [CI], 0.14–1.17), 0.84 (95% CI, 0.40–1.27), 0.85 (95% CI, 0.60–1.10), and 0.87 (95% CI, 0.53–1.21) after the first, second, third, and fourth or more injections, respectively. The overall effect of dextrose was better than control injections (effect size, 0.36; 95% CI, 0.10–0.63). Dextrose prolotherapy had a superior effect compared with local anesthesia (effect size, 0.38; 95% CI, 0.07–0.70) and exercise (effect size, 0.71; 95% CI, 0.30–1.11). There was an insignificant advantage of dextrose over corticosteroids (effect size, 0.31; 95% CI, –0.18 to 0.80) which was only estimated from one study.
Conclusion: Dextrose injections decreased pain in osteoarthritis patients but did not exhibit a positive dose–response relationship following serial injections. Dextrose prolotherapy was found to provide a better therapeutic effect than exercise, local anesthetics, and probably corticosteroids when patients were retested 6 months following the initial injection.
Keywords: dextrose, prolotherapy, knee, hand, osteoarthritis
Introduction
Osteoarthritis is a chronic degenerative disorder that causes pain, stiffness, and limited range of motion in affected joints.1 Causes of osteoarthritis include cartilage degradation, subchondral bone outgrowths, synovial hypertrophy, and altered ligament integrity.2 Injection therapies are most commonly administered when pain or functional limitations of osteoarthritis are significant despite oral medication or exercise.3 While injection of corticosteroids is the most common regimen for musculoskeletal disorders, it merely provides short-term improvement of symptoms while posing the risk of aggravating cartilage damage and producing tissue atrophy.4 Administration of hyaluronic acid is another common approach to replenishing dysfunctional synovial fluid in joints affected by osteoarthritis. However, according to the results of a recent large-scale meta-analysis, this approach offers only a
clinically irrelevant benefit.5
Regenerative therapy involves the injection of a small volume of solution into multiple sites of painful ligament and tendon insertions (entheses) and adjacent joint spaces, with the goal of reducing pain and ostensibly promoting tissue repair and growth.6 Hyperosmolar dextrose, the most commonly used solution, was first examined in the treatment of osteoarthritis in randomized controlled trials (RCTs) in 2000.7,8 The proposed mechanism by which dextrose prolotherapy alleviates joint degenerative disorders includes creating a hyperosmolar environment to induce cell rupture and release platelet-derived growth factor.9 In recent years, increasing evidence has supported the use of dextrose prolotherapy for patients with osteoarthritis.10 However, the magnitude of benefit of prolotherapy may be affected by variations in treatment protocols, evaluation intervals, and therapeutic measurement tools. Therefore, we designed a meta-analysis to evaluate the effect of using dextrose prolotherapy in the treatment of osteoarthritis.
Methods
Two electronic databases, PubMed and Scopus, were explored from their earliest records until February 2016.11–15 The Cochrane Collaboration Central Register of Controlled Clinical Trials, Cochrane Systematic Reviews (ClinicalTrials.gov), which includes bibliographies of included trials and related systematic reviews, was also checked for pertinent references. The key terms encompassed prolotherapy, dextrose, cartilage, degeneration, and osteoarthritis, and were entered as medical subject headings and text words for searches.
We included single-arm prospective studies, studies with non-randomized assignment to control or active treatment (quasi-experimental studies), and RCTs. Case series lacking a predestinated therapeutic protocol and a follow-up plan were excluded. The inclusion criteria for studies and RCTs were as follows: (1) they should involve adult participants with degenerative cartilage disorders, regardless of the affected sites; (2) they should involve adults receiving serial dextrose injections to the involved joints in at least one treatment arm of a study; and (3) they should be studies providing quantitative measurement of functional change or pain reduction before and after interventions.
Data extraction and quality assessment
Two authors (C-YH and M-YH) independently assessed all the relevant literature and extracted the following data from selected studies: patient demographics, dosage and interval of dextrose administrations, injection techniques, and changes in functional outcome measurements. The quality of the RCTs was appraised by the Jadad scale, the composite scores of which ranged from 0 to 5 points. Studies that scored fewer than 3 points were regarded to have poorly designed methodology.12–14 The Newcastle–Ottawa Scale, which has a maximal score of 9 points, was used to evaluate the quality of selection, comparability, exposure, and outcome in single-arm and quasi-experimental studies.12–14 A total score less than 4 points was assumed to signify a study that was low in quality. Discrepancies in opinions between the two evaluators were resolved by discussion or a ruling by the corresponding author.
