Back to Journals » International Journal of General Medicine » Volume 14
Comparative Analysis of Clinicopathological Characteristics, Survival Features, and Protein Expression Between Basaloid and Squamous Cell Carcinoma of the Esophagus
Authors Xu Y, Zhao H, Tong Y, Wang W, Huang J, Zhu W
Received 2 April 2021
Accepted for publication 26 May 2021
Published 26 July 2021 Volume 2021:14 Pages 3929—3939
DOI https://doi.org/10.2147/IJGM.S314054
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yingying Xu,1 Huanyu Zhao,2 Yusuo Tong,1 Wanwei Wang,1 Jing Huang,1 Weiguo Zhu1
1Department of Radiation Oncology, The Affiliated Huaian No.1 People’s Hospital of Nanjing Medical University, Huai’an, Jiangsu, 223300, People’s Republic of China; 2Department of Pathology, The Affiliated Huaian No.1 People’s Hospital of Nanjing Medical University, Huai’an, Jiangsu, 223300, People’s Republic of China
Correspondence: Weiguo Zhu Email [email protected]
Background: Basaloid squamous cell carcinoma (BSCC) is a rare variant of squamous cell carcinoma (SCC) of the esophagus. This study aimed to assess the discrepancy in clinicopathological characteristics and protein expression between esophageal BSCC and typical esophageal SCC.
Study Design: We reviewed 40 cases of esophageal BSCC. As controls, 63 well-differentiated SCC (WSCC) patients, 70 moderately differentiated SCC (MSCC) patients, and 51 poorly differentiated SCC (PSCC) patients were selected. The clinicopathologic characteristics and immunoreactivity of Ki-67, p53, p63, and epidermal growth factor receptor (EGFR) were then evaluated in the BSCC and typical SCC patients.
Results: The 5-year survival rates for the BSCC patients were 27.5%. The prognostic outcomes of the BSCC group were similar to those of the PSCC and MSCC groups but worse than that of the WSCC group, with a significant difference (P=0.045). Ki-67 expression was significantly higher in the BSCC group than that in the WSCC group (P < 0.05). Meanwhile, there were no significant differences in the expression of the other molecular markers (p53, p63, and EGFR) between the typical SCC and BSCC groups (P > 0.05). The median survival time of esophageal the BSCC patients with low p53 expression was significantly longer than that of the patients with high p53 expression (P=0.026). Further, the median survival time of the esophageal BSCC patients with high p63 expression was significantly longer than that of the patients with low p63 expression (P=0.041). Meanwhile, Ki-67 and EGFR expressions were not correlated with OS in the BSCC group.
Conclusion: Esophageal BSCC has a more clinically virulent course. Notably, p53 and p63 expression are associated with prognosis in BSCC. These findings conject that evaluation of multiple cancer biomarkers might be a promising auxiliary diagnostic indicator in BSCC.
Keywords: basaloid squamous cell carcinoma, Ki-67, p53, p63, EGFR
Introduction
Esophageal cancer (EC) is one of the most common cancers worldwide.1 More than 450,000 incident cases of EC are diagnosed annually, and this is projected to increase by 140% over the next 10 years.2,3 Squamous cell carcinoma (SCC) remains the most common type of EC globally. Basaloid squamous cell carcinoma (BSCC) is a rare and poorly differentiated variant of the typical SCC. BSCC is histologically defined as an infiltrating carcinoma with dense cells, hyperchromatic nuclei, and sparse cytoplasm.4 In previous reports, the incidence of esophageal BSCC ranged from 0.77% to 5.0%.5–11
Pathological manifestations of esophageal BSCC were local invasive growth, poor differentiation, high proliferative activity, and high incidence of distant metastasis.12 The prognostic outcomes of BSCC in comparison to those of esophageal SCC are yet to be clarified. According to the high histological characteristics of BSCC, some researchers have suggested that patients with esophageal BSCC have poorer prognosis than those with esophageal SCC. In contrast, others have detected similar outcomes.5,13–15 However, comparative analyses of survival outcomes between esophageal SCC and BSCC have been rarely reported.
