Back to Journals » Clinical Ophthalmology » Volume 16
Combined Photoactivated Chromophore for Infectious Keratitis-Corneal Collagen Cross-Linking (PACK-CXL) and Therapeutic Penetrating Keratoplasty for Resistant Bacterial Keratitis
Authors Bor'i A , El-Haig WM
Received 18 November 2021
Accepted for publication 16 January 2022
Published 3 February 2022 Volume 2022:16 Pages 273—279
DOI https://doi.org/10.2147/OPTH.S348835
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Ashraf Bor’i,1,2 Wael M El-Haig1,2
1Ophthalmology Department, Zagazig University, Zagazig, Sharkia, Egypt; 2Alfat’h Eye Hospital, Zagazig, Sharkia, Egypt
Correspondence: Ashraf Bor’i
Ophthalmology Department, Zagazig University, PO Box 44286, Zagazig, Sharkia, Egypt
, Tel +20 1065338080
, Email [email protected]
Purpose: To report the results of treating resistant bacterial keratitis by corneal collagen cross-linking followed by therapeutic penetrating keratoplasty and to compare with those of therapeutic penetrating keratoplasty alone.
Methods: Retrospective analysis of the medical records of 33 eyes of 33 patients diagnosed with resistant bacterial keratitis. Fourteen eyes (14 patients) were treated with photoactivated chromophore for infectious keratitis corneal collagen cross-linking (PACK-CXL) followed by therapeutic penetrating keratoplasty (TPK) (group I) and 19 eyes (19 patients) were treated by TPK alone (group II). The main outcome measures were graft clarity and the mean best corrected visual acuity at 1, 3, 6, 12 and 18 months after penetrating keratoplasty.
Results: The mean age of the patients was 53.6 ± 1.9 years and 52.3 ± 1.8 years in group I and group II, respectively (p = 0.374), the mean ulcer size was 49.9 ± 16.2 mm2 and 54.7.1 ± 15.1 mm2 in group I and group II, respectively (p = 0.239), the mean corneal infiltrate size was 58.2 ± 17mm2 and 59.9 ± 15.7 mm2 in group I and group II, respectively (p = 0.384). Hypopyon was seen in 6 eyes (41.7%) in group I and in 8 eyes (42.1%) in group II. At the last follow-up visit, 12 corneal grafts (85.7%) maintained their clarity in group I while 13 corneal grafts (68.4%) maintained their clarity in group II (p = 0.037) and the mean best corrected visual acuity was 0.84 ± 0.63 log MAR in group I and 1.27 ± 0.81 log MAR in group II (p = 0.024). Postoperatively, one eye (7%) showed graft reinfection in group I that was controlled medically while 5 eyes (26.3%) showed resistant graft reinfection and ended in graft opacification in group II (p = 0.042).
Conclusion: In resistant bacterial keratitis, priming infected corneas with PACK-CXL before performing TPK improve the results in such cases.
Keywords: resistant bacterial keratitis, therapeutic penetrating keratoplasty, PACK-CXL and graft failure
Microbial keratitis is a significant cause of visual disability in the rural areas of the developing world. Despite the advances in the corneal diagnostic tools and the array of available antimicrobial agents and their different methods of application, many cases show resistance.1–4
Antimicrobial resistance is usually developed due to multiple factors including the improper diagnosis, the injudicious use of antibiotics, the presence of untreated ocular or systemic risk factors and the antibiotic resistance or toxicity.3,4 All these factors may lead to progression of the disease with eventual severe corneal scarring, perforation or melting.2–6
Many surgical options are proposed in the context of managing refractory microbial keratitis to medical therapy, of which TPK.7 The decision to carry out TPK in such cases is usually delayed though it was shown to be effective in eliminating the infectious disease process. TPK is challenging intraoperatively and postoperatively but enables the corneal surgeon to get rid of the infected cornea and in cases of corneal perforation to restore the structural integrity of the globe by the newly transplanted graft.8–10
Many published data have proved the effective antibacterial actions of PACK-CXL through the released reactive oxygen species from photoactivated riboflavin that can destroy microbial nucleic acids and inhibit their replication and the sterilizing effect of ultraviolet A irradiation to the cornea. Moreover, it increases the resistance of corneal collagen to proteolysis by the microbial enzymes.11–15
The incidence of graft failure after TPK was reported to be about 22%, 42% and 63% at 1, 3, and 5 years, respectively, after surgery, owing to the possible residual infection at the host bed, corneal vascularization, large graft size, low graft endothelial cell count, corneal perforation, and postoperative shallowing of the anterior chamber or glaucoma.16,17
In this study, we aimed at comparing the clinical outcomes of performing PACK-CXL followed by TPK to those of TPK in managing refractory bacterial keratitis.
