Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Combined Treatment with Micro-Focused Ultrasound with Visualization and Intradermal Incobotulinumtoxin-A for Enlarged Facial Pores: A Retrospective Study in Asians
Authors Park JY, Lee JS, Lee SR , Lee DH
Received 28 December 2022
Accepted for publication 1 May 2023
Published 15 May 2023 Volume 2023:16 Pages 1249—1255
DOI https://doi.org/10.2147/CCID.S402001
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Je-Young Park,1,* Ji Su Lee,2,* Soo Ran Lee,2 Dong Hun Lee2– 4
1Apkoo-Jung Oracle Dermatology Center, Seoul, Republic of Korea; 2Department of Dermatology, Seoul National University Hospital, Seoul, Republic of Korea; 3Department of Dermatology, Seoul National University College of Medicine, Seoul, Republic of Korea; 4Institute of Human-Environment Interface Biology, Medical Research Center, Seoul National University, Seoul, Republic of Korea
*These authors contributed equally to this work
Correspondence: Dong Hun Lee, Department of Dermatology, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul, 03080, Republic of Korea, Tel +82-2-2072-4916, Fax +82-2-742-7344, Email [email protected]
Background: Despite the increasing need for the improvement of enlarged facial pores, the treatment remains challenging. A few previous studies have reported the effects of micro-focused ultrasound with visualization (MFU-V) or intradermal incobotulinumtoxin-A (INCO) on enlarged facial pores.
Objective: To evaluate the efficacy and safety of combined treatment with superficial MFU-V and intradermal INCO for enlarged facial pores.
Methods: This single-center retrospective study included 20 patients treated with MFU-V and intradermal INCO to improve enlarged facial pores. Outcomes were evaluated 1, 4, 12, and 24 weeks after a single session of the combined procedure. Pore count and density were objectively quantitated using a three-dimensional scanner, and improvement was assessed using the physician and patient Global Aesthetic Improvement Scale (GAIS).
Results: The mean pore count and density decreased after one week and decreased by up to 62% until 24 weeks. After one week, almost all patients (100% in physician GAIS and 95% in patient GAIS) showed improvement with a grade 3 (much improved) or higher. All adverse events were transient.
Conclusion: Combined treatment with MFU-V and intradermal INCO could be effective and safe for reducing enlarged facial pores; the improvements can be sustained for up to 24 weeks.
Keywords: pores, enlarged facial pores, incobotulinumtoxin-A, micro-focused ultrasound with visualization
Introduction
‘Enlarged facial pores’ are visible topographic features on the skin surface, corresponding to enlarged openings of pilosebaceous follicles.1 They are one of the greatest cosmetic concerns for many individuals, as evidenced by numerous cosmetic products designed to conceal enlarged pores.2 Enlarged facial pores can deteriorate facial skin quality, negatively affecting an individual’s appearance and self-image, which can impede social interaction and ruin the quality of life.2 Although the pathogenesis of enlarged facial pores is still unclear, three factors are thought to be the main pathology: 1) high sebum production, 2) decreased skin elasticity around pores, and 3) increased hair follicle volume.1,3–7 Other factors, including chronic recurrent acne, sex hormones, and skin care regimens, such as inappropriate use of cosmetics, washing habits, and sun exposure, also affect pore enlargement.1,8
Diverse treatment methods to reduce pores have been reported, including topical/systemic retinoids, chemical peeling, botulinum toxin, filler, and energy-based devices, such as intense pulse light, diode, radiofrequency, fractional laser, and micro-focused ultrasound with visualization (MFU-V).1,2,9–19 However, there are no established treatments, and treatment of enlarged facial pores is still challenging.1,9 Particularly, enlarged pores easily recur after treatment discontinuation, which negatively impacts treatment satisfaction.20 Given the multifactorial pathophysiology of enlarged facial pores, combining treatments might be more effective than a single modality.1 MFU-V and intradermal incobotulinumtoxin-A (INCO) have been shown to independently improve enlarged pores to varying extents,10–19 but the combined use of both modalities has not been assessed. Both modalities are reported to involve minimal downtime, potentially making this combination a suitable treatment option for patients who cannot afford long recovery periods. As such, this study aimed to investigate the efficacy and safety of combined treatment with MFU-V and INCO for enlarged facial pores in Asians.
