Back to Journals » Cancer Management and Research » Volume 12
Comparative Diagnostic Evaluation with Contrast-Enhanced Ultrasound, Computed Tomography and Magnetic Resonance Imaging in Patients with Pancreatic Cystic Neoplasms
Authors Sun Y, Yang S, Qi E, Liu F, Zhou F, Lu Y, Liang P , Ye H, Yu X
Received 22 January 2020
Accepted for publication 9 April 2020
Published 28 April 2020 Volume 2020:12 Pages 2889—2898
DOI https://doi.org/10.2147/CMAR.S246564
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Ya Sun,1,2,* Shuo Yang,3,* Erpeng Qi,1 Fangyi Liu,1 Fubo Zhou,1 Yuhan Lu,1 Ping Liang,1 Huiyi Ye,4 Xiaoling Yu1
1Department of Interventional Ultrasound, Chinese PLA General Hospital, Beijing 100853, People’s Republic of China; 2Department of Ultrasound, Aerospace Central Hospital, Beijing 100049, People’s Republic of China; 3Chinese PLA Medical School, Beijing, 100853, People’s Republic of China; 4Radiology Department, Chinese PLA General Hospital, Beijing 100853, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaoling Yu
Department of Interventional Ultrasound, Chinese PLA General Hospital, 28 Fuxing Road, Beijing 100853, People’s Republic of China
Email [email protected]
Purpose: The purpose of our study was to evaluate the role of contrast-enhanced ultrasound (CEUS) with magnetic resonance imaging (MRI) and computed tomography (CT) in the pathological diagnosis of pancreatic cystic neoplasms (PCNs).
Methods: A total of 90 patients (66 women, 24 men) aged 18– 71 years were studied prospectively. CEUS was performed in all patients, whereas MRI was performed in 85 patients and CT in 69 patients. We analyzed the sensitivity and accuracy of these three imaging modalities to diagnose the PCNs. Neoplasm size, location, shape, intralesional mural nodules, septa and duct dilatation were also assessed by different radiologists.
Results: There were no significant differences in sensitivity for discriminating PCNs from pancreatic cystic lesions between CEUS and MRI (p=0.614) or between CEUS and CT (p=0.479). The diagnostic accuracy of CEUS for classifying PCNs was 64.4% (58/90), which was higher than that of CT (53.6%, 37/69, P=0.017), and lower than that of MRI (70.6%, 60/85, p=0.791). Regarding tumor size for lesions larger than 3 cm, CEUS was superior to CT in differentiating the specific type of PCN (p=0.041), and CEUS had the same value as MRI (p=0.774). Furthermore, CEUS is valuable for precisely characterizing internal structures, for instance, septa (p=0.003, compared with CT; p=0.443, compared with MRI) and nodules (p= 0.018, compared with CT; p=0.033, compared with MRI). The number of septa (p=0.033) and cyst morphology (p=0.016) were meaningful indicators in differentiating serous and mucinous adenoma. There was no significant difference in evaluating size and detecting duct dilatation among the three imaging methods.
Conclusion: CEUS compares favorably with MRI in displaying the inner structure of PCNs and offers advantages over CT. CEUS can contribute in an important way to the diagnosis of pancreatic cystic neoplasms.
Keywords: contrast-enhanced ultrasound, computed tomography, magnetic resonance imaging, pancreatic cystic neoplasms, diagnostic evaluation
Introduction
High-quality cross-sectional imaging examinations and medical checkups have significantly increased the detection of pancreatic cystic lesions (PCLs). With this imaging development, it is challenging to manage PCLs, which are mostly incidentally discovered.1,2 The pooled rate of PCLs was higher in studies conducted in the US than in Asia (12.6% vs 3.1%).3 PCLs commonly encountered in clinical practice include pancreatic cystic neoplasms (PCNs) and nonneoplasms, which mainly refer to true cysts and pseudocysts. In the past, among all PCLs, pseudocysts were the most common; however, with imaging being more sensitive, PCNs are more frequently detected, and the prevalence of PCNs accounts for up to 60% of all PCLs.4,5 The most common PCNs consist of serous cystadenomas (SCAs), mucinous cystadenomas (MCAs), intraductal papillary mucinous neoplasms (IPMNs), and solid pseudopapillary neoplasms (SPNs).6,7 Neuroendocrine neoplasms (NENs) and cystadenocarcinomas are relatively less common PCNs.8,9 The differential diagnosis of these cystic lesions ranges from benign to potentially or truly malignant lesions. Although most PCLs are considered benign, particularly those that are small in size, they have the potential to become malignant.10,11 Thus, differentiating PCNs from nonneoplastic cysts and improving the diagnostic performance of classification the different PCNs is critical.