Data synthesis and analysis
The main outcome was determined by the severity of pain, derived from the visual analog scale or knee pain scale. The first priority of pain measurement extraction was the pain score during movement/walking because of a stronger association with patient’s function. If pain score with movement/walking was not available in the trial, we used the pain subscale from Western Ontario and McMaster Universities Arthritis Index as the substitute. To assess the effectiveness of dextrose prolotherapy compared with the baseline condition, we used the standardized mean difference between the baseline and status after treatment in the treatment arm using dextrose prolotherapy. Data were calculated from the ratio of the difference between baseline and posttreatment pain to the standard deviation (SD) of pooled results, (Painbaseline – Painposttreatment)/pooled SD. Pooled SD equaled the square root of {[(participant numbers in baseline – 1)*(standard deviation of pain scales in baseline)2 + (participant numbers after treatment – 1)*(standard deviation of pain scales after treatment)2]/[(participant numbers in baseline – 1) + (participant numbers after treatment – 1)]}.12,13 A positive value of the effect size indicated a decrease in pain compared with baseline.
In terms of the effect size of dextrose prolotherapy compared with other injection treatments, we used the ratio of the difference in reduction of pain between the dextrose and reference groups to the pooled SD, (Pain reductiondextrose – Pain reductionreference)/pooled SD. Pooled SD was generated from the square root of {[(participant numbers in the dextrose group – 1)*(standard deviation of pain reduction in the dextrose group)2 + (participant numbers in the control group – 1)*(standard deviation of changes in pain in the control group)2]/[(participant numbers in the dextrose group – 1) + (participant numbers in the control group – 1)]}.12,13 A positive value of the effect size indicated a favorable result after dextrose prolotherapy.
The random effect model was used to pool the effect sizes, and the heterogeneity was determined by I-square and Cochran’s Q methods.16 Whether the effect sizes were modified by the involved joints and difference in control groups was assessed by the subgroup analysis. The funnel plot and Egger test were used to examine the publication bias, defined as the tendency for positive trials to be published and the tendency for negative and null trials not to be published.17 All analyses were performed using Stata 10.0 (StataCorp LP, College Station, TX, USA), and p < 0.05 was considered statistically significant.
Results
Study search and patient characteristics
Of the 170 non-duplicate citations identified from the literature, eleven studies were screened for eligibility (Figure 1). We excluded one single-arm trial, due to lack of quantitative pain evaluation in patients with patellar chondro-arthropathy,18 one RCT comparing single injection of dextrose with erythropoietin,19 one RCT targeting nonspecific chronic low back pain without definite radiologic evidence of lumbar spine osteoarthritis,20 and two observational studies analyzing data from a published RCT exploring knee osteoarthritis.21,22 The final meta-analysis included one single-arm follow-up trial23 and five RCTs,7,8,24–26 four of which probed knee osteoarthritis7,23,24,26 and two of which examined hand osteoarthritis.8,25 Regarding the comparative injection regimens in the five RCTs, one used two serial saline injections followed by one shot of corticosteroids,25 three used sequential administrations of local anesthetics,7,8,24 and one used a crossover design by implementing the dextrose injection at different time points.26
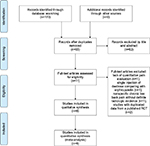  | Figure 1 Flow diagram of the evaluation process for the inclusion or exclusion of studies. Adapted from Moher et al.31 Abbreviation: RCT, randomized controlled trial. |
The meta-analysis enrolled a total of 326 participants, 190 (58.3%) of whom were females. Ages ranged from 56.4 to 64.5 years. The duration from the onset of symptoms to being registered in the study was from 10.7 to 108.0 months. The diagnosis of osteoarthritis was verified by radiographic findings in each trial. As regards the quality assessment, all the RCTs obtained a maximal 2 points in the aspect of “randomization” in Jadad scale since appropriate randomization methods were mentioned. In the aspect of “blinding”, all the RCTs had appropriate blinding methods (maximal 2 points) except the study by Dumais et al26 which did not mention a blinded design of intervention and evaluation (earning 0 points). In the aspect of “an account of all patients”, the two studies conducted by Reeves and Hassanein,7,8 the one by Rabago et al,24 and the one by Dumais et al26 had descriptions of the outcome of all the patients at the end of the studies (earning 1 point). The results of the quality assessment are listed in Table 1. The preparations and injection details of each retrieved study are summarized in Table 2.