Patients and Methods
We retrospectively reviewed the medical records of 33 patients (33 eyes) who were diagnosed with resistant bacterial keratitis and treated by TPK since April 2016. Two groups of patients were identified. Group I: 14 patients (14 eyes) managed by PACK-CXL followed by TPK with the objective to enhance the clinical outcomes of TPK. Group II: 19 patients (19 eyes) treated by TPK solely. The protocol of the study was approved by the IRB of Alfat’h Eye Hospital (Zagazig, Egypt). All the patients were operated by AB in Alfat’h Eye Hospital (Zagazig, Egypt) in accordance with the WMA Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subjects and the treatment protocol was fully discussed with all patients who then provided written consent about the procedure and the release of their medical records.
The inclusion criteria were eyes diagnosed with resistant bacterial keratitis involving the visual axis and not exceeding the middle third of the corneal stroma that were primarily treated with the proper topical antibiotics for two weeks and showed no improvement or getting worse during treatment. All patients were subjected to careful history taking, thorough ophthalmological examination including visual acuity assessment, slit-lamp biomicroscopy and dilated fundus examination when feasible. Anterior segment optical coherence tomography (AS-OCT) was used to evaluate the ulcer and infiltrate depth, corneal thickness and anterior chamber content and depth, and B-scan ultrasonography to assess the posterior segment.
Patients included in this study presented with resistant infectious keratitis as evident by the duration of illness and the multiplicity of the previously prescribed antimicrobial drugs. Corneal scrapings, obtained under topical anesthesia from all patients 48 hours after cessation of their previous medications, were sent for microbiological examinations. Patients with culture-proven bacterial keratitis and who fulfilled the other inclusion criteria were included.
PACK-CXL Technique
PACK-CXL was done under topical anaesthesia. Corneal epithelium was removed in 10 mm diameter using Bard-Parker blade No.15 with removal of necrotic tissue at the site of ulcer, corneal thickness was measured at the floor of the ulcer using PachPen (Accutome, Inc., Malvern, Pennsylvania, USA). If the corneal thickness was more than 400 um, isotonic riboflavin (MedioCROSS M 0.1% riboflavin, 1.1% hydroxypropyl methylcellulose (HPMC); Peschke Meditrade GmbH, Huenenberg, Switzerland) was used and if the thickness was less than 400 um and above 350 um hypotonic riboflavin (MedioCROSS H 0.1%; Peschke Meditrade GmbH) was used. Riboflavin was instilled topically on the cornea every 2 minutes for a duration of 30 minutes, after that the cornea was irradiated by UVA 365 nm (3 mW/cm2, total dose of 5.4 J/cm2 for 30 minutes, and diameter of irradiation = 10 mm) using a Phoenix UVA system (VEGA, UV EMITTER, FIRENZE, ITALY) with continuous instillation of riboflavin every 2 minutes, finally, soft bandage contact lens was placed and the patient was discharged with continuation of the same topical medications that were used prior to PACK-CXL. All patients were examined daily until TPK was done. In every visit, slit-lamp biomicroscopy was done to detect re-epithelialization of the cornea, size and depth of corneal infiltrates, AC inflammation and any complication was reported and managed.