Materials and Methods
Study Subjects
This was a single-center, retrospective clinical study. The patients who visited Apkoo-Jung Oracle Dermatology Center between April and November 2021 and received facial treatments with combined intradermal INCO and MFU-V for enlarged facial pores were reviewed. Among these patients, those who had regular follow-ups at the clinic four times (after 1 week, 4 weeks, 12 weeks, and 24 weeks) after the procedure were included. If the date of follow-up differed by more than 3 days (after 1 week), 5 days (after 4 weeks), or 7 days (after 12 weeks or 24 weeks) of the scheduled date, the results for the patient were not included. Patients who underwent botulinum toxin injections or other cosmetic procedures in the face in the preceding 12 months were also excluded. The present study was conducted in the respect of the Declaration of Helsinki. This study received approval from the Institutional Review Board (IRB) of the Korea National Institute for Bioethics Policy (No.: P01-202210-01-007). As this research was a retrospective study, the IRB waived the requirement for obtaining informed consent. However, we obtained consent forms, which included the review and publication of medical records and photographs, from patients and ensured the confidentiality of all patient data.
Treatment
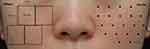
The face was cleansed with isopropyl alcohol, and anesthetic cream (Lidocan cream [lidocaine 96 mg/g]; Daehan Newpharm, Gyeonggi, South Korea) was applied for 30 min before injection. First, MFU-V (Ultherapy®; Ulthera, Inc. and Merz North America, Inc. Raleigh, NC, USA) was performed on the clinically identified enlarged pore area on both anterior cheeks. We marked the area with a surgical pen before treating it with MFU-V, followed by an intradermal injection of INCO. Twenty-five treatment lines per 2.5×2.5 cm2 square (ie, 100 lines per cheek) were delivered to the deep dermis using a 10-MHz/1.5 mm transducer at a setting of energy level 2 (0.18J), focusing on the areas where the pores were most noticeable and avoiding the medial part of nasolabial fold (Figure 1). Treatment was delivered in at least two passes or a cross-hatched pattern to minimize patient discomfort and prevent excessive stacking. Then, intradermal injections with INCO (Xeomin) were administered to the same areas that received MFU-V therapy. Each 100-U vial of INCO was reconstituted using 5 mL of bacteriostatic saline. Then, the reconstituted INCO was administered at 20 injection points per side intradermally, 0.5 U (0.025 mL) per injection point. The injection dose was 10 U per side (Figure 1).
Assessment
The clinical outcome was evaluated at baseline and 1, 4, 12, and 24 weeks after a single session of the combined treatment with MFU-V and INCO. Facial pores (count and density) were objectively evaluated using a three-dimensional scanner (Morpheus 3D; Morpheus Co., Gyeonggi, South Korea). This device contained software that automatically analyzed three-dimensional facial images, including the measurement of facial pores. The Global Aesthetic Improvement Scale (GAIS) was scored by the physician (physician GAIS, assessed by JY Park) and each patient (patient GAIS), using a 5-grade scale as follows: 0, worsened; 1, no change; 2, improved; 3, much improved; and 4, very much improved.10,13
Statistical Analysis
Statistical analysis was performed using SPSS version 27.0 (IBM Co., Armonk, NY, USA). Repeated measures analysis of variance (RM-ANOVA) was performed to compare the baseline and follow-up periods. Differences between each assessment point were compared using independent T-tests with Bonferroni correction. All continuous variables are presented as the mean ± standard deviation. P values of <0.05 were considered statistically significant.
Results
A total of 20 patients were included in the analysis. Most of the patients (95%) were females. The mean age was 36.9±5.5 years. The mean of pore count was significantly decreased after 1 week (870.2±236.2, p<0.001), 4 weeks (673.3±209.2, p<0.001), 12 weeks (529.5±190.0, p<0.001), and 24 weeks (429.3±128.5, p<0.001) post-treatment compared to baseline (1130.6±288.4) (Table 1 and Figure 2). The differences between each assessment point were significant between week 1 vs week 4 (p<0.001) and week 4 vs week 12 (p<0.001), but not between week 12 vs week 24 (p=0.024). Similarly, the mean of pore density also showed a significant decrease at 1 week (23.1±8.1%, p<0.001), 4 weeks (17.8±6.9%, p<0.001), 12 weeks (14.0±5.7%, p<0.001), and 24 weeks (11.1±3.5%, p<0.001) after the treatment compared to the baseline (29.7±8.8%) (Table 1 and Figure 2). The differences between week 1 vs week 4 (p<0.001) and week 4 vs week 12 (p<0.001) were significant, but not between week 12 vs week 24 (p=0.017). Both pore count and density continued to decrease over the entire study period, with maximum improvement at 24 weeks and no rebound increment observed (Figure 3). Both physician and patient GAIS scores showed the best results 1 week after the treatment, with almost all patients (100% in physician GAIS and 95% in patient GAIS) having grade 3 (much improved) or higher (Table 2 and Figure 4). The improvement was generally well-maintained up to 24 weeks after the treatment, with all patients having at least a score of 2 (improved) (Table 2 and Figure 4). Only minor and transient adverse events were reported immediately after treatment; erythema and edema were seen in 16 patients (80%), and bruising in 2 patients (10%). However, no adverse events were reported at 1, 4, 12, and 24 weeks after treatment.