Currently, the imaging methods used to diagnose PCNs mainly include conventional ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI), magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasound (EUS) with or without fine-needle aspiration (FNA).8 As the most convenient and inexpensive examination, US can detect cystic lesions with high sensitivity, but its diagnostic accuracy is low.12,13 CT, due to its short scanning duration and high-quality multiplanar image display, has been regarded as a preferred imaging modality for evaluating PCLs,14,15 but it is radioactive and has lower image resolution. MRI is deemed necessary when a pancreatic cyst is identified on cross-sectional imaging. Although MRI is considered the gold standard imaging method to evaluate these cysts in follow-up visits, it possesses some limits, which include its high costs and time-consuming process,16–18 and the contrast agent of CT and MRI was both nephrotoxicity. Recently, contrast-enhanced sonography (CEUS), which is performed with microbubbles, a blood-pool contrast agent, is not nephrotoxic and has been increasingly used in the evaluation of pancreatic lesions.19 There are several published reports of using CEUS in PCLs, which proved that CEUS can play a vital role in improving the diagnostic rate of pancreatic cystic lesions.12,13,20-22 However, no study has compared the diagnostic performance of CEUS with CT and MRI in pancreatic neoplasms. In this study, we prospectively compared the diagnostic capability of CT, MRI, and CEUS of 90 patients with PCNs who were finally confirmed surgicopathologically and analyzed the mainly detailed characterization of these lesions. In addition, some diagnostic indicators of serous cystadenomas and mucinous cystadenomas were analyzed.
Materials and Methods
Patient Population
Undetermined pancreatic cystic lesions detected by any examination were prospectively evaluated for possible study enrollment. The inclusion criteria were as follows: 1) being at least 18 years old; 2) undergoing CEUS examination; 3) undergoing CT or MRI examination; and 4) surgicaopathologically diagnosed with PCNs. The exclusion criteria were as follows: patient with acute pancreatitis; patient being allergic to the intravenous contrast agent; patient with pseudocyst, which is highly suspected clinically.
Imaging Examination
The ultrasound instrument was Sequoia 512 (Siemens Ultrasound, Mountain View, CA, USA); the probe frequency was 1–4 MHz; and the mechanical index was lower than 0.12. SonoVue (Bracco, Milan, Italy), the contrast agent, was dissolved in 5 mL saline, and a bolus of 2.4 mL of this solution was injected into the antecubital vein quickly, followed by a 5 mL saline flush. A GE Lightspeed 64-slice spiral CT scan or Siemens SOMATOM Sensation 64-Slice CT scanner was applied with a slice thickness of 5 mm. Plain CT was followed by contrast-enhanced CT with nonionic iodinated contrast material. Philips Achieva 1.5 T machine or GE3.0T machine was applied for MRI examination. Postgadolinium contrast-enhanced images were obtained in all cases.
Study Design
There were several reference standards for our study. 1) The location of the lesion was categorized into 2 groups: head and body/tail location, and the superior mesenteric vessels were the dividing landmark, whose right part defined as the head portion of the pancreas encompassed the uncinate, head, and neck portion; and the left aspect included body and tail. 2) For tumor size, the cross-section plane and coronal section plane on CT or MRI both need to be measured, and the larger dimension was selected as the size of the cyst. Otherwise, CEUS was conducted through various planes, and the longest one was selected as its size. 3) When the widest part of the pancreatic duct was more than 3 mm, it was defined as duct dilatation. 4) Regular shape was defined when the lesion was approximately round or oval, and the rest were irregular. 5) A septum was identified as a fibrous structure that started from the wall and ended at the other side, and a nodule was identified as a solid component from the wall protruding into the inner cyst.
Patient who was suspected of having pancreatic cystic neoplasm was recommended to undergo CEUS, CT, and MRI. CT and MRI images were individually read by radiologists who had more than 10 years of experience and were aware of our study design. US and CEUS were performed by two ultrasound physicians who were blinded to the CT and MRI results—one with more than 20 years and the other with more than 10 years of experience with CEUS diagnoses.