TPK Technique
All TPK procedures were done under general anesthesia. The donor cornea was punched out from the endothelial side using a Barron donor punch (Katena Products, Denville, NJ) with a diameter 0.25 mm to 0.5 mm larger than that of the recipient. The recipient cornea was trephined using a Hessburg–Barron vacuum trephine (Katena Products, Denville, NJ) including the infected area and about 0.25 mm larger. The corneal button was then excised with curved corneal scissors. Any pus in the anterior chamber was irrigated, and the membranes over the iris and/or lens were removed gently by forceps if existed. After suturing the donor cornea with the main 4 interrupted 10–0 nylon sutures, 12 interrupted 10–0 nylon sutures were added. Postoperatively, all eyes received topical antibiotic eye drops that were stopped one month after surgery and steroid eye drops that were tapered along 6 months duration. We intensified the frequency of topical steroids in the early postoperative period after TPK, in addition to the use of cycloplegic eye drops to minimize the possibility of postoperative inflammation and its consequences together with the same antimicrobial therapy that was used before performing TPK. All patients were examined on the first postoperative day and every week during the first 3 months and monthly till the end of the follow-up period. At each follow-up visit, eyes were examined for manifestations of infectious keratitis, corneal clarity, BCVA and refractive error. According to the graft status at the last follow-up visit, graft survival was classified into clear, endothelial decompensation and appearance microbial keratitis. Statistical analysis of preoperative and postoperative data was performed using the Student paired t-test and Chi square. Graft survival was analyzed using the Fisher's exact test. P < 0.05 was considered statistically significant.
Results
Thirty-three eyes of 33 patients were enrolled in this study, 2 groups of patients were identified. Group I included 14 eyes who received TPK shortly after PACK-CXL and group II included 19 eyes who received TPK alone.
Baseline Clinical Findings
There was no statistically significant difference between both groups as regards patients’ age, maximum ulcer size, corneal infiltrate size, anterior chamber reaction, hypopyon, preoperative BCVA, thinnest pachymetry measured with AS-OCT and causative bacterial agent (Table 1). The most commonly encountered causative bacterial agents were Pseudomonas aeruginosa (4 eyes) (28.6%) followed by Staphylococcus aureus (3 eyes) (21.4%) in group I and were also Pseudomonas aeruginosa (4 eyes) (21%) and Staphylococcus aureus (4 eyes) (21%) in group II (Table 2).
 |
Table 1 Basic Characteristics of Patients in the Study Groups |
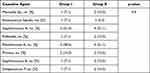 |
Table 2 Causative Bacterial Agents in the Study Groups |
Results of PACK-CXL
Isotonic riboflavin was used in 7 eyes (50%) in which the thinnest pachymetry was ≥400 u and hypotonic riboflavin was used in 7 eyes (50%) in which the corneal thickness was <400 u and ≥350 u measured with the PachPen. Complete epithelial healing was seen in 4 eyes (28.6%) after PACK-CXL, the mean epithelial healing time was 10.25±5.36 days (range 6–13 days). Incomplete epithelial healing was seen in 10 eyes (71.4%). After PACK-CXL, no eye showed progression of infiltration, increase in the ulcer size or perforation. Before PACK-CXL, the mean BCVA was 1.78±0.48 log MAR (range 1.0–3.0 log MAR). After PACK-CXL and shortly before PK the mean BCVA was 1.69±0.44 log MAR (range 1.0–2.4 log MAR).
Results of Penetrating Keratoplasty
The mean time from PACK-CXL to PK was 8±2 days (range 7–15 days). The mean donor graft preservation time was 11±1.8 days (range 8–14 days) in group I and was 11.76±1.6 days (range 9–14 days) in group II (p-value=0.151). The mean graft donor age was 59±14.25 years (range 26–75 years) in group I and was 59.9±13.67 years (range 33–79 years) in group II w (p-value=0.410). The mean graft endothelial cell count was 2389.85±176.75 cells/mm2 (range 2121–2704 cells/mm2) in group I and was 2509.76±205.48 cells/mm2 (range 2189–2903 cells/mm2) (p-value=0.082). The mean graft diameter was 8.21±0.47 mm (range 7.5–9 mm) in group I and was 8.26±0.45 mm (range 7.5–9 mm) in group II (p-value=0.422). The mean follow-up period after TPK was 19.43±1.39 months (range 19–23 months) in group I and was 18.88±1.45 months (range 18–22 months) in group II with no statistically significant difference between both groups (p-value=0.060).