Given that BSCC is a rare distinct variant of SCC, further assessments (eg, cancer biomarkers), in addition to those conducted for preliminary diagnosis, are needed. Ki-67, a well-known marker of cell proliferation, has been discovered to be significantly connected with poor prognosis in a great many cancers. P63 plays a part in cell differentiation, development, and cancerization. Epidermal growth factor receptor (EGFR) play a major role in the growth and maintenance of organisms by regulating cell division, apoptosis, differentiation, and migration. Studies have shown that Ki-67, p53, p63, and EGFR are associated with EC tumorigenesis and progression. However, evidence on the role of Ki-67, p53, p63, and EGFR in esophageal BSCC are scarce.
Thus, this study aimed to assess the discrepancy in clinicopathological characteristics and prognosis between esophageal BSCC and typical esophageal SCC. Moreover, we assessed the protein expression of Ki-67, p53, p63, and EGFR to determine their prognostic impact in esophageal BSCC.
Materials and Methods
Study Design and Patients
This study was approved by the Ethics Committee of the Affiliated Huaian No. 1 People’s Hospital of Nanjing Medical University and was conducted according to the tenets of the 1064 Declaration of Helsinki and its later amendments. Informed consent was exempted due to the retrospective nature of the study. All patients’ health information, such as patient’s name, age, diagnosis, and medical history were treated strictly confidentially.
The subjects were 1681 patients with esophageal carcinoma who underwent surgical resection in the Affiliated Huai’an No. 1 People’s Hospital of Nanjing Medical University between December 2012 and June 2015. Esophageal cancer was classified according to the 7th edition of the Union for International Cancer Control-American Joint Committee on Cancer (UICC-AJCC) tumor, node, metastasis (TNM) staging system. In this cohort, there are only 40 patients (2.38%) were histologically diagnosed with BSCC. These 40 BSCC patients included 7 patients with stage IA, 4 patients with stage IB, 19 patients with stage IIA, 3 patients with stage IIB, 5 patients with stage IIIA, and 2 patients with stage IIIC. Except for these 40 BSCC patients, we diagnosed and collected 63 well-differentiated SCC (WSCC) patients, 70 moderately differentiated SCC (MSCC) patients, and 51 poorly differentiated SCC (PSCC) patients in other 1641 patients by two independent pathological doctors. Finally, we collected all these 224 patients for further comparisons and analysis in order to distinguish their clinical and molecular pathological features (Table 1).
 |
Table 1 Comparison of Clinicopathologic Characteristics Among the Patients with BSCC and with Different Histologic Grades of SCC |
Pathologic Review
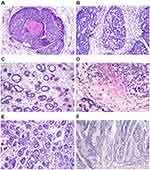
Formalin-fixed paraffin-embedded tissue sections stained with hematoxylin and eosin were reviewed by pathologists. BSCC is mainly consist of basaloid cells (BCs), with high nucleo-cytoplasm ratio and obvious mitotic characteristics. BCs are mainly arranged in the shape of solid-contoured lobules. Solid sheets, anastomosing trabeculae, or microcystic structures are present in some cases. The frequencies of solid nests with central necrosis (Figure 1A), cribriform patterns (Figure 1B), ductal differentiation (Figure 1C), amorphous hyaline substance (Figure 1D), microcyst nests (Figure 1E) and trabecular nests (Figure 1F) were evaluated.
Immunohistochemistry
Immunohistochemical analysis were performed using the streptavidin-biotin-peroxidase complex technique, according to the manufacturer’s protocol. The sections were incubated with primary antibodies as follows: Ki-67, 1:40 (Neomarkers, USA); p53, 1:80 (Dako, Glostrup, Denmark); p63, 1:50 (Dako, Glostrup, Denmark); and EGFR, 1:20 (Novocastra, UK). For all antibodies, slides were then incubated in liquid DAB+chromogen system (Dako, Glostrup, Denmark) and counterstained with hematoxylin.