ALL patients underwent CEUS, most patients (n = 85) underwent MRI, and 69 patients performed CT. A total of 53 patients underwent both CT and MRI. Once all of the confirmed PCNs were enrolled, the sensitivity and accuracy of CEUS, MRI and CT in diagnosing the cysts were assessed. When the lesion was diagnosed as “cyst lesion” or “cyst-solid lesion”, it would be categorized as an undetermined PCL; when the lesion was diagnosed with “cystadenoma” or “cyst tumor”, it would be categorized as an unclassified PCN.
Statistical Analysis
All statistics were analyzed using the SPSS 17.0 software package (SPSS, Chicago, IL). The Pearson chi-square test, Fisher’s exact test and continuity correction were applied to compare the differences in numbers between two groups. Quantitative data are expressed as the mean or median; differences were tested using a nonparametric test or a t-test. A two-tailed P-value of less than 0.05 was considered statistically significant.
Results
Basic Characteristics
From April 2015 to July 2019, 96 patients were evaluated for the presence of PCLs, and 90 patients (66 women, 24 men; mean age 42.6±13.3 years; 18–71 years) who were pathologically diagnosed with PCNs were enrolled. The other six PCLs included 2 pseudocysts, 2 retention cysts, 1 epidermoid cyst and 1 lymphoepithelial cyst. Among the 90 enrolled patients, 36 patients had SCAs, 29 had MCAs, 11 had IPMNs, 8 had SPNs, 3 had NENs, and 3 had cystadenocarcinomas. Ten patients were examined because of abdominal pain, 12 were detected due to abdominal distension, and 68 had no symptoms. The cysts were detected in the head location in 35 patients and in the body–tail location in 52 patients. Two patients had diffuse involvement of IPMNs, and one had multiple cysts. Cyst size was assessed by CEUS for 90 cysts, 85 by MRI and 69 by CT. One patient had multiple cysts, and only the largest cyst was assessed using both MRI and CEUS. The mean size was 4.6 cm (1.6–13 cm; 2.5 cm) by CEUS, 4.1 cm (1.2–13.5 cm; 2.9 cm) by MRI, and 4.9 cm (2.3–10.7 cm; 2.1 cm) by CT. There was no statistically significant difference in the assessment of cyst size using these three imaging techniques (Table 1).
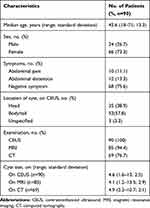 |
Table 1 Characteristics of the Enrolled Study Population |
The Diagnostic Performance of the Three Imaging Examinations in PCNs
Regarding the 90 PCNs, CEUS was able to discriminate PCNs from nonneoplastic PCLs with a sensitivity of 88.9% (80/90) and an accuracy for differentiating the specific type of PCN of 64.4% (58/90); MRI was able to discriminate PCNs from nonneoplastic PCLs with a sensitivity of 91.8% (78/85) and an accuracy of 70.6% (60/85) for differentiating the specific type of PCN; and CT was able to discriminate PCNs from nonneoplastic PCLs with a sensitivity of 84.1% (58/69) and an accuracy for differentiating the specific type of PCN of 53.6% (37/69). There were no significant differences in sensitivity for discriminating PCNs from nonneoplastic PCLs between CEUS and MRI (p=0.614), between CEUS and CT (P=0.479), or between MRI and CT (P=0.207). Regarding the accuracy for differentiating the specific type of PCN, CEUS and MRI were both higher than that of CT (p=0.017, p=0.03), but there was no significant difference between CEUS and MRI (p=0.791) (Figure 1, Table 2).