 |
Table 1 Changes in Facial Pore Count and Density at Baseline and 1, 4, 12, and 24 Weeks After Combined Treatment with MFU-V and Intradermal INCO |
 |
Table 2 Changes in Physician and Patient Global Aesthetic Improvement Scale Scores at Baseline and 1, 4, 12, and 24 Weeks After Combined Treatment with MFU-V and Intradermal INCO |
 |
Figure 2 Facial pore count and density at baseline and each follow-up visit after combined treatment with MFU-V and intradermal INCO. |
 |
Figure 4 Trends in physician and patient Global Aesthetic Improvement Scale scores before and after combined treatment with MFU-V and intradermal INCO. |
Discussion
Although various treatment modalities have been employed to reduce enlarged facial pores, they are still challenging to treat and easily recur after treatment discontinuation.1,2,9–20 To the best of our knowledge, this is the first study showing the efficacy and safety of combined treatment with MFU-V and intradermal INCO for enlarged facial pores.
MFU-V, also known as Ultherapy®, is cleared by the United States (US) Food and Drug Administration (FDA) for noninvasive skin lifting.21 The mechanism of action of MFU-V is the delivery of ultrasound energy to target skin layers causing thermal coagulation points, triggering the production of new collagen and elastin and the increase of perifollicular structural support.2,22–24 MFU-V provides real-time visualization and allows the user to see where ultrasound energy will be applied, enabling precise delivery of ultrasound energy to the targeted tissues such as the deep dermis and superficial musculoaponeurotic system.21,23–26 Only a few studies have demonstrated the effect of MFU-V on enlarged facial pores.11–13 Vachiramon et al reported that the mean pore volume reduced by approximately 15% at 1 month and by 25% 4 months after a single session of MFU-V and slightly rebounded to 22% reduction compared to the baseline at 6 months after the procedure.11 Similarly, Lu et al observed the maximum improvement of pores at 3 months after MFU-V treatment, which returned to baseline 6 months after the treatment.13 In our study, pore count and density decreased by approximately 40% at 1 month, 53% at 3 months, and 62% at 6 months after the combined treatment. The decrease in pore count and density was sustained with no rebound increment. This difference could be attributed to more treatment lines (100 lines on each cheek) than in the previous study (50 lines on each cheek)11 and the combination treatment with intradermal INCO in this study.