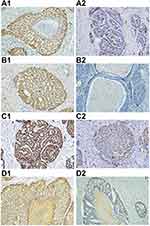
The cutoff values for positivity were as follows. High and low Ki-67 expression were defined as nuclear immunoreactivity of less than and at least 50% staining, respectively.16 P53, p63, and EGFR were scored depending on the staining intensity (0–3+), as no staining (0), weak (1+), moderate (2+), and strong (3+) staining. Cases with scores of 0–1+ were considered to have low expression, whereas those with a score of 2+-3+ were considered to have high expression (Figure 2).17,18
Statistical Analysis
Survival time was calculated from the start of treatment to death or the most recent follow-up. Clinicopathologic data between esophageal BSCC and esophageal SCC patients were statistically compared using the x2 test or Fisher’s exact test, as appropriate. The cumulative survival of the patients was calculated using the Kaplan-Meier method compared between two groups using the log-rank method. Data from patients who were still alive by the time of analysis were censored. All statistical analysis were performed using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). P values <0.05 were considered significant.
Results
Clinicopathologic Characteristics and Prognosis
The clinicopathologic characteristics, including sex, age, tumor location, depth of invasion, lymph node metastasis, and clinical stage (P > 0.05), were not significantly different between the four groups. The clinicopathological characteristics of the BSCC group and different histologic grades of the SCC group are shown in Table 1.
The 1-, 3-, and 5-year survival rates of the BSCC group were 77.5%, 42.5%, and 27.5%, respectively; the WSCC group, 87.3%, 60.3%, and 44.4%; the MSCC group, 84.3%, 52.9%, and 38.6%; and the PSCC group, 82.4%, 51.0%, and 37.3%, respectively. The prognostic outcomes of the BSCC group were similar to those of the PSCC and MSCC groups, but worse than that of the WSCC group, with the difference being significant (P=0.045) (Figure 3).
 |
Figure 3 Comparison of survival curves between patients with esophageal BSCC and esophageal SCC (BSCC vs WSCC, P = 0.045; BSCC vs MSCC, P = 0.191; BSCC vs PSCC, P = 0.331). |
Prognostic Factors for Survival
Univariate analysis of risk factors for survival in BSCC showed significant correlations between patient survival and lymph node metastasis (P=0.013) and clinical stage (P=0.002) (Table 2).
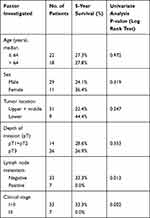 |
Table 2 Univariate Analysis of Prognostic Factors |
Histopathological Features
The main component of BSCC is BCs with high nucleo-cytoplasmic ratio. Mitotic figures were prominent. Some cases also presented with solid sheets, anastomosing trabeculae, or microcystic structures. Solid nests with central necrosis and amorphous hyaline substance were present in all cases of BSCC. Cribriform patterns were observed in 18 cases (45%). Ductal differentiation was found in 17/40 (42.5%) cases, microcyst nests and trabecular nests was found in 33/40 (82.5%) cases.
Expression of Ki-67, P53, P63, and EGFR
Ki-67 expression was significantly higher in the BSCC and PSCC groups than that in the WSCC group (P < 0.05). Meanwhile, there were no significant differences in p53, p63, and EGFR expressions between the typical SCC and BSCC groups (P > 0.05) (Table 3).
 |
Table 3 Comparison of Immunohistochemical Status of Ki-67, P53, P63, and EGFR Between the Esophageal BSCC Group and Esophageal SCC Groups |
Clinicopathologic Variables and Survival in Relation to Protein Expression in Esophageal BSCC
In the BSCC group, the expressions of Ki-67, p53, p63, and EGFR protein did not correlate with sex, age, tumor location, depth of invasion, lymph node metastasis, and clinical stage (P > 0.05) (Table 4). However, the median survival time of the BSCC patients with low p53 expression was significantly longer than that of patients with high p53 expression (42 months vs 16 months, P = 0.026). Our data also showed a significant correlation between p63 expression and OS. The median survival time of the BSCC patients with high p63 expression was significantly longer than that of patients with low p63 expression (33 months vs 14 months, P = 0.041). Ki-67 and EGFR were not correlated with OS in the BSCC group (Figure 4).