Pooled effect sizes
Regarding the treatment arm using dextrose prolotherapy, the effect sizes compared with baseline were 0.65 (95% confidence interval [CI], 0.14–1.17), 0.84 (95% CI, 0.40–1.27), 0.85 (95% CI, 0.60–1.10), and 0.87 (95% CI, 0.53–1.21) after the first, second, third, and fourth or more injections, respectively (Figure 2). In terms of the comparisons between dextrose prolotherapy and other injected solutions, we extracted the data closest to the sixth month after the first injection, which was the last follow-up point available in most studies. The effect size of dextrose prolotherapy compared with other injected solutions was 0.36 (95% CI, 0.10–0.63) (Figure 3A), while that compared with exercise was 0.71 (95% CI, 0.30–1.11) (Figure 3B).
  | Figure 2 Forest plot of the effect of pain reduction from baseline after the first, second, third, and fourth or more injections. Abbreviations: ES, effect size; CI, confidence interval. |
The subgroup analysis showed that dextrose prolotherapy had a superior effect to local anesthesia (effect size, 0.38; 95% CI, 0.07–0.70) and an insignificant advantage over corticosteroids (effect size, 0.31; 95% CI, –0.18 to 0.80) (Figure 3C). There was no difference in the effect sizes regarding the use of dextrose prolotherapy compared with control injection between knee and hand joints (Figure 3D). No publication bias (p=0.8, determined by the Begg’s test) and funnel plot asymmetry were detected in terms of the effect size comparing dextrose prolotherapy and other injected solutions.
Discussion
Our meta-analysis included one single-arm study and five RCTs, and found that dextrose injection reduced pain in patients with hand and knee osteoarthritis compared with their pretreated baseline levels. However, we did not identify a positive dose–response relationship following serial dextrose injections. Dextrose prolotherapy was more effective than local anesthetics injection and exercise, and had an insignificant advantage over corticosteroids. The subgroup analysis further showed that the effect of prolotherapy did not differ between hand and knee osteoarthritis.
Dextrose prolotherapy has been used for treating musculoskeletal pain for decades, but the number of clinical trials investigating its effectiveness is still limited. In 2005, a systematic review summarized case reports, case series, and clinical trials using prolotherapy in the treatment of chronic musculoskeletal pain and found a potential benefit of prolotherapy on osteoarthritis but an inconsistent outcome on low back pain.27 In 2011, a narrative review investigated the same issue, disclosing a favorable trend of prolotherapy in treating osteoarthritis and chronic tendinopathy.6 In 2016, Sit et al conducted a meta-analysis regarding the use of dextrose in the treatment of symptomatic osteoarthritis, mentioning a potential positive effect of prolotherapy. However, their analysis did not include patients with hand osteoarthritis.28 Since 2013, three more RCTs have been published regarding the use of dextrose injection for hand and knee osteoarthritis.19,24,25 Two of these studies showed a superior effect of dextrose administration over the reference regimen. The inconsistency might have arisen from heterogeneity in enrolled participants, differences in injection techniques, and volumes and selections of injected joints. Therefore, a quantitative analysis was required to examine the dose–response relationship and true effect of dextrose prolotherapy in the treatment of osteoarthritis.
We selectively analyzed the serial changes between the baseline and posttreatment status within the dextrose group. The purpose was to examine whether additional injections could reduce more pain or sustain the duration of symptomatic relief. We were aware that the effect size derived from the comparison with the pretreatment condition was overestimated due to the placebo effect and could not be used as true effectiveness of a treatment and was only used as a surrogate approach for evaluation of a potential dose–response relationship (Figure 2).