 |
Table 2 Sensitivity and Accuracy of CEUS, MRI and CT to Diagnose the PCNs |
Diagnostic Accuracy of Different Cyst Sizes and Detection Rates of Nodules, Septa, and Duct Dilatation in PCNs by CEUS, MRI and CT
In this study, there were 15 patients who underwent both CEUS and CT with small (<3 cm) cysts and 54 patients with large (≥3 cm) cysts. CEUS was superior to CT in differentiating the specific type of PCN for large lesions (77.8% vs 59.3%, p=0.041), while these two imaging modalities showed no large differences for small lesions (40.0% vs 33.3%, p=1.000). For CEUS and MRI, the result was the opposite. Twenty-two patients underwent both CEUS and MRI with small cysts and 63 patients with large cysts. There were no significant differences between the two modalities in differentiating the specific type of PCN for large lesions (74.6% vs 71.4%, p=0.774); however, MRI was superior to CEUS for small lesions (36.4% vs 68.2%, p=0.039). Septa were detected in 37 of 69 cysts by CEUS and in 20 of 69 cysts by CT (53.6% vs 29.0%, p= 0.003). Among the patients who underwent both CEUS and MRI, septa were detected in 46 of 85 cysts by CEUS and in 41 of 85 cysts by MRI (54.1% vs 48.2%, p=0.443). With regard to nodules, the detection rate by CEUS (24.6%, 17/19; 31.8%, 27/85) was higher than that by CT (8.7%, 6/69; p=0.018) or MRI (17.6%, 15/85;p=0.033). There were no significant differences in the detection rates of duct dilation between CEUS and CT (14.5% vs 11.6%, p=0.625) or between CEUS and MRI (14.1% vs 15.3%, p=1.000) (Table 3).
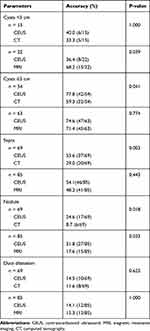 |
Table 3 Comparison of Diagnostic Accuracy of Different Cysts Size and Detection Rates of Nodule, Septa, Duct Dilatation in Pancreatic Cystic Neoplasms by CEUS, MRI and CT |
Comparison of Location, Shape and Septa Between SCAs and MCAs by CEUS
There were 15/36 lesions detected in the head location and 21/36 in the body–tail location with SCA. For MCAs, the lesion was located in the head region in 11/29 patients (one SCA had multiple cysts, and only the largest one was assessed in the location research) and 18/29 patients in the body–tail region. There was no significant difference in terms of location between SCAs and MCAs. For shape, a regular shape was noted in 14/36 patients with SCA, and in 19/29 patients with MCA, and an irregular shape was detected in 22/36 patients with SCA, and in 10/29 patients with MCA. Zero to two septa were present in 14/36 cases of SCA and in 20/29 cases of MCA, and ≥2 septa were present in 22/36 cases of SCA and 9/29 patients with MCA. There was a significant difference in the shape and number of septa between SCAs and MCAs using CEUS (Table 4).
 |
Table 4 Characteristics of Serous Cystadenomas and Mucinous Cystadenomas Observed by Contrast-Enhanced Ultrasonography |
Discussion
The American Gastroenterological Association guidelines4 have indicated that the risk of malignancy of PCLs in asymptomatic patients is significantly increased in patients with a cyst >3 cm, in patients with a solid component, and in patients with a dilated duct. Nevertheless, this guideline does not include symptomatic patients with PCLs or patients with SPNs and NENs. Asymptomatic patients with small (<1 cm) PCNs also need a diagnostic work-up because malignancy can also occur (2%).6 As a consequence, the timely distinguishing PCNs from nonneoplastic PCLs and accurate diagnosis of these patients are also of importance to help clinical management. As a consequence, we use “cyst size”, “nodule”, “septa”, and “duct dilatation” as research indicators. It should be noted that “septa” is an indicator to help discriminate between neoplastic and nonneoplastic cysts, SCA and MCA.22
The accepted first-line imaging modalities for PCN surveillance are CT and MRI, and the Korean Society of Abdominal Radiology recommends that both contrast-enhanced MRI with MRCP and contrast-enhanced CT with multiplanar reformation be used as imaging modalities for the follow-up of incidental pancreatic cystic lesions. However, some cystic lesions display similar morphologic characteristics, complicating the differentiation of neoplastic from nonneoplastic PCLs.4
In our study, MRI was determined to be the best diagnostic imaging modality for these patients, outperforming CEUS and CT. However, CEUS showed no significant diagnostic differences with MRI in whether discriminating PCNs from nonneoplastic PCLs or differentiating the specific type of PCN. In characterizing the specific type of PCNs, MRI and CEUS both excel in CT. A previous study reported that the accuracy of CT and MRI in making the correct diagnosis for PCLs ranges from 40% to 60%,23–25 which is lower than that reported herein. Fan et al12 reported that CEUS showed substantial agreement with enhanced CT for the diagnostic classification accuracy of pancreatic cystic lesions. In this study, although the ultrasound physician was blinded to the CT and MRI results, it is difficult to avoid receiving related information from the patient or their relatives. This may have led to the higher diagnostic performance observed for CEUS.