Eighteen months after TPK, the mean BCVA was 0.84±0.63 log MAR (range 0.2–3.0 log MAR) in group I and the mean BCVA was 1.27±0.81 log MAR (range 0.2–3.0 log MAR) in group II. Significant improvement of the BCVA was noticed in both groups with statistically significant improvement in the PACK-CXL TPK group when compared to the TPK group (p = 0.024) (Figure 1).
 |
Figure 1 Changes in the BCVA of patients in the study groups up to 18 months postoperative. |
Postoperatively, in group I, increased IOP was recorded in 4 eyes (28.6%), which was controlled medically in 2 eyes. The other two eyes (14.3%) went into further complications and ended into graft failure. One eye (7%) showed graft ulcer with superficial infiltration diagnosed during the second month with slit lamp biomicroscopy and documented with AS-OCT, culture and sensitivity tests were negative and managed with fortified ceftazidime and vancomycin eye drops with cessation of steroid eye drops. It showed complete healing after 11 days from modification of treatment.
In group II, increased IOP was recorded in 5 eyes (26.3%), 4 eyes of which were controlled medically and the fifth eye needed surgical interference that ended into graft failure. Five eyes (26.3%) developed resistant graft ulcers despite topical treatment diagnosed during the first 3 postoperative months and ended into opacification and graft failure (Table 3).
 |
Table 3 Postoperative Findings of Patients in the Study Groups |
Discussion
Microbial keratitis represents a clinical challenge that mandates prompt interference to avoid deleterious complications and visual handicap. The emergence of resistant bacterial strains and the indiscriminate use of antimicrobial agents resulted in the appearance of recalcitrant forms of keratitis with eventual anatomical and functional ocular damage.
Surgical intervention in the form of TPK is usually needed to eradicate the infection, restoration of the ocular anatomy and regain useful vision.18,19 TPK represents about 40% of all indications of corneal grafts in some developing countries.20 However, resorting to corneal transplantation in eyes with microbial keratitis has its inherent complications as it has a higher incidence of graft infection, rejection or failure. Recurrent keratitis after TPK is very resistant and may be caused by clinically undetectable residual infection in the host corneal rim and may destroy the graft with the need for repeat graft.21–24
In light of the good results of using PACK-CXL to eliminate microbial keratitis11,14,25 a novel approach was pursued to manage resistant bacterial keratitis, which entailed performing PACK-CXL in one session followed shortly by TPK in order to improve the results of hot keratoplasty.
The present study was conducted on 33 eyes of 33 patients who presented with resistant bacterial keratitis. Two groups of patients were identified, group I (14 eyes) received TPK after Pack-CXL and group II (19 eyes) received TPK alone.
The number of eyes that maintained graft clarity was significantly higher in the group of patients managed by sequential than those treated by TPK alone (p = 0.037). In the present study, rates of graft clarity after only TPK group (68%) were comparable to those of Chen et al (68.7%) and Ti et al (76.6%).18,26
Superior functional results were also achieved by PACK-CXL and TPK treatment when compared to those treated by only TPK. Ten eyes (71%) in group I and 11 eyes (57.9%) in group II had BCVA better than 1.00 log MAR (p = 0.024). BCVA more than 1.00 log MAR was reported to range from 25% to 80% in previous studies that used TPK only for resistant bacterial keratitis. The difference in results could be attributed to the delay in performing TPK till limbal extension of infection or operating on perforated corneal ulcers.17,20,21
The value of performing PACK-CXL before TPK was translated to lower recurrent graft infection rate in group I as compared to group II (p 0.042). Graft reinfection rate in group II (26%) was not different from those reported in the previous studies (7–29%) by Yalniz-Akkaya et al, Bajracharya and Gurung, and Sharma et al.19,20,22
Increased IOP was reported in 4 eyes (28.6%) in group I and in 5 eyes (26.3%) in group II which was similar to that reported in previous studies.19,22
In group I, TPK was performed 7 days or more after PACK-CXL, this lag period was attributed to the time taken till the corneal graft became available and also waiting for subsidence of the CXL-induced corneal inflammation. The mean time from PACK-CXL to PK was 8±2 days (range 7–15 days).