In our study, the effect of dextrose prolotherapy was positive compared with the reference regimen and exercise at 6 months following the first injection. The result from the comparison between dextrose and exercise was within expectations because injection and needling carry a strong placebo effect, which usually leads to superior response to the noninvasive treatment. The values of the effect size indicated moderately superior treatment effect of dextrose to local anesthetics and corticosteroids.29 Corticosteroids was known to provide short-term pain relief for osteoarthritic knees in a previous meta-analysis,4 and the effect did not last for more than 3 months after administration. In our subgroup analysis, dextrose showed an insignificant benefit over corticosteroids. The reason for lacking statistical significance might be attributed to insufficient study numbers (only one trial) using corticosteroids as reference. Our data implied that dextrose prolotherapy was potentially more effective than an anti-inflammatory (corticosteroids) regimen or placebo (local anesthetics). Furthermore, although the injection volumes of dextrose for small joints differed remarkably from those for large joints, the subgroup analysis did not reveal a significant difference between hand and knee osteoarthritis.
Study limitations
Our study had several limitations. First, the number of trials eligible for meta-analysis was limited, and heterogeneity
existed in the patient populations, injection protocols, comparative regimens, and outcome assessment. Second, we did not analyze effect sizes of functional improvements because the data were not available in each retrieved trial. Third, although previous research proposed that the benefit of dextrose prolotherapy derived from its chondo-protective effect or modulation of intra-articular cytokines, these theories could not be proved by our meta-analysis since there were few data about the measurement of cartilage thickness and intra-articular cytokine level in the retrieved articles. Fourth, hyaluronic acid and platelet-rich plasma are known to counteract osteoarthritis.13,30 Although dextrose prolotherapy appeared to be more effective than the use of corticosteroids and local anesthetics, the comparison with commonly used regimens like hyaluronic acid or platelet-rich plasma was lacking in our literature search. This subject should be investigated in future prospective studies. Fifth, the treatment responsiveness using binary data, which considered the standard outcome variable in pain medicine, is not reported in the included studies. Therefore, we used the effect sizes retrieved from continuous variables like changes in pain or function instead in the quantitative analysis. Finally, the interpretation of the effect of dextrose compared with corticosteroids should be cautious because the finding was derived from only a single study.
Conclusion
Compared with pretreatment baseline, dextrose injections decreased pain in osteoarthritis patients but did not exhibit a positive dose–response relationship following serial injections. Dextrose prolotherapy was found to provide a better therapeutic effect than exercise, local anesthetics, and probably corticosteroids when patients were retested 6 months following the initial injection. The effect of prolotherapy did not differ between hand and knee osteoarthritis
Acknowledgments
This research was supported by grants from the National Science Council (104-2314-B-002-022-MY2) and by funding from National Taiwan University Hospital, Bei-Hu branch (Beihu 10501).
Disclosure
The authors report no conflicts of interest in this work.
References
Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21(9):1145–1153. | ||
Mobasheri A, Matta C, Zakany R, Musumeci G. Chondrosenescence: definition, hallmarks and potential role in the pathogenesis of osteoarthritis. Maturitas. 2015;80(3):237–244. | ||
Ayhan E, Kesmezacar H, Akgun I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J Orthop. 2014;5(3):351–361. | ||
Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005328. | ||
Jevsevar D, Donnelly P, Brown GA, Cummins DS. Viscosupplementation for osteoarthritis of the knee: a systematic review of the evidence. J Bone Joint Surg Am. 2015;97(24):2047–2060. | ||
Vora A, Borg-Stein J, Nguyen R. Regenerative injection therapy for osteoarthritis: fundamental concepts and evidence-based review. PM R. 2012;4(5 Suppl):S104–S109. | ||
Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000;6(2):68–74, 77–80. | ||