Botulinum toxin has been widely used for various indications, such as glabellar lines, eyelid wrinkles, and masseter hypertrophy, by utilizing its muscle-paralyzing properties.10 Aside from muscle paralysis, several recent studies have reported its off-label indications, including enlarged facial pores and excess sebum production.10,14–19 Particularly, our group first demonstrated the effect of intradermal INCO in treating enlarged facial pores.10 According to our previous results, pore count and density decreased by approximately 16% at 1 week and 24% at 1 month after the treatment.10 However, after then, pore count and density rebounded to a 12% reduction compared to the baseline at 3 months after injection.10 In the present study, pore count and density decreased by approximately 23% at 1 week, 40% at 1 month, and 53% at 3 months after the combined treatment. The underlying mechanism through which intradermal botulinum toxin injection improves enlarged facial pores is still unclear. It could be mediated directly by paralysis of arrector pili muscles and indirectly by reducing sebum production.2,3,18,19 Sebum production seems to decrease by the botulinum toxin blocking acetylcholine release.2,15 In addition, the botulinum toxin relaxes lymphatic channels, leading to fluid retention in the dermis and ensuing pore reduction.27 Other botulinum toxin type A, such as Abobotulinumtoxin-A or Onaboulinumtoxin-A, could also be effective in facial pore reduction.14–19 However, considering that intradermal injection of botulinum toxin could be more immunogenic than an intramuscular or subcutaneous injection, treatment of enlarged pores with INCO might be safer due to its lower antigenicity compared to other botulinum toxin formulations.2,10,28,29
In this study, pore count and density decreased by approximately 62% at 6 months after a single session of combined treatment with MFU-V and intradermal INCO. Compared to the reduction of up to 25% at 4 months after MFU-V monotherapy11 and the reduction of up to 24% at 1 month after INCO monotherapy,10 the combined treatment led to even greater improvement. Notably, there was no rebound increase in pore count or density over the entire follow-up period. Pore count and density started to decrease 1-week post-treatment, and continued decreasing until 24 weeks. The combination of MFU-V and intradermal INCO may produce a synergistic effect in reducing enlarged facial pores due to different mechanisms. The slower-onset and sustained efficacy of MFU-V and faster-acting and relatively short-lived effects of INCO may together contribute to prolonged pore reduction compared to MFU-V or INCO alone. In line with the objective improvement, both physician and patient GAIS scores showed good results up to week 24, especially with 95% of patients responding with a grade 3 (much improved) or higher at week 24. However, there was a slight difference in the results that GAIS scores reached their best result at week 1, despite the maximum objective improvement being observed at week 24. This discrepancy may be due to skin swelling immediately after the treatment rather than the actual reduction in pore count and density. Furthermore, all adverse events were transient.
This study has some limitations. First, this study was a single-center retrospective study. Second, the efficacy of the procedures was investigated without a control group and each procedure alone (MFU-V alone or INCO alone). However, this is the first study to demonstrate the effects of combined treatment with superficial MFU-V and intradermal INCO for enlarged facial pores up to 24 weeks after the procedures using both objective measurements and subjective rating scales. Further studies involving prospective split-face studies of MFU-V and INCO and investigations of other combinations of treatment modalities for enlarged pores are needed.
In conclusion, combined treatment with MFU-V and intradermal INCO could effectively and safely reduce enlarged facial pores. The improvements may be sustained for up to 24 weeks after a single treatment.
Funding
Funding and products were provided by Merz Asia Pacific Pte. Ltd.
Disclosure
The authors have nothing to declare.
References
1. Lee SJ, Seok J, Jeong SY, Park KY, Li K, Seo SJ. Facial pores: definition, causes, and treatment options. Dermatologic Surgery. 2016;42:277–285.
2. Park JY, Chen JF, Choi H, et al. Insights on skin quality and clinical practice trends in Asia pacific and a practical guide to good skin quality from the inside out. J Clin Aesthet Dermatol. 2022;15:10–21.
3. Roh M, Han M, Kim D, Chung K. Sebum output as a factor contributing to the size of facial pores. Br J Dermatol. 2006;155:890–894.
4. Sugiyama-Nakagiri Y, Sugata K, Iwamura M, Ohuchi A, Kitahara T. Age-related changes in the epidermal architecture around facial pores. J Dermatol Sci. 2008;50:151–154.
5. Mizukoshi K, Takahashi K. Analysis of the skin surface and inner structure around pores on the face. Skin Res Technol. 2014;20:23–29.
6. Sugata K, Nishijima T, Kitahara T, Takema Y. Confocal laser microscopic imaging of conspicuous facial pores in vivo: relation between the appearance and the internal structure of skin. Skin Res Technol. 2008;14:208–212.
7. Kim SJ, Shin MK, Back JH, Koh JS. Pore volume is most highly correlated with the visual assessment of skin pores. Skin Res Technol. 2014;20:429–434.
8. Uhoda E, Piérard-Franchimont C, Petit L, Piérard GE. The conundrum of skin pores in dermocosmetology. Dermatology. 2005;210:3–7.
9. Dong J, Lanoue J, Goldenberg G. Enlarged facial pores: an update on treatments. Cutis. 2016;98:33–36.
10. Park JY, Cho SI, Hur K, Lee DH. Intradermal microdroplet injection of diluted incobotulinumtoxin-a for sebum control, face lifting, and pore size improvement. J Drugs Dermatol. 2021;20:49–54.
11. Vachiramon V, Namasondhi A, Anuntrangsee T, Kositkuljorn C, Jurairattanaporn N. A study of combined microfocused ultrasound and hyaluronic acid dermal filler in the treatment of enlarged facial pores in Asians. J Cosmet Dermatol. 2021;20:3467–3474.