With the current treatment regimen used in group I, only 2 eyes (14%) showed graft failure and the rest of eyes (12 eyes) maintained their clarity at least 18 months after TPK. According to previous studies, graft failure can range from 10% to 50% and this wide range of variability may be due to the different patients’ characteristics included in these studies. In the present study, the higher incidence of graft survival and clarity in group I can be attributed to the effect of PACK-CXL in eradicating infection particularly all eyes included were bacterial in etiology, and the early surgical interference before corneal perforation or limbal spread. The effect of PACK-CXL was also maximized by using a large irradiation diameter of 10mm thus covering the host residual corneal rim which may have harbored any bacterial colonies.
In conclusion, PACK-CXL followed by early TPK can lead to increased graft clarity, graft survival rate and reduced rate of graft reinfection. Accordingly, combined PACK-CXL and TPK may offer a new management option in the armamentarium combating recalcitrant bacterial keratitis. However, the study has its own limitations, being retrospective and including small cohort of patients. A randomized clinical trial is recommended to support the results of the current work.
Disclosure
The manuscript was read and approved by the authors. The authors did not receive any funds throughout the whole work. The authors declare that they do not have any conflicts of interest for this work.
References
1. Keay L, Edwards K, Naduvilath T, et al. Microbial keratitis predisposing factors and morbidity. Ophthalmology. 2006;113:109–116. doi:10.1016/j.ophtha.2005.08.013
2. Austin A, Lietman T, Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmology. 2017;124:1678–1689. doi:10.1016/j.ophtha.2017.05.012
3. Ting DSJ, Ho CS, Deshmukh R, DG Said, HS Dua. Infectious keratitis: an update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance. Eye. 2021;35(4):1084–1101. doi:10.1038/s41433-020-01339-3
4. Egrilmez S, Yildirim-Theveny Ş. Treatment-resistant bacterial keratitis: challenges and solutions. Clin Ophthalmol. 2020;29(14):287–297. doi:10.2147/OPTH.S181997
5. Asbell PA, Sanfilippo CM, Pillar CM, DeCory HH, Sahm DF, Morris TW. Antibiotic resistance among ocular pathogens in the United States: five-year results from the antibiotic resistance monitoring in ocular microorganisms (ARMOR) surveillance study. JAMA Ophthalmol. 2015;133:1445–1454. doi:10.1001/jamaophthalmol.2015.3888
6. Sharma N, Sachdev R, Jhanji V, Titiyal JS, Vajpayee RB. Therapeutic keratoplasty for microbial keratitis. Curr Opin Ophthalmol. 2010;21(4):293–300. doi:10.1097/ICU.0b013e32833a8e23
7. Khor WB, Prajna VN, Garg P, et al. The Asia cornea society infectious keratitis study: a prospective multicenter study of infectious keratitis in Asia. Am J Ophthalmol. 2018;195:161–170. doi:10.1016/j.ajo.2018.07.040
8. Henry CR, Flynn HW
9. Hossain P, Tourkmani AK, Kazakos D, Jones M, Anderson D. Emergency corneal grafting in the UK: a 6-year analysis of the UK transplant registry. Br J Ophthalmol. 2018;102:26–30. doi:10.1136/bjophthalmol-2016-309870