For evaluating the size and detection of duct dilatation, there were no significant differences among CEUS, MRI and CT. This finding suggests that if we follow-up changes in size or duct dilatation, these imaging methods have the same value. Nevertheless, when these lesions were bounded by 3 cm, CEUS had a relatively poor diagnostic performance in group<3 cm, which actually comprised6 cysts smaller than 2 cm and 14 located in the unicate or tail. For patients with small cystic pancreatic lesions smaller than 2 cm, it is sometimes difficult to judge the inner structure, especially when these lesions are located in the unicate or tail where the presence of gastrointestinal gas can obscure imaging.
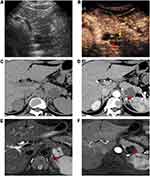
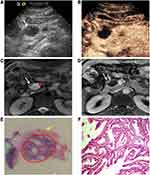
The septa and nodule detection rates by CEUS in our research were both much higher than those by CT and MRI, especially good at the nodule detection ability. Previously, M. D’Onofrio et al20 reported that the difference between the diagnostic accuracy of CEUS and that of MRI was not significant in the identification of septa and nodules. Septa and nodules were always observed more clearly on T2-weighted MRI images than on contrast-enhanced MRI images (Figures 2 and 3). These inner structures are more difficult to detect on plain CT images, so their identification is mainly depend on contrast-enhanced CT (Figure 2). The slice thickness or artifacts from the breathing movement may have led to missed diagnosis on CT or MRI. During contrast-enhanced sonography, due to its real-time dynamic properties, it is easy to visualize these structures as the contrast agent passes into the capillary beds of the septa (Figures 2 and 3) or nodule (Figure 3) inside the lesion.12,20,21 This finding explains the larger number of detections of these inner structures on CEUS than on CT or MRI in our study. The ability to detect septa and nodules can contribute to the differentiation of a cystic neoplasm from a nonneoplasm, as well as the determination of their possible malignant potential.20,26
As the most common types of PCNs, SCAs and MCAs have adverse biological behaviors. Therefore, the ability to differentiate SCAs from MCAs is important. In agreement with our previous study,22 there was a significant difference in the shape and number of septa in discriminating these two adenomas, but the statistics of “location” were inconsistent with our previous results, mainly because of the two different samples used. SCAs were originally identified as “microcystic adenomas”, but this classification has been criticized soon on account of reports of macrocystic variants, which are similar to the main type of MCAs.27 In our study, there were 14 oligocyst SCAs with regular morphology and 9 had no septa.
In the western hemisphere, SCNs account for 32% to 39%, MCNs for 10% to 45%, IPMNs for 21% to 33%, and SPNs for less than 10% of all PCNs.4,6 Nevertheless, IPMNs accounted for only 12.2% (11/90) in our cohort. The relatively low prevalence of IPMNs may be due to the following two aspects. First, unlike SCAs and MCNs, IPMNs are found in older individuals between 60 and 70 years of age.28 In our study, we included only patients with a median age of 42.6 (18–71) years who underwent surgery. Some elderly individuals who were unwilling or unsuitable for surgical treatment might cause a lack of IPMNs. Second, patients with acute pancreatitis were excluded from this work; however, it was reported that the typical symptoms of IPMNs include abdominal pain (55%), weight loss (45%), jaundice (17%), and acute pancreatitis (15%).28
Notably, some of the patients in this study underwent EUS with or without FNA. As mentioned in some articles and guidelines,4,6,28-30 if the results of the traditional technique indicate nondiagnostic or “suspicious” morphological aspects, EUS with or without FNA is recommended, which is valuable for the differential diagnosis and prediction of the malignancy of pancreatic cysts. However, as EUS is an invasive examination that poses greater risk and may also not be readily available in some hospitals, we compared only these traditional imaging modalities in our study. Moreover, the role of FNA is still limited, especially in discriminating IPMN from MCA;31 therefore, surgical pathology is regarded as the diagnostic standard.