 |
Table 4 Relationship of Ki-67, P53, P63, and EGFR Expressions with the Clinicopathological Characteristics of Esophageal BSCC |
Discussion
BSCC is a rare histological type of EC with distinct characteristics. In our study, only 40 of the 1681 (2.38%) patients with esophageal carcinoma had BSCC. The incidence is consistent with those published previously.5–11 The histopathological characteristics of BSCC differ from those of typical SCC of the esophagus. BSCC itself has typical histological components: a solid nest with central necrosis, cribriform pattern, amorphous hyaline substance, microcyst nests and trabecular nests.19
The diagnosis of esophageal BSCC is dependent upon histopathological examination. Endoscopic biopsy is the most commonly used method for pretreatment diagnosis. Considering the differing histological characteristics of esophageal BSCC, some cases are confused with small cell carcinoma, adenoid cystic carcinoma, poorly differentiated SCC, or adenosquamous carcinoma.5,15,20,21 Furthermore, endoscopic biopsy has low diagnostic accuracy for BSCC because in many cases of BSCC, the tumor surface is covered by normal epithelium or SCC. Oguma et al reported that endoscopic biopsy only has 32% accuracy for diagnosing BSCC.22 In our study, the accuracy of preoperative endoscopic biopsy for esophageal BSCC only 7.5% accuracy. A total of 36 cases were misdiagnosed as SCC, and one was misdiagnosed as small cell esophageal carcinoma. A previous study reported that some modalities can help improve the accuracy of diagnosis. For example, if BSCC is suspected during endoscopy, a biopsy should be performed from the erosive lesion.22 Biopsy samples should be obtained from multiple sites in the deep portion of the tumor to determine the histological characteristics of BSCC.23
Due to the low incidence of esophageal BSCC and the lack of relevant data, no standard treatment for esophageal BSCC has been established. So far, the treatment for esophageal BSCC is akin to that for typical SCC, with surgical resection being an important modality for esophageal BSCC. In our study, we observed no significant differences in sex, age, tumor location, depth of invasion, lymph node metastasis, or clinical stage between BSCC and SCC, consistent with previous findings.5,24–28
The prognostic differences between BSCC and typical esophageal SCC are unclear. Sarbia et al5 have observed that no significant difference in clinical prognosis between patients with BSCC and with SCC who have a similar clinical stage and undergo curative resection. In contrast, Some previous reports have suggested that the prognosis of esophageal BSCC with locally invasive growth pattern and high proliferation activity is worse than that of typical SCC.13–15 In our study, among the patients who underwent thoracic esophagectomy, the prognostic outcomes of the BSCC group were similar to those of the PSCC and MSCC group. Meanwhile, they were worse than those of the WSCC group, with a significant difference. Our results are consistent with those previously reported by Imamhasan et al.19 Importantly, these findings support that early stage BSCC could have a lower clinically virulent course. In addition, the histologic grade of esophageal BSCC may be considered comparable to that of poorly or moderately differentiated esophageal SCC.
Molecular diagnosis is becoming increasingly important in the therapeutic decisions for esophageal BSCC. Immunohistochemical analysis could be used to distinguish between BSCC and SCC. Many antibodies have been previously found to have a diagnostic value in BSCC. These include type IV collagen, S-100, Bcl-2, and so on.5,21,27,29 However, none of these are specific for BSCC.
Molecular markers (Ki-67, p53, p63, and EGFR) have been recently widely studied in SCC.18,30–32 However, the patterns of Ki-67, p53, p63, and EGFR expressions have yet to be extensively analyzed in BSCC. In this study, we assessed the proliferative activity of Ki-67, p53, p63, and EGFR expression via immunohistochemical analysis to determine the differences in biological behaviors between BSCC and other esophageal SCC. We found that Ki-67, p53, p63, and EGFR expressions were not significantly correlated with any clinicopathological characteristics of BSCC.
The Ki-67 antigen is associated with cell proliferation. It has been previously reported that esophageal BSCC has higher proliferative activity than typical esophageal SCC.19,25 Our results also showed significantly higher Ki-67 expression in BSCC and PSCC than in WSCC. These results are consistent with the high proliferation activity and high biological malignancy of BSCC. However, Ki-67 expression did not influence the OS rates in the BSCC group, as has also been reported in previous studies.7,19
Normal p53 induces apoptosis in detecting DNA damage plays a crucial part in many human cancers. In our study, we examined p53 expression via immunohistochemistry and detected no significant difference in expression status between BSCC and SCC tumors. Similar results were found previously by Baba et al.11 With respect to the prognostic impact of p53 expression, Shimaya et al33 reported that cumulative survival of patients with p53-negative esophageal SCC was significantly better than that of patients with p53-positive esophageal SCC. In addition, Wang et al17 detected that high p53 expression in esophageal SCC was an independent predictor of unfavorable OS. Similarly, our data showed that esophageal BSCC patients with high p53 expression had significantly worse survival than those with low p53 expression.