10. Abbouda A, Abicca I, Alió JL. Current and future applications of Photoactivated Chromophore for Keratitis-Corneal Collagen Cross-Linking (PACK-CXL): an overview of the different treatments proposed. Semin Ophthalmol. 2016. doi:10.3109/08820538.2015.1123731
11. Hsia YC, Moe CA, Lietman TM, et al. Expert practice patterns and opinions on corneal cross-linking for infectious keratitis. BMJ Open Ophthalmol. 2018;3:e000112. doi:10.1136/bmjophth-2017-000112
12. Makdoumi K, Mortensen J, Crafoord S. Infectious keratitis treated with corneal crosslinking. Cornea. 2010;29(12):1353–1358. doi:10.1097/ICO.0b013e3181d2de91
13. Chan TCY, Lau TWS, Lee JWY, Wong IYH, Jhanji V, Wong RLM. Corneal collagen cross-linking for infectious keratitis: an update of clinical studies. Acta Ophthalmol. 2015;93(8):689–696. doi:10.1111/aos.12754
14. Knyazer B, Krakauer Y, Baumfeld Y, Lifshitz T, Kling S, Hafezi F. Accelerated corneal cross-linking with photoactivated chromophore for moderate therapy-resistant infectious keratitis. Cornea. 2018;37(4):528–531. doi:10.1097/ICO.0000000000001498
15. McLeod SD, LaBree LD, Tayyanipour R, et al. The importance of initial management in the treatment of severe infectious corneal ulcers. Ophthalmology. 1995;102:1943–1948. doi:10.1016/S0161-6420(95)30771-3
16. Maguire MG, Stark WJ, Gottsch JD, et al. Risk factors for corneal graft failure and rejection in the collaborative corneal transplantation studies. Ophthalmology. 1994;101(9):1536–1547. doi:10.1016/S0161-6420(94)31138-9
17. Tan DT, Janardhanan P, Zhou H, et al. Penetrating keratoplasty in Asian eyes: the Singapore corneal transplant study. Ophthalmology. 2008;115:975–982. doi:10.1016/j.ophtha.2007.08.049
18. Chen WL, Wu CY, Hu FR, Wang IJ. Therapeutic penetrating keratoplasty for microbial keratitis in Taiwan from 1987 to 2001. Am J Ophthalmol. 2004;137(4):736–743. doi:10.1016/j.ajo.2003.11.010
19. Yalniz-Akkaya Z, Burcu A, Dogan E, Onat M, Ornek F. Therapeutic penetrating keratoplasty for infectious and non-infectious corneal ulcers. Int Ophthalmol. 2015;35:193–200. doi:10.1007/s10792-014-9931-y
20. Bajracharya L, Gurung R. Outcome of therapeutic penetrating keratoplasty in a tertiary eye care center in Nepal. Int Ophthalmol. 2015;9:2299–2304.
21. Sony P, Sharma N, Vajpayee RB, Ray M.Therapeutic keratoplasty for infectious keratitis: a review of the literature. CLAO J. 2002;28(3):111–118.
22. Sharma N, Jain M, Sehra SV, et al. Outcomes of therapeutic penetrating keratoplasty from a tertiary eye care centre in Northern India. Cornea. 2014;33:114–118. doi:10.1097/ICO.0000000000000025
23. Bates AK, Kirkness CM, Ficker LA, Steele AD, Rice NS. Microbial keratitis after penetrating keratoplasty. Eye. 1990;4(Pt 1):74–78. doi:10.1038/eye.1990.8
24. Sun J-P, Chen W-L, Huang J-Y, Hou Y-C, Wang I-J, Hu F-R. Microbial keratitis after penetrating keratoplasty. Am J Ophthalmol. 2017;178:150–156. doi:10.1016/j.ajo.2017.03.022
25. Ting DSJ, Henein C, Said DG, Dua HS. Photoactivated chromophore for infectious keratitis - corneal cross-linking (PACK-CXL): a systematic review and meta-analysis. Ocul Surf. 2019;17(4):624–634. doi:10.1016/j.jtos.2019.08.006
26. Ti SE, Scott JA, Janardhanan P, Tan DT. Therapeutic keratoplasty for advanced suppurative keratitis. Am J Ophthalmol. 2007;143(5):755–762. doi:10.1016/j.ajo.2007.01.015
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