Reeves KD, Hassanein K. Randomized, prospective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DIP, PIP, and trapeziometacarpal) joints: evidence of clinical efficacy. J Altern Complement Med. 2000;6(4):311–320. | ||
Rabago D, Best TM, Zgierska AE, Zeisig E, Ryan M, Crane D. A systematic review of four injection therapies for lateral epicondylosis: prolotherapy, polidocanol, whole blood and platelet-rich plasma. Br J Sports Med. 2009;43(7):471–481. | ||
Rabago D, Yelland M, Patterson J, Zgierska A. Prolotherapy for chronic musculoskeletal pain. Am Fam Physician. 2011;84(11):1208–1210. | ||
Slobogean GP, Verma A, Giustini D, Slobogean BL, Mulpuri K. MEDLINE, EMBASE, and Cochrane index most primary studies but not abstracts included in orthopedic meta-analyses. J Clin Epidemiol. 2009;62(12):1261–1267. | ||
Chang KV, Hsiao MY, Chen WS, Wang TG, Chien KL. Effectiveness of intra-articular hyaluronic acid for ankle osteoarthritis treatment: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2013;94(5):951–960. | ||
Chang KV, Hung CY, Aliwarga F, Wang TG, Han DS, Chen WS. Comparative effectiveness of platelet-rich plasma injections for treating knee joint cartilage degenerative pathology: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95(3):562–575. | ||
Chang KV, Hung CY, Chen WS, Lai MS, Chien KL, Han DS. Effectiveness of bisphosphonate analogues and functional electrical stimulation on attenuating post-injury osteoporosis in spinal cord injury patients- a systematic review and meta-analysis. PLoS One. 2013;8(11): | ||
Chang KV, Hung CY, Han DS, Chen WS, Wang TG, Chien KL. Early versus delayed passive range of motion exercise for arthroscopic rotator cuff repair: a meta-analysis of randomized controlled trials. Am J Sports Med. 2015;43(5):1265–1273. | ||
Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res. 1993;2(2):121–145. | ||
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. | ||
Hauser RA, Sprague IS. Outcomes of prolotherapy in chondromalacia patella patients: improvements in pain level and function. Clin Med Insights Arthritis Musculoskelet Disord. 2014;7:13–20. | ||
Rahimzadeh P, Imani F, Faiz SH, Entezary SR, Nasiri AA, Ziaeefard M. Investigation the efficacy of intra-articular prolotherapy with erythropoietin and dextrose and intra-articular pulsed radiofrequency on pain level reduction and range of motion improvement in primary osteoarthritis of knee. J Res Med Sci. 2014;19(8):696–702. | ||
Yelland MJ, Glasziou PP, Bogduk N, Schluter PJ, McKernon M. Prolotherapy injections, saline injections, and exercises for chronic low-back pain: a randomized trial. Spine (Phiia Pa 1986). 2004;29(1):9–16; discussion 16. | ||
Rabago D, Kijowski R, Woods M, et al. Association between disease-specific quality of life and magnetic resonance imaging outcomes in a clinical trial of prolotherapy for knee osteoarthritis. Arch Phys Med Rehabil. 2013;94(11):2075–2082. | ||
Rabago D, Patterson JJ, Mundt M, et al. Dextrose and morrhuate sodium injections (prolotherapy) for knee osteoarthritis: a prospective open-label trial. J Altern Complement Med. 2014;20(5):383–391. | ||
Rabago D, Zgierska A, Fortney L, et al. Hypertonic dextrose injections (prolotherapy) for knee osteoarthritis: results of a single-arm uncontrolled study with 1-year follow-up. J Altern Complement Med. 2012;18(4):408–414. | ||
Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11(3):229–237. | ||
Jahangiri A, Moghaddam FR, Najafi S. Hypertonic dextrose versus corticosteroid local injection for the treatment of osteoarthritis in the first carpometacarpal joint: a double-blind randomized clinical trial. J Orthop Sci. 2014;19(5):737–743. | ||
Dumais R, Benoit C, Dumais A, et al. Effect of regenerative injection therapy on function and pain in patients with knee osteoarthritis: a randomized crossover study. Pain Med. 2012;13(8):990–999. | ||
Rabago D, Best TM, Beamsley M, Patterson J. A systematic review of prolotherapy for chronic musculoskeletal pain. Clin J Sport Med. 2005;15(3):376–380. | ||
Sit RW, Chung VCh, Reeves KD, et al. Hypertonic dextrose injections (prolotherapy) in the treatment of symptomatic knee osteoarthritis: a systematic review and meta-analysis. Sci Rep. 2016;6:25247. | ||
Sullivan GM, Feinn R. Using effect size—or why the P value is not enough. J Grad Med Educ. 2012;4(3):279–282. | ||
Richette P, Chevalier X, Ea HK, et al. Hyaluronan for knee osteoarthritis: an updated meta-analysis of trials with low risk of bias. RMD Open. 2015;1(1):e000071. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.