P63, an important transcription factor, is engaged in a broad spectrum of biological activities, including cell proliferation, development, differentiation, survival, senescence, and apoptosis. P63 expression is often altered in a range of human cancers has suggested that altered p63 expression is linked to tumor progression. The p63 protein plays a crucial part in the carcinogenesis of esophageal SCC. Several investigators have suggested that high p63 expression is an early incident in esophageal SCC.34,35 P63 is significantly overexpressed in some cancers, but its association with overall survival and disease-free survival outcomes is unclear. Some reports show that increased expression of p63 in squamous cell carcinomas correlates with good prognosis.36–38 Massion et al36 demonstrated that there was early and frequent genomic amplification of p63 in the development of squamous carcinoma of the lung and that patients showing amplification and overexpression of p63 had prolonged survival. Takahashi et al38 reported that 5-year OS was significantly longer in patients with p63-positive esophageal SCC than in patients with p63-negative esophageal SCC at the 50% cut-off value for p63 expression. Patients with negative p63 expression had markedly unfavorable survival. In the current study, we found similar p63 expression status between BSCC and SCC. Moreover, the survival prognosis of patients with high p63 expression in esophageal BSCC was significantly better than that of patients with low p63 expression. The results of our study on p63 are similar to those of previous studies. P63 could be an important prognostic marker in esophageal BSCC.
EGFR is a membrane-bound tyrosine kinase receptor that mediates cell growth and survival signals. As such, EGFR has been widely studied in cancer, and numerous studies have demonstrated that EGFR overexpression is related to poor prognosis in esophageal SCC.39 Although EGFR overexpression appeared to be associated worse survival in esophageal SCC, our data showed that EGFR expression does not independently influence the clinical prognosis of BSCC of the esophagus. Similar results were previously reported by Sato-Kuwabara et al.7
This study has some limitations, including the small number of BSCC cases. A multicenter study with a larger number of esophageal cancer cases will help to further verify our findings. Despite these limitations, our data on the pathological characteristics of esophageal BSCC can be helpful for its early and accurate diagnosis.
Conclusions
The prognosis of BSCC is similar to that of PSCC and MSCC, but worse than that of WSCC. Notably, Ki-67 expression is higher in BSCC than in WSCC. High p63 expression is correlated with favorable prognosis in BSCC, while high p53 expression is significantly related to poor OS.
Funding
Funding information Science and technology development fund of Nanjing Medical University, Grant/AwardNumber: NMUB2020135.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gupta B, Kumar N. Worldwide incidence, mortality and time trends for cancer of the oesophagus. Eur J Cancer Prev. 2017;26(2):107–118. doi:10.1097/CEJ.0000000000000249
2. Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet. 2013;381(9864):400–412. doi:10.1016/S0140-6736(12)60643-6
3. Napier KJ, Scheerer M, Misra S. Esophageal cancer: a Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol. 2014;6(5):112–120. doi:10.4251/wjgo.v6.i5.112
4. Huang Z, Liang Y, Wu X. [Basaloid squamous carcinoma of the oesophagus: a distinctive clinico-pathological entity]. Zhonghua Bing Li Xue Za Zhi. 1995;24(2):90–92. Chinese.