12. Lee HJ, Lee KR, Park JY, Yoon MS, Lee SE. The efficacy and safety of intense focused ultrasound in the treatment of enlarged facial pores in Asian skin. J Dermatolog Treat. 2015;26:73–77.
13. Lu PH, Yang CH, Chang YC. Quantitative Analysis of Face and Neck Skin Tightening by Microfocused Ultrasound With Visualization in Asians. Dermatologic Surgery. 2017;43:1332–1338.
14. Sayed KS, Hegazy R, Gawdat HI, et al. The efficacy of intradermal injections of botulinum toxin in the management of enlarged facial pores and seborrhea: a split face-controlled study. J Dermatolog Treat. 2021;32:771–777.
15. Shuo L, Ting Y, KeLun W, Rui Z, Rui Z, Hang W. Efficacy and possible mechanisms of botulinum toxin treatment of oily skin. J Cosmet Dermatol. 2019;18:451–457.
16. Min P, Xi W, Grassetti L, et al. Sebum Production Alteration after Botulinum Toxin Type A Injections for the Treatment of Forehead Rhytides: a Prospective Randomized Double-Blind Dose-Comparative Clinical Investigation. Aesthetic Surgery j. 2015;35:600–610.
17. Rose AE, Goldberg DJ. Safety and efficacy of intradermal injection of botulinum toxin for the treatment of oily skin. Dermatologic Surgery. 2013;39:443–448.
18. Shah AR. Use of intradermal botulinum toxin to reduce sebum production and facial pore size. J Drugs Dermatol. 2008;7:847–850.
19. Rho NK, Gil YC. Botulinum Neurotoxin Type A in the Treatment of Facial Seborrhea and Acne: evidence and a Proposed Mechanism. Toxins. 2021;13:45.
20. Palawisuth S, Manuskiatti W, Apinuntham C, Wanitphakdeedecha R, Cembrano KAG. Quantitative assessment of the long-term efficacy and safety of a 1064-nm picosecond laser with fractionated microlens array in the treatment of enlarged pores in Asians: a case-control study. Lasers Surg Med. 2022;54:348–354.
21. Park JY, Lin F, Suwanchinda A, et al. Customized Treatment Using Microfocused Ultrasound with Visualization for Optimized Patient Outcomes: a Review of Skin-tightening Energy Technologies and a Pan-Asian Adaptation of the Expert Panel’s Gold Standard Consensus. J Clin Aesthet Dermatol. 2021;14:E70–e9.
22. Suh DH, Shin MK, Lee SJ, et al. Intense focused ultrasound tightening in Asian skin: clinical and pathologic results. Dermatologic Surgery. 2011;37:1595–1602.
23. White WM, Makin IR, Slayton MH, Barthe PG, Gliklich R. Selective transcutaneous delivery of energy to porcine soft tissues using Intense Ultrasound (IUS). Lasers Surg Med. 2008;40:67–75.
24. Laubach HJ, Makin IR, Barthe PG, Slayton MH, Manstein D. Intense focused ultrasound: evaluation of a new treatment modality for precise microcoagulation within the skin. Dermatologic Surgery. 2008;34:727–734.
25. Shome D, Vadera S, Ram MS, Khare S, Kapoor R. Use of Micro-focused Ultrasound for Skin Tightening of Mid and Lower Face. Plastic Reconstructive Surgery Global Open. 2019;7:e2498.
26. Fabi SG, Joseph J, Sevi J, Green JB, Peterson JD. Optimizing Patient Outcomes by Customizing Treatment With Microfocused Ultrasound With Visualization: gold Standard Consensus Guidelines from an Expert Panel. J Drugs Dermatol. 2019;18:426–432.
27. Kandhari R, Kaur I, Gupta J, Al-Niaimi F. Microdroplet botulinum toxin: a review. J Cutan Aesthet Surg. 2022;15:101–107.
28. Park JY, Sunga O, Wanitphakdeedecha R, Frevert J. Neurotoxin impurities: a review of threats to efficacy. Plastic Reconstructive Surgery Global Open. 2020;8:e2627.
29. Dressler D. Five-year experience with incobotulinumtoxinA (Xeomin(®): the first botulinum toxin drug free of complexing proteins. Eur J Neurol. 2012;19:385–389.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.