There were several limitations to this study. First, there was a high sensitivity for discriminating PCN from nonneoplastic PCL probability because the enrolled patients were all pathologically diagnosed with PCNs, and the lesions clinically diagnosed with nonneoplasms, mainly pseudocysts, were mostly suggested to occur during follow-up. Second, CEUS is highly operator dependent, and the ultrasound physicians were both experienced in CEUS, particularly in PCNs, which could have had affected the final diagnosis. Third, the detection of septa and nodules has not been completely correlated with the anatomic appearance of the specimens, which could lead to subjective evaluations. Last, as this is a prospective study, a pilot study was conducted for measuring the diagnostic capacity of these three imaging modalities to estimate the group size. According to the statistical formula, we needed 100 patients to evaluate the diagnostic capacity of CEUS, 109 patients for CT and 92 patients for MRI, so an additional study in a larger population is needed.
Altogether, the results of our study suggest that, as an economic, radiation-free, and effective imaging modality, CEUS can be used as an optional examination method and contribute to the diagnosis and classification of PCNs.
Ethics
The Ethics Committee at the General Hospital of the Chinese People’s Liberation Army approved this prospective study. Written informed consent was obtained from all patients who participated in this study.
Acknowledgments
This study is supported by Scientific Research Fund of Army of China, No. 14BJZ01, the Chinese National Nature Science Foundations (81471683 and 81671710). No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Disclosure
The authors report that they have no conflicts of interest related to this work.
References
1. Nougaret S, Mannelli L, Pierredon MA, Schembri V, Guiu B. Cystic pancreatic lesions: from increased diagnosis rate to new dilemmas. Diag Interv Imag. 2016;97(12):1275–1285. doi:10.1016/j.diii.2016.08.017
2. de Jong K, Nio CY, Hermans JJ, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8(9):806–811. doi:10.1016/j.cgh.2010.05.017
3. Zerboni G, Signoretti M, Crippa S, Falconi M, Arcidiacono PG, Capurso G. Systematic review and meta-analysis: prevalence of incidentally detected pancreatic cystic lesions in asymptomatic individuals. Pancreatology. 2019;19(1):2–9. doi:10.1016/j.pan.2018.11.014
4. Scheiman JM, Hwang JH, Moayyedi P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148(4):824–848.e822. doi:10.1053/j.gastro.2015.01.014
5. Basturk O, Coban I, Adsay NV. Pancreatic cysts: pathologic classification, differential diagnosis, and clinical implications. Arch Pathol Lab Med. 2009;133(3):423–438.
6. Buscarini E, Pezzilli R, Cannizzaro R, et al. Italian consensus guidelines for the diagnostic work-up and follow-up of cystic pancreatic neoplasms. Dig Liver Dis. 2014;46(6):479–493. doi:10.1016/j.dld.2013.12.019
7. Lennon AM, Canto MI, Pancreatic C. Part 2: should we be less cyst centric? Pancreas. 2017;46(6):745–750. doi:10.1097/MPA.0000000000000841
8. Morelli L, Guadagni S, Borrelli V, et al. Role of abdominal ultrasound for the surveillance follow-up of pancreatic cystic neoplasms: a cost-effective safe alternative to the routine use of magnetic resonance imaging. World J Gastroenterol. 2019;25(18):2217–2228. doi:10.3748/wjg.v25.i18.2217
9. Khan A, Khosa F, Eisenberg RL. Cystic lesions of the pancreas. AJR. 2011;196(6):W668–677. doi:10.2214/AJR.10.4378
10. Khashab MA, Shin EJ, Amateau S, et al. Tumor size and location correlate with behavior of pancreatic serous cystic neoplasms. Am J Gastroenterol. 2011;106(8):1521–1526. doi:10.1038/ajg.2011.117
11. Lee ES, Kim JH, Yu MH, et al. Diagnosis and Surveillance of Incidental Pancreatic Cystic Lesions: 2017 Consensus Recommendations of the Korean Society of Abdominal Radiology. Korean J Radiol. 2019;20(4):542–557. doi:10.3348/kjr.2018.0640
12. Fan Z, Yan K, Wang Y, et al. Application of contrast-enhanced ultrasound in cystic pancreatic lesions using a simplified classification diagnostic criterion. Biomed Res Int. 2015;2015:974621. doi:10.1155/2015/974621