5. Sarbia M, Verreet P, Bittinger F, et al. Basaloid squamous cell carcinoma of the esophagus: diagnosis and prognosis. Cancer. 1997;79(10):1871–1878. doi:10.1002/(SICI)1097-0142(19970515)79:10<1871::AID-CNCR5>3.0.CO;2-J
6. Morice WG, Ferreiro JA. Distinction of basaloid squamous cell carcinoma from adenoid cystic and small cell undifferentiated carcinoma by immunohistochemistry. Hum Pathol. 1998;29(6):609–612. doi:10.1016/S0046-8177(98)80011-7
7. Sato-Kuwabara Y, Fregnani JHTG, Jampietro J, et al. Comparative analysis of basaloid and conventional squamous cell carcinomas of the esophagus: prognostic relevance of clinicopathological features and protein expression. Tumour Biol. 2016;37(5):6691–6699. doi:10.1007/s13277-015-4551-3
8. Lam KY, Law S, Luk JM, et al. Oesophageal basaloid squamous cell carcinoma: a unique clinicopathological entity with telomerase activity as a prognostic indicator. J Pathol. 2001;195(4):435–442. doi:10.1002/path.984
9. Cho C, et al. Basaloid squamous carcinoma of the oesophagus: a distinct neoplasm with multipotential differentiation. Histopathology. 2000;36(4):331–340. doi:10.1046/j.1365-2559.2000.00851.x
10. Li TJ, Zhang Y-X, Wen J, et al. Basaloid squamous cell carcinoma of the esophagus with or without adenoid cystic features. Arch Pathol Lab Med. 2004;128(10):1124–1130. doi:10.5858/2004-128-1124-BSCCOT
11. Baba Y, Ishimoto T, Harada K, et al. Molecular characteristics of basaloid squamous cell carcinoma of the esophagus: analysis of KRAS, BRAF, and PIK3CA mutations and LINE-1 methylation. Ann Surg Oncol. 2015;22(11):3659–3665. doi:10.1245/s10434-015-4445-z
12. Shibata Y, Baba E, Ariyama H, et al. Metastatic basaloid-squamous cell carcinoma of the esophagus treated by 5-fluorouracil and cisplatin. World J Gastroenterol. 2007;13(26):3634–3637. doi:10.3748/wjg.v13.i26.3634
13. Zhang B-H, Cheng G-Y, Xue Q, et al. Clinical outcomes of basaloid squamous cell carcinoma of the esophagus: a retrospective analysis of 142 cases. Asian Pac J Cancer Prev. 2013;14(3):1889–1894. doi:10.7314/APJCP.2013.14.3.1889
14. Tripathi M, Swanson PE. Rare tumors of esophageal squamous mucosa. Ann N Y Acad Sci. 2016;1381(1):122–132. doi:10.1111/nyas.13108
15. Chen S-B, Weng H-R, Wang G, et al. Basaloid squamous cell carcinoma of the esophagus. J Cancer Res Clin Oncol. 2012;138(7):1165–1171. doi:10.1007/s00432-012-1180-8
16. Deng H-Y, Chen Z-H, Wang Z-Q, et al. High expression of Ki-67 is an independent favorable prognostic factor for esophageal small cell carcinoma. Oncotarget. 2017;8(33):55298–55307. doi:10.18632/oncotarget.19426
17. Wang H, Zhou Y, Qian L, et al. Prognostic value of SOX2, Cyclin D1, P53, and ki-67 in patients with esophageal squamous cell carcinoma</em>. Onco Targets Ther. 2018;11:5171–5181. doi:10.2147/OTT.S160066
18. Yu W, Yang X, Chu L, et al. Prognostic value of EGFR family expression in lymph node-negative esophageal squamous cell carcinoma patients. Pathol Res Pract. 2018;214(7):1017–1023. doi:10.1016/j.prp.2018.04.017
19. Imamhasan A, Mitomi H, Saito T, et al. Immunohistochemical and oncogenetic analyses of the esophageal basaloid squamous cell carcinoma in comparison with conventional squamous cell carcinomas. Hum Pathol. 2012;43(11):2012–2023. doi:10.1016/j.humpath.2012.02.010
20. Akagi I, Miyashita M, Makino H, et al. Basaloid squamous cell carcinoma of the esophagus: report of two cases. J Nippon Med Sch. 2008;75(6):354–360. doi:10.1272/jnms.75.354
21. Nishimura W, Naomoto Y, Hamaya K, et al. Basaloid-squamous cell carcinoma of the esophagus: diagnosis based on immunohistochemical analysis. J Gastroenterol Hepatol. 2001;16(5):586–590. doi:10.1046/j.1440-1746.2001.02424.x
22. Oguma J, Ozawa S, Kazuno A, et al. Clinicopahological features of superficial basaloid squamous cell carcinoma of the esophagus. Dis Esophagus. 2017;30(12):1–5. doi:10.1093/dote/dox076
23. Tada T, Honma R, Imai J-I, et al. A novel gene expression scoring system for accurate diagnosis of basaloid squamous cell carcinoma of the esophagus. Int J Oncol. 2017;51(3):877–886. doi:10.3892/ijo.2017.4075
24. Zhang XH, Sun GQ, Zhou XJ, Guo HF, Zhang TH. Basaloid squamous carcinoma of esophagus: aclinicopathological, immunohistochemical and electron microscopic study of sixteen cases. World J Gastroenterol. 1998;4(5):397–403. doi:10.3748/wjg.v4.i5.397
25. Sarbia M, Loberg C, Wolter M, et al. Expression of Bcl-2 and amplification of c-myc are frequent in basaloid squamous cell carcinomas of the esophagus. Am J Pathol. 1999;155(4):1027–1032. doi:10.1016/S0002-9440(10)65203-0
26. Bellizzi AM, Woodford RL, Moskaluk CA, et al. Basaloid squamous cell carcinoma of the esophagus: assessment for high-risk human papillomavirus and related molecular markers. Am J Surg Pathol. 2009;33(11):1608–1614. doi:10.1097/PAS.0b013e3181b46fd4
27. Ohashi K, Horiguchi S, Moriyama S, et al. Superficial basaloid squamous carcinoma of the esophagus. A clinicopathological and immunohistochemical study of 12 cases. Pathol Res Pract. 2003;199(11):713–721. doi:10.1078/0344-0338-00487
28. Salami A, Abbas AE, Petrov R, et al. Comparative analysis of clinical, treatment, and survival characteristics of basaloid and squamous cell carcinoma of the esophagus. J Am Coll Surg. 2018;226(6):1086–1092. doi:10.1016/j.jamcollsurg.2017.10.019
29. Kobayashi Y, Nakanishi Y, Taniguchi H, et al. Histological diversity in basaloid squamous cell carcinoma of the esophagus. Dis Esophagus. 2009;22(3):231–238. doi:10.1111/j.1442-2050.2008.00864.x
30. Wang L, Yang HY, Zheng YQ. Personalized medicine of esophageal cancer. J Cancer Res Ther. 2012;8(3):343–347. doi:10.4103/0973-1482.103510
31. Kawamura T, Goseki N, Koike M, et al. Acceleration of proliferative activity of esophageal squamous cell carcinoma with invasion beyond the mucosa: immunohistochemical analysis of Ki-67 and p53 antigen in relation to histopathologic findings. Cancer. 1996;77(5):843–849. doi:10.1002/(SICI)1097-0142(19960301)77:5<843::AID-CNCR6>3.0.CO;2-F
32. Hara T, Kijima H, Yamamoto S, et al. Ubiquitous p63 expression in human esophageal squamous cell carcinoma. Int J Mol Med. 2004;14(2):169–173.
33. Shimaya K, Shiozaki H, Inoue M, et al. Significance of p53 expression as a prognostic factor in oesophageal squamous cell carcinoma. Virchows Arch a Pathol Anat Histopathol. 1993;422(4):271–276. doi:10.1007/BF01608335
34. Glickman JN, Yang A, Shahsafaei A, et al. Expression of p53-related protein p63 in the gastrointestinal tract and in esophageal metaplastic and neoplastic disorders. Hum Pathol. 2001;32(11):1157–1165. doi:10.1053/hupa.2001.28951
35. Hu H, Xia S-H, Li A-D, et al. Elevated expression of p63 protein in human esophageal squamous cell carcinomas. Int J Cancer. 2002;102(6):580–583. doi:10.1002/ijc.10739
36. Massion PP, Taflan PM, Jamshedur Rahman SM, et al. Significance of p63 amplification and overexpression in lung cancer development and prognosis. Cancer Res. 2003;63(21):7113–7121.
37. Massion PP, Taflan PM, Rahman SMJ, et al. Role of p63 amplification and overexpression in lung cancer development. Chest. 2004;125(5):102S. doi:10.1378/chest.125.5_suppl.102S-a
38. Takahashi Y, Noguchi T, Takeno S, et al. Reduced expression of p63 has prognostic implications for patients with esophageal squamous cell carcinoma. Oncol Rep. 2006;15(2):323–328.
39. Yu WW, Guo Y-M, Zhu M, et al. Clinicopathological and prognostic significance of EGFR over-expression in esophageal squamous cell carcinoma: a meta-analysis. Hepatogastroenterology. 2011;58(106):426–431.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.