13. Xu M, Xie XY, Liu GJ, et al. The application value of contrast-enhanced ultrasound in the differential diagnosis of pancreatic solid-cystic lesions. Eur J Radiol. 2012;81(7):1432–1437. doi:10.1016/j.ejrad.2011.03.048
14. Sahani DV, Sainani NI, Blake MA, Crippa S, Mino-Kenudson M. del-Castillo CF. Prospective evaluation of reader performance on MDCT in characterization of cystic pancreatic lesions and prediction of cyst biologic aggressiveness. AJR. 2011;197(1):W53–61. doi:10.2214/AJR.10.5866
15. Cho HW, Choi JY, Kim MJ, et al. Pancreatic tumors: emphasis on CT findings and pathologic classification. Korean J Radiol. 2011;12(6):731–739. doi:10.3348/kjr.2011.12.6.731
16. Kim YC, Choi JY, Chung YE, et al. Comparison of MRI and endoscopic ultrasound in the characterization of pancreatic cystic lesions. AJR. 2010;195(4):947–952. doi:10.2214/AJR.09.3985
17. Pinho DF, Rofsky NM, Pedrosa I. Incidental pancreatic cysts: role of magnetic resonance imaging. TMRI. 2014;23(2):117–128. doi:10.1097/RMR.0000000000000018
18. Megibow AJ, Baker ME, Morgan DE, et al. Management of incidental pancreatic cysts: a white paper of the ACR incidental findings committee. JACR. 2017;14(7):911–923. doi:10.1016/j.jacr.2017.03.010
19. Lin LZ, Li F, Liu Y, Xing LX, Du LF. Contrast-Enhanced Ultrasound for differential diagnosis of pancreatic mass lesions: a meta-analysis. Med Ultrason. 2016;18(2):163–169. doi:10.11152/mu.2013.2066.182.ceu
20. D’Onofrio M, Megibow AJ, Faccioli N, et al. Comparison of contrast-enhanced sonography and MRI in displaying anatomic features of cystic pancreatic masses. AJR. 2007;189(6):1435–1442. doi:10.2214/AJR.07.2032
21. Beyer-Enke SA, Hocke M, Ignee A, Braden B, Dietrich CF. Contrast enhanced transabdominal ultrasound in the characterisation of pancreatic lesions with cystic appearance. JOP. 2010;11(5):427–433.
22. Sun Y, Zhou F, Liu F, et al. Discrimination of serous cystadenoma from mucinous cystadenoma in the pancreas with contrast-enhanced ultrasonography: a prospective study in 61 patients. Onco Targets Ther. 2017;10:1285–1294. doi:10.2147/OTT.S125497
23. Visser BC, Yeh BM, Qayyum A, Way LW, McCulloch CE, Coakley FV. Characterization of cystic pancreatic masses: relative accuracy of CT and MRI. AJR. 2007;189(3):648–656. doi:10.2214/AJR.07.2365
24. Sainani NI, Saokar A, Deshpande V, Fernandez-del Castillo C, Hahn P, Sahani DV. Comparative performance of MDCT and MRI with MR cholangiopancreatography in characterizing small pancreatic cysts. AJR. 2009;193(3):722–731. doi:10.2214/AJR.08.1253
25. Lu X, Zhang S, Ma C, Peng C, Lv Y, Zou X. The diagnostic value of EUS in pancreatic cystic neoplasms compared with CT and MRI. Endos Ultrasound. 2015;4(4):324–329. doi:10.4103/2303-9027.170425
26. Jais B, Rebours V, Malleo G, et al. Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the international association of pancreatology and European pancreatic club (European study group on cystic tumors of the pancreas). Gut. 2016;65(2):305–312. doi:10.1136/gutjnl-2015-309638
27. Chu LC, Singhi AD, Haroun RR, Hruban RH, Fishman EK. The many faces of pancreatic serous cystadenoma: radiologic and pathologic correlation. Diag Interv Imag. 2017;98(3):191–202. doi:10.1016/j.diii.2016.08.005
28. Fusaroli P, Kypraios D, Eloubeidi MA, Caletti G. Levels of evidence in endoscopic ultrasonography: a systematic review. Dig Dis Sci. 2012;57(3):602–609. doi:10.1007/s10620-011-1961-y
29. Serrani M, Lisotti A, Caletti G, Fusaroli P. Role of contrast harmonic-endoscopic ultrasound in pancreatic cystic lesions. Endos Ultrasound. 2017;6(1):25–30. doi:10.4103/2303-9027.190931
30. Moris M, Raimondo M, Woodward TA, et al. diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration cytology, carcinoembryonic antigen, and amylase in intraductal papillary mucinous neoplasm. Pancreas. 2016;45(6):870–875. doi:10.1097/MPA.0000000000000559
31. Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12(3):183–197. doi:10.1016/j.pan.2012.04.004